Consequences of Recurrence of Major Depressive Disorder: Is Stopping Effective Antidepressant Medications Ever Safe?
Abstract
According to the World Health Organization, major depressive disorder is the world’s leading cause of disability. If clinical remission is not attained and sustained, episodes tend to recur with greater severity and with lessening responsivity to conventional treatments. Reasonably well-established clues and guidelines are presented about the high risk and profound consequences of recurrence of major depressive disorder if successful antidepressant treatments are discontinued. The authors describe actions required to achieve a “lifetime wellness” focus for treatment. Current approaches would need to be transformed from attainment of clinical remission to attainment and maintenance of lifetime wellness, with the knowledge that some individuals may need continuous treatment. Risk factors would need to be assessed and used to formulate clinical treatment guidelines for risk of recurrence. Clinical trials would need to be greatly lengthened. Measurement-based care and precision medicine would be the foundation for informing clinical decisions. The authors provide guidance in determining how to discontinue antidepressants if that decision is made despite risks.
Major depressive disorder is a pervasive, toxic, highly prevalent, early-onset illness, ignominiously holding the World Health Organization’s (WHO’s) ranking as the leading cause of disability (1, 2). Its litany of devastation is frightening. High prevalence, heterogeneous causes of depressions, young ages of onset, and variable treatment responses are well understood as leading reasons for disability.WHO data estimate that more than 300 million people worldwide struggle with depressive illnesses. Depressive illnesses tend to start young, with onsets peaking between ages 15 and 24 (3). An estimated 30% of persons with depressive illnesses fail to respond to multiple, evidence-based interventions (4), and the depression is then categorized as “treatment resistant.”
In addition, depressive illnesses are too-often fatal. WHO data document that more than 800,000 individuals worldwide die from suicide each year (5), and the United States is currently struggling to contain what could be labeled a suicide epidemic (6). Although comorbid disorders may be the norm for those who die by suicide, depression is perceived as the most common underlying illness (2). Mortality from nonsuicide causes also occurs more frequently and earlier among those with depressive illnesses; in fact, those with untreated depression die at a rate 20 times higher than the rate in the general population (7).
Financial consequences of recurrent depression are equally disturbing. As summarized by Grazier (2), “A World Economic Forum/Harvard School of Public Health estimated that the cumulative global impact of mental disorders in terms of lost economic output will amount to $16.3 trillion between 2011, and 2030.” Brain-behavior illnesses are at the root of $30 to $50 billion in estimated annual salary equivalency loss (8, 9). This explains why major depressive disorder has been labeled the second-costliest disorder in the United States (2), second only to heart diseases. The disability, mortality, and financial costs associated with major depressive disorder are augmented further by family turmoil, divorces, and social upheaval (2, 3).
Although most of the reasons for the high social and financial costs of major depressive disorder described above important role of recurrence is understudied and often overlooked. Recognizing and seeking to prevent recurrent longitudinal episodes of major depressive disorder to help diminish the huge burdens associated with major depressive disorder is the focus of this article.
Recurrence of Depressive Episodes: Definitions, Key Variables, and Consequences
The proposed consensus definition for the term “recurrence” consists of a new-onset depressive episode following sustained clinical remission, meaning following recovery (10, 11). The pattern of recurrence is clinically variable but with some consistent features. In a 10-year prospective, naturalistic study by the National Institute of Mental Health, the probability of recurrence increased by 16% with each successive recurrence but showed a progressive decline as the duration of recovery lengthened (12).
Depressive episodes, once started, sometimes fade spontaneously, but they more often require treatment and sometimes, but not always, are successfully treated with psychotherapies, pharmaceutical agents, and other modalities (11, 13, 14). Even if clinical remission is attained, it is not always sustained. In fact, a recurring longitudinal course tends to develop among most individuals who experience a major depressive episode (11, 15, 16). Most frequently, when successful treatments are discontinued, after a variable time interval during which the individual does well, another new episode develops (17) (Figure 1).
