Complex Combination Pharmacotherapy for Bipolar Disorder: Knowing When Less Is More or More Is Better
Abstract
Combination pharmacotherapy for bipolar disorder is commonplace and often reflects the severity and complexity of the illness and the comorbid conditions frequently associated with it. Across treatment settings, about one-fifth of patients with bipolar disorder appear to receive four or more psychotropic medications. Practice patterns often outpace the evidence-based literature, insofar as few systematic studies have examined the efficacy and safety of two or more medications for any given phase of illness. Most randomized trials of combination pharmacotherapy focus on the utility of pairing a mood stabilizer with a second-generation antipsychotic for prevention of either acute mania or relapse. In real-world practice, patients with bipolar disorder often take more elaborate combinations of mood stabilizers, antipsychotics, antidepressants, anxiolytics, stimulants, and other psychotropics for indefinite periods that do not necessarily arise purposefully and logically. In this article, I identify clinical factors associated with complex combination pharmacotherapy for patients with bipolar disorder; describe approaches to ensuring that each component of a treatment regimen has a defined role; discuss the elimination of unnecessary, ineffective, or redundant drugs in a regimen; and address complementary, safe, rationale-based drug combinations that target specific domains of psychopathology for which monotherapies often provide inadequate benefit.
Combination pharmacotherapy—sometimes derisively called polypharmacy to connote drugs that are deemed unnecessary or inappropriate in a regimen—has long been a cornerstone of treatment for complex medical conditions ranging from hypertension to infectious disease to oncology. Psychiatry has lagged behind other areas of medicine by fostering the idea that psychiatric disorders somehow ought to be treatable with only one medication, no matter how complex the problem may be, and that the deliberate juxtaposition of two or more drugs likely reflects shoddy prescribing rather than the pursuit of pharmacodynamic synergy. In the case of bipolar disorder, in which comorbid conditions are more common than rare (1) and symptom targets are often heterogeneous, the idea that one medication will reliably and effectively resolve all features of a complex clinical presentation is often unrealistic and naïve. In part, such fanciful expectations reflect a dubious assumption that a single underlying neurobiological process accounts for the entirety and diversity of psychopathology features shown by people with bipolar disorder. Seldom does one all-encompassing intervention ameliorate all signs of psychopathology as comprehensively as an antibiotic for pneumonia treats cough, fever, weakness, and dyspnea or a nitrate for angina eliminates chest pain, nausea, diaphoresis, and weakness. Accordingly, in this article, I provide an overview of the varied symptoms of psychopathology for which patients with bipolar disorder are prescribed medications so that practitioners can devise regimens that are complementary, nonredundant, purposeful, and evidence based.
Targets of Pharmacotherapy
Rather than speak of medications in a narrow fashion as simply treating mania or depression, it is often more useful to identify their utility for specific targets of pharmacotherapy according to dimensions of psychopathology, as illustrated in Figure 1.
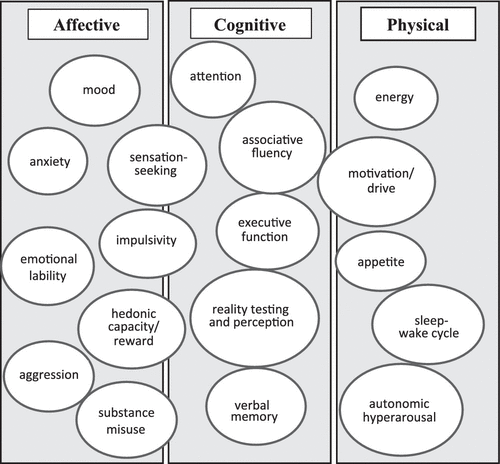
FIGURE 1. Domains of psychopathology
Ideally, a pharmacotherapy regimen for bipolar disorder includes as many components as necessary to successfully address all the relevant aspects of psychopathology described in Figure 1. Elegant combination pharmacotherapy is economical (making use of one drug with multiple effects when feasible), purposeful (with every component serving a definable function), and efficient (with dosing and the number of drugs no higher than necessary to address the intended targets). Less elegant are assemblages of medications that accrue over time without regard to intended effects, lack of efficacy, mechanistic redundancies, pharmacokinetic interactions, or ongoing relevance.
Prevalence of Prescription of Complex Combination Pharmacotherapy
Cross-sectional descriptive studies have suggested that about one-fifth (2) to one-third (3) of patients with bipolar disorder are taking four or more psychotropic medications at the time of hospitalization. Among 4,035 mostly outpatient entrants to the National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD), 18% were taking four or more psychotropic drugs, and 40% took three or more (4). One European study found that nearly 80% of outpatients with bipolar disorder took a mean of 3.8 medications, most often a mood stabilizer (92%), followed by an antidepressant (59%), benzodiazepines (43%), antipsychotics (39%), and thyroid hormone (21%; 5). Another report from the United Kingdom found that, in 2009, nearly half of patients with bipolar disorder were prescribed two or more psychotropic drugs on an ongoing basis; nearly half of that group chronically took lithium plus an antipsychotic drug (6).
Separate from merely counting how many medications a patient takes is determining whether the components of a pharmacotherapy regimen are appropriate and complementary (as opposed to inappropriate and pharmacologically conflictual). Considerations of drug appropriateness include the following:
Is the intended use of a drug aligned with its known pharmacodynamic effects? For example, lamotrigine would not be considered relevant to treat acute mania; chronic sleep aids may be unnecessary and counterproductive in patients with hypersomnia.
Are the known pharmacodynamic effects of a drug contrary to current symptoms? Antidepressants generally have no value during acute mania; and psychostimulants are likely counterproductive when treating acute psychosis.
Do