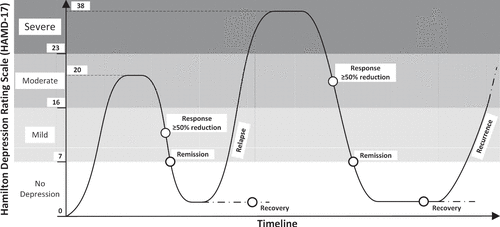
FIGURE 1. Longitudinal course of major depressive disorder, severity scores on the 17-item Hamilton Depression Rating Scale, and response terminology
Although adequate data have never been collected to quantify how much of the disability, mortality, and financial consequences associated with major depressive disorder are attributable to recurrent episodes, available evidence logically and strongly confirms that every new episode contributes to consequences. Well-documented morbidity data serve as a siren call for focusing more attention on beginning to treat major depressive disorder as a lifetime illness, more comparable to diabetes and bipolar illness. For bipolar illness, expert clinicians consider maintenance of wellness and adherence to mood-stabilizing interventions as integral and fundamental. Most would consider it anathema to stop lithium or mood-stabilizing medications soon after stability is achieved (18). However, similar long-term continuation of treatments has failed to become a core principle of treating most individuals with major depressive disorder.
Clinical Calls to Action for Objective Monitoring of Major Depressive Disorder
Figure 1 schematically portrays a recurrent episode of major depressive disorder. The figure makes several points and outlines the calls to action that will be required for objective monitoring of major depressive disorder and its recurring course to achieve the needed perspective of lifetime maintenance of wellness.
Measurement-based care, established scales, and mobile monitoring tools should be used in the treatment of all those with major depressive disorder.
The Hamilton Depression Rating Scale is used in Figure 1. The scale is one of many established measures to identify and quantify the longitudinal course of major depressive disorder, including recurrences (19, 20). When established rating scales are not used, clinicians are unable to accurately assess longitudinal changes and develop strategies for lifetime wellness. A comparable paradigm would be treating hypertension without measuring blood pressure. As described in many recent articles (21, 22), including some in this issue, the ever-increasing presence of mobile health applications, sensor-based health monitoring system coupled with other technological aids, can augment longitudinal and remote monitoring. Such tools should be adopted by all clinicians treating those with major depressive disorder and empower patients to become more engage in their overall care.
Severity terminology should be standardized and linked with objective measures.
As illustrated in the figure, conventional quantification of severity of recurrent episodes relies on ratings, such as Hamilton Depression Rating Scale scores—i.e. no depression, mild, moderate, and severe. Lacking such scores, no comparability is possible, and longer-term assessments following well-orchestrated antidepressant trials will never be reliable.
Degree of response terminology should be standardized.
Conventional clinical terms used to categorize degree of response following an episode are as follows: improvement (percentage decrease from baseline severity); response (≥50% decrease in rating scales from initial severity levels); remission (a score of ≤7 on the 17-item Hamilton Depression Rating Scale); and recovery (commonly used in clinical settings as a substitute generic term, generally unmeasured, to describe “remission” when traditional depressive, bipolar disorder, or anxiety symptoms have seemingly resolved; ratings have not been collected but clinicians deem that “wellness” has occurred).
Data-based prediction algorithms of risk of recurrences should be developed.
Risk algorithms similar to the Framingham risk prediction calculations for cardiovascular disease and prediction algorithms for cancer recurrences (23, 24) would be remarkably valuable—and potentially lifesaving for many. Such data could not be included in Figure 1 as they have yet to be collected.
Factors Indicating Higher Risks of Recurrence
Brain-behavior researchers are beginning to develop rudimentary prognostic models on which individuals’ quantifiable risks of developing future recurrence of major depressive disorder can be calculated (25). These are summarized in Figure 2 and are generally based on the following: partial symptomatic response or treatment failures, number of previous depressive episodes, co-occurring psychiatric or general medical illnesses that may intensify the major depressive disorder, psychosocial difficulties linked with persistent major depressive disorder, and certain demographic characteristics (15, 25–27). Premorbid risk factors for major depressive disorder were examined across the range of important domains in a large community-based sample of parents and offspring (N=2,764 subjects) and are also included in Figure 2. Almost all of the studied risk factors predicted the development of a subsequent major depressive episode. When the authors delineate the effects of onset of major depressive disorder on its course and course on onset, the risk of recurrence was found to be profoundly affected by earlier onset of recurrence. (26)
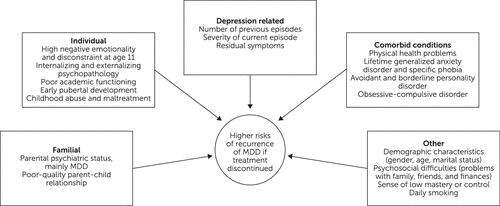
FIGURE 2. Factors suggesting higher risk of major depressive disorder (MDD) recurrence with treatment discontinuation
Although these risk factors could assist in determining appropriate treatment interventions, their absence—or the presence of opposite factors—do not equate with a low risk of major depressive disorder. Additional independent predictors and mediators of major depressive disorder entail genetic, epigenetic, biochemical, and anatomical factors (8, 9, 28, 29).
Guideline Programs to Help Prevent Recurrence
Lacking the desired robust database to formulate mathematical, artificial intelligence or machine learning–aided predictions of risk, “expert opinion” guidelines have been compiled to aid clinicians. A number of professional societies have forged clinical recommendations to guide clinicians in how, when, and for how long to prescribe maintenance treatment for major depressive disorder. Their various recommendations have similarities (Figure 3). They are predominantly based on “expert opinion” rather than long-term, data-driven studies.
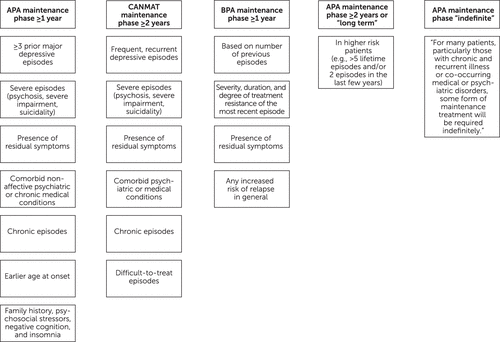
FIGURE 3. Clinical guidelines informing antidepressant maintenance therapya
aAPA, American Psychiatric Association (11); CANMAT, Canadian Network for Mood and Anxiety Treatments (30); British Association for Psychopharmacology (32).
Traditionally, maintenance therapy takes place after the acute phase and continuation phase treatment and is highly recommended to prevent recurrence of major depressive disorder (11). The American Psychiatric Association practice guideline for the treatment of major depressive disorder suggests three different time frames for maintenance therapy based on individuals’ risk factors: 1 year or longer, 2 years or longer, and indefinite (11). The guidelines of the Canadian Network for Mood and Anxiety Treatments recommend that patients with certain risk factors should maintain antidepressant treatment for 2 years or longer (30). Treatment guidelines for major depressive disorder from other countries do not differ much, particularly in their judgment of who should maintain therapy (31, 32). For patients considered higher-risk, the British Association for Psychopharmacology guidelines support 2 or more years of maintenance therapy (32).
Major depressive disorder risk factors should be embedded in clinicians’ daily practice and decision making. They should be discussed openly and collaboratively, as early as possible, with patients and families, with the goal of educating them and obtaining their involvement in order to tailor treatment accordingly (11, 30–32). Only then is adherence attainable, and adherence is vital for the prevention of recurrence.
Although these treatment guidelines are vague and less precise than desired, they fill a void. In our clinical experience, they tend to be conservative and somewhat duplicative, and perhaps they inadvertently buttress the erroneous concept that major depressive disorder is a short-term illness, one that can be eliminated or considered cured once remission has been achieved, even though there are few data to support that concept.
A “Lifetime Wellness” Focus for Major Depressive Disorder: The Time Has Come
The time has come for major depressive disorder to be categorized as a chronic illness, such as diabetes, heart diseases, and schizophrenia, for which prevention and maintenance of wellness interventions are imperative. The strategy is as follows: the earlier the detection, the sooner the longer-term planning for intervention and evidence-based maintenance treatment—and therefore, the better the outcome (Table 1). A look back at the incremental breakthroughs in functional and structural brain imaging tells us how far we have come in understanding the long-term neurobiological and neuroanatomical signatures of index episodes and cumulative recurrences of major depressive disorder (8, 9, 28, 29). Major depressive disorder is a complex, chronic illness, with unique pathophysiological mechanisms that runs a neuroprogressive course (29, 33). A hallmark of treating such illnesses is to halt or delay progression. For many individuals, perhaps most, the optimal approach might entail not stopping successful antidepressant interventions that have maintained stability. Perhaps the prototype medical parallel is continuation of and close adherence to a successful insulin regimen for those with diabetes.
| Characteristic | Diabetes | Cardiovascular diseases | Schizo-phrenia | Bipolar disorder | Major depressive disorder |
|---|---|---|---|---|---|
| Presence of prodromal symptoms | Yes | Yes | Yes | Yes | Yes |
| Months to years of symptoms progression before diagnosis | Yes | Yes | Yes | Yes | Yes |
| Predictive risks factors are identified and can inform treatment | Yes | Yes | Yes | Yes | Yes |
| Delayed detection and lack of treatment-induced tissue damage or illness progression | Yes (multiple organs) | Yes (multiple organs) | Yes (brain) | Yes (brain, heart) | Yes (brain, heart) |
| Illness captured through specific laboratory workup or imaging | Yes | Yes | Yes | Yes | Probably |
| Laboratory tests available to aid diagnosis | Yes | Yes | No | No | No |
| Stigma most likely delays detection and makes treatment more difficult | No | No | Yes | Yes | Yes |
| Possibility of treatment resistance | Yes | Yes | Yes | Yes | Yes |
| Early detection and treatment prevent tissue deterioration and treatment resistance | Yes | Yes | Yes | Yes | Yes |
| Effective treatments reverse tissue deterioration among patients with chronic and severe illnesses | Probably | Probably | Probably | Probably (inflammatory processes) | Probably (inflammatory processes) |
| Extended maintenance treatments available to sustain wellness | Yes | Yes | Yes | Yes | Yes |
| Pharmacotherapy or continuation psychotherapy or other interventional treatments are widely recognized as essential to maintain wellness and regularly maintained | Yes | Yes | Yes | Yes | No |
| Adherence problem when maintenance treatment prescribed | Yes | Yes | Yes | Yes | Yes |
TABLE 1. Comparison of major depressive disorder and other chronic, progressive illnessesa
Aided by strong epidemiological studies and highly beneficial biomarkers, the medical field is making commendable strides toward development of precision treatments that will be more capable of differentiating and effectively treating the heterogeneous causes of major depressions and attaining and helping maintain wellness and preventing recurrences. This is similar to treatment of other major medical illnesses, such as cancers and cardiovascular illnesses. Cancer clinicians have long espoused targeting long-term outcomes, such as “five-year survival” (34). In contrast, the duration of conventional clinical studies and outcome reports for major depressive disorder has been limited to weeks. This perspective ideally should be eliminated.
A Vision for The Future: Pursuit of Lifetime Wellness
Conceptualizing major depressive disorder as warranting a chronic-illness focus rather than an episode focus urges us to elevate treatment goals to vigorous pursuit and attainment and maintenance of lifetime wellness. Goals of clinical trials for the treatment of major depressive disorder will need to be revised to be similar to those for cancers, with established definitions of recurrence, confirmed by biomarkers, and “5-week improvement” being replaced by “5-year wellness.” Achieving wellness embodies a full restoration of premorbid levels of functioning and quality of life, beyond clinical remission (35, 36).
Although depressed individuals and their family members view functional remission as a key component of treatment goals (37), most of the well-designed efficacy trials for major depressive disorder treatments tend to emphasize symptom reduction as the primary aim at the expense of functional remission. When functional remission is taken into account, such data tend to significantly reduce the overall success rate of therapy (38, 39). In an observational study of 1,297 patients with major depressive disorder, Novick and colleagues (38) noticed that whereas 70.6% of patients achieved clinical remission, only 52.1% achieved both clinical and functional remission.
Maintaining Lifetime Wellness With Long-Term Antidepressant Therapy
The preponderance of data suggests that even with successful treatments for major depressive disorder, 50% to 85% of individuals will experience at least one lifetime recurrence (11). The effectiveness of long-term antidepressant treatment in preventing recurrent major depressive disorder, often modestly augmented by accompanying therapies, such as interpersonal therapy (40, 41), was convincingly established during the tricyclic antidepressant era. Newer classes of antidepressants seem to provide equal benefit in maintenance therapy, with a reasonable safety profile, although a consensus has yet to be reached (16, 42–44). A meta-analysis of 35 trials of antidepressants (N=7,253 subjects) spanning more than 2 years (odds ratio=2.27 years, 95% confidence interval=1.88–2.62), perhaps the largest published analysis of data on maintenance therapy, underscored a significant reduction in the recurrence of major depressive disorder by 50.7%, with a low number needed to treat (NNT=3.8, p<0.0001) (16). The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) study showed accrued benefits with venlafaxine over time in terms of prevention of recurrent major depressive disorder and improvement of psychosocial outcomes for outpatients with recurrent unipolar depression (45). Recurrence rates in the second maintenance year of the PREVENT study markedly decreased, compared with the first year (8% versus 23%). Such reduction of recurrent major depressive disorder also applied to treatment-naïve patients who achieved remission with initial monotherapy and opted to maintain antidepressant therapy (46). Such data may be further improved when coupled with pharmacogenomic data (47).
Effectiveness in preventing recurrence and maintaining lifetime wellness has yet to be investigated with long-term studies (multiple years in duration). In a study examining the efficacy data from all antidepressant maintenance trials submitted to the U.S. Food and Drug Administration between 1987 and 2012, almost all clinical trials lasted about 24 to 44 months, with only three continuing up to 52 weeks. Inadequate long-term data prevent a definitive statement of frequency and timing of recurrences and how effective maintenance treatments are in preventing them. The few longer-term treatment outcome studies that have been conducted are highly informative (41, 48). Many more similar, well-designed, long-term studies comparable to cancer trials are needed (41).
Potential Challenges to Long-Term Antidepressant Therapy
The decision to stop or maintain successful antidepressant treatment can be life-changing. Sadly, the pervasive stigma about mental illness and its treatment may contribute to premature discontinuation of treatment and higher risk of relapse (49). Data from 900 patients followed in a naturalistic treatment setting showed a proclivity to antidepressant discontinuation after 6 months, mainly through missed follow-up visits (50). From a patient’s perspective, common adverse effects, such as weight gain or sexual dysfunction, are strong signals encouraging their requests for discontinuation of antidepressant maintenance therapy—and so are fear and uncertainty about the long-term consequences of treatment (50–52). Nevertheless, overall documented “remission” benefits of treatment with regard to symptom reduction and improvement in quality of life very often exceed patients’ concerns about safety (50, 52, 53), and many come to recognize these benefits. Such recognition was supported in a survey of 180 long-term users of antidepressants (3–15 years) in which only 11% expressed a desire to stop antidepressants, even though more than half reported common adverse effects (51).
From a clinical provider’s perspective, emerging data appear to challenge the view that it is routinely safe to stop antidepressant treatment for patients who have responded and achieved short-term remission. Clinical practice guidelines have yet to enlighten us on the optimal length of antidepressant therapy for major depressive disorder. Perhaps, for some, successful antidepressant treatment can be terminated if reasonably justified by patients’ strongly stated preference, persisting and intolerable side effects, drug-drug interactions, development of adverse consequences that interfere with treatments used for other medical conditions (50, 54, 55), and a low profile of risk factors for recurrent major depressive disorder (Figure 2). There may also be a proclivity to wean first-time antidepressant responders who have reached sustained clinical and functional remission. A thoughtful and stepwise process, using the “WIRE” acronym, can be implemented to hopefully aid the discontinuation process (Box 1). The addition of cognitive-behavioral therapy during the weaning process has been found to reduce the risk of recurrence of major depressive disorder (55).
BOX 1. “WIRE” Guide to Discontinuing Antidepressants
Warn patients, their families, support systems, and other therapists about the frequency of recurrences and other possible risks following antidepressant discontinuation.
Involve family members in ongoing monitoring, measurement-based care as well as the development and implementation of a safety plan; and insist that monitoring continues into the future.
Recommend a slow taper of antidepressant over 4–6 months, with monitoring during that period.
Educate on importance of other preventive measures, continuation of psychotherapy, exercise, sleep monitoring, and safety plan for suicide thoughts and plans.
The Crucial Role of Precision Treatment
Precision medicine is currently changing the way we think about treatment and replacing the traditional reactive medicine approach. Precision medicine encourages us to expand our understanding of the heterogeneous causes of major depressive disorder, such as sleep apnea or substance misuse, and pay closer attention to co-occurring conditions when deciding treatment. It also includes the valuable addition of pharmacogenomics to guide psychotropic medication selection, minimize the potential for medication side effects, and identify certain genetic variations that perhaps could be corrected with oral supplements such as l-methylfolate for the methylenetetrahydrofolate reductase (MTHFR) gene variants (47, 56, 57). As recently demonstrated in a large, randomized, blindly-rated pharmacogenomics study, genetic “congruency matters” for successful treatment (47).
Adding to the existing data, more studies are being conducted to help predict remission of major depressive disorder and enable clinicians to make educated decisions about treatment selection, targeted interventions, and personalized long-term therapy (46, 58, 59). In the Veterans Affairs Augmentation and Switching Treatments for Improving Depression Outcomes (VAST-D) study, depressed patients with longer durations of the index medication trial, who had better quality of life and positive mental health and were employed, less severely and chronically depressed, less anxious, and who reported no childhood adversity or complicated grief symptoms were found to be more likely to achieve remission from a depressive episode with treatment (58). Within the same predictive model, researchers were able to delineate characteristics that would call for specific next-step treatment intervention, such as augmentation, combination, or a switching strategy. Neuroimaging biomarkers, such as posterior hippocampal volumes, have also been reported in early studies as potentially useful predictors of remission following antidepressant treatment (59). More studies are under way, but very few findings have been replicated in a manner that can affect clinical practice. Data translation into clinical practice remains a paramount component of precision medicine.
Conclusions
The focus of this article is primarily to highlight, alert, and summarize for clinicians reasonably well-established clues and guidelines about the high risk and profound consequences of recurrence of major depressive disorder for many when successful antidepressant treatments are discontinued. To attack the huge burdens of major depressive disorder, current medical approaches need to be transformed from the goal of attaining clinical remission and then using remission as justification for stopping treatments to the goal of attaining and maintaining lifetime wellness, with the knowledge that an unknown number of individuals may require indefinite continuation of treatment.
A vast array of future, longer-term research studies is needed to address these vital, potentially lifesaving questions. The heterogeneity of causes of major depressive disorder and bipolar illnesses must be recognized and treatments tailored to these causes. Risk variables should be assessed and used to formulate clinical guidelines for risk of recurrence. Investigation of biomarkers, such as sleep apnea studies, is required, with wider use of biomarkers among clinicians. Clinical guidelines have yet to enlighten us on the optimal length of antidepressant therapy for major depressive disorder, and to gain such wisdom, clinical trials will need to be greatly lengthened. Measurement-based care with accepted scales should be employed by all. And precision medicine will need to be front and center in informing clinical decisions. Such studies will be costly, but hypothetically cost-saving and life-saving.
As documented by previous studies, for individuals with moderate-to-severe major depressive disorder, combinations of antidepressant medications and established psychotherapies produce better outcomes than either approach alone (11). This article focuses on the role of antidepressant medications in preventing recurrent major depressive disorder and the risks of discontinuing successful antidepressant medication treatment; however, it warrants repeating that the roles of concomitant psychotherapy and psychosocial interventions, exercise, avoidance of substance misuse, healthy sleep patterns, and other approaches remain paramount. Continuation of successful “packages” of interventions is commendable. They help prevent relapse and recurrence, promote recovery from major depressive disorder’s sequelae, bolster safety interventions, restore quality of life, and enhance the possibilities of a lifetime of wellness experiences.
1 Major Depression. Bethesda, MD, National Institute of Mental Health, 2019. www.nimh.nih.gov/health/statistics/major-depression.shtml. Accessed Nov 21, 2019Google Scholar
2 (eds): Mental Health in the Workplace: Strategies and Tools to Optimize Outcomes. Basel, Switzerland, Springer, 2019Crossref, Google Scholar
3 : The costs of depression. Psychiatr Clin North Am 2012; 35:1–14Crossref, Google Scholar
4 : Treatment of Recurrent Depression. Washington, DC, American Psychiatric Publishing, 2001Google Scholar
5 Suicide Data. Geneva, World Health Organization, 2019. www.who.int/mental_health/prevention/suicide/suicideprevent/en. Accessed Jan 8, 2020Google Scholar
6 : Death Rates Due to Suicide and Homicide Among Persons Aged 10–24: United States, 2000–2017. NCHS Data Brief 352. Hyattsville, MD, National Center for Health Statistics, Oct 2019Google Scholar
7 : The increasing burden of depression. Neuropsychiatr Dis Treat 2011; 7(suppl 1):3–7Google Scholar
8 : Major depressive disorder. Nat Rev Dis Primers 2016; 2:16065Crossref, Google Scholar
9 : The neurobiology of depression: an integrated view. Asian J Psychiatr 2017; 27:101–111Crossref, Google Scholar
10 : Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry 1991; 48:851–855Crossref, Google Scholar
11 : Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Am J Psychiatry 2010; 167(suppl 10):1–152Google Scholar
12 : Multiple recurrences of major depressive disorder. Am J Psychiatry 2000; 157:229–233Crossref, Google Scholar
13 : Definitions of recovery and outcomes of major depression: results from a 10-year follow-up. Acta Psychiatr Scand 2008; 117:35–40Google Scholar
14 : The natural course and outcome of major depressive disorder in primary care: the PREDICT-NL study. Soc Psychiatry Psychiatr Epidemiol 2012; 47:87–95Crossref, Google Scholar
15 : The burden of recurrent depression: causes, consequences, and future prospects. J Clin Psychiatry 2001; 62(suppl 22):5–9Google Scholar
16 : Predictors of long-term prognosis of depression. CMAJ 2011; 183:1969–1976Crossref, Google Scholar
17 : Prevention of relapse and recurrence in adults with major depressive disorder: systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol 2015; 19:
18 : Practice guideline for the treatment of patients with bipolar disorder, 2nd ed. Am J Psychiatry 2002; 159(suppl 4):1–82Google Scholar
19 : Severity classification on the Hamilton Depression Rating Scale. J Affect Disord 2013; 150:384–388Crossref, Google Scholar
20 : Validation of the 17-item Hamilton Depression Rating Scale definition of response for adults with major depressive disorder using equipercentile linking to Clinical Global Impression scale ratings: analysis of Pharmacogenomic Research Network Antidepressant Medication Pharmacogenomic Study (PGRN-AMPS) data. Hum Psychopharmacol 2016; 31:185–192Crossref, Google Scholar
21 : Mobile sensing and support for people with depression: a pilot trial in the wild. JMIR Mhealth Uhealth 2016; 4:
22 : Mobile apps for mood tracking: an analysis of features and user reviews. AMIA Annu Symp Proc 2018; 2017:495–504Google Scholar
23 : Framingham risk score for estimation of 10-years of cardiovascular diseases risk in patients with metabolic syndrome. J Health Popul Nutr 2017; 36:36Crossref, Google Scholar
24 : Development and validation of risk prediction algorithms to estimate future risk of common cancers in men and women: prospective cohort study. BMJ Open 2015; 5:
25 : Development and validation of a prediction algorithm for use by health professionals in prediction of recurrence of major depression. Depress Anxiety 2014; 31:451–457Crossref, Google Scholar
26 : Premorbid risk factors for major depressive disorder: are they associated with early onset and recurrent course? Dev Psychopathol 2014; 26:1477–1493Crossref, Google Scholar
27 : Multiple risk factors predict recurrence of major depressive disorder in women. J Affect Disord 2015; 180:52–61Crossref, Google Scholar
28 : The neurobiology of depression. Br Med Bull 2012; 101:127–145Crossref, Google Scholar
29 : The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 2009; 24:27–53Crossref, Google Scholar
30 : Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. Can J Psychiatry 2016; 61:540–560Crossref, Google Scholar
31 : Clinical practice guidelines for the management of depression. Indian J Psychiatry 2017; 59(suppl 1):S34–S50Crossref, Google Scholar
32 : Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 2015; 29:459–525Crossref, Google Scholar
33 : The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med 2015; 13:68Crossref, Google Scholar
34 : Trends in 5-year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res Treat 2014; 147:609–616Crossref, Google Scholar
35 : Helping patients with depression achieve wellness. J Clin Psychiatry 2009; 70:
36 : Full remission: a return to normal functioning. J Psychiatry Neurosci 2002; 27:233–234Google Scholar
37 : Treatment goals of depressed outpatients: a qualitative investigation of goals identified by participants in a depression treatment trial. J Psychiatr Pract 2010; 16:425–430Crossref, Google Scholar
38 : Recovery in patients with major depressive disorder (MDD): results of a ‐6-month, multinational, observational study. Patient Prefer Adherence 2017; 11:1859–1868Crossref, Google Scholar
39 : Predictors of functional response and remission with desvenlafaxine 50 mg/d in patients with major depressive disorder. CNS Spectr 2014; 19:519–527Crossref, Google Scholar
40 : Efficacy of interpersonal psychotherapy as a maintenance treatment of recurrent depression: contributing factors. Arch Gen Psychiatry 1991; 48:1053–1059Crossref, Google Scholar
41 : Five-year outcome for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1992; 49:769–773Crossref, Google Scholar
42 : Comparative efficacy and acceptability of antidepressants in the long-term treatment of major depression: protocol for a systematic review and networkmeta-analysis. BMJ Open 2019; 9:
43 : The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) study: outcomes from the acute and continuation phases. Biol Psychiatry 2007; 62:1371–1379Crossref, Google Scholar
44 : Review of maintenance trials for major depressive disorder: a 25-year perspective from the US Food and Drug Administration. J Clin Psychiatry 2014; 75:205–214Crossref, Google Scholar
45 : Maintenance therapy to prevent recurrence of depression: summary and implications of the PREVENT study. Expert Rev Neurother 2008; 8:737–742Crossref, Google Scholar
46 : Follow-up of monotherapy remitters in the PReDICT study: maintenance treatment outcomes and clinical predictors of recurrence. J Consult Clin Psychol 2018; 86:189–199Crossref, Google Scholar
47 : Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res 2019; 111:59–67Crossref, Google Scholar
48 : Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1990; 47:1093–1099Crossref, Google Scholar
49 : Mental health-related stigma in health care and mental health-care settings. Lancet Psychiatry 2014; 1:467–482Crossref, Google Scholar
50 : Times to discontinue antidepressants over 6 months in patients with major depressive disorder. Psychiatry Investig 2016; 13:440–446Crossref, Google Scholar
51 : Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence 2016; 10:1401–1407Crossref, Google Scholar
52 : Patient experiences of taking antidepressants for depression: a secondary qualitative analysis. Res Social Adm Pharm 2013; 9:884–902Crossref, Google Scholar
53 : Consumer-related factors influencing antidepressant adherence in unipolar depression: a qualitative study. Patient Prefer Adherence 2018; 12:1863–1873Crossref, Google Scholar
54 : A review of the management of antidepressant discontinuation symptoms. Ther Adv Psychopharmacol 2015; 5:357–368Crossref, Google Scholar
55 : Managing antidepressant discontinuation: a systematic review. Ann Fam Med 2019; 17:52–60Crossref, Google Scholar
56 : Pharmacogenetics and psychiatric care: a review and commentary. J Ment Health Clin Psychol 2018; 2:17–24Crossref, Google Scholar
57 : A pharmacogenomic-based antidepressant treatment for patients with major depressive disorder: results from an 8-week, randomized, single-blinded clinical trial. Clin Psychopharmacol Neurosci 2018; 16:469–480Crossref, Google Scholar
58 : General predictors and moderators of depression remission: a VAST-D report. Am J Psychiatry 2019; 176:348–357Crossref, Google Scholar
59 : Hippocampal tail volume as a predictive biomarker of antidepressant treatment outcomes in patients with major depressive disorder: a CAN-BIND report. Neuropsychopharmacology 2020; 45:283–291Crossref, Google Scholar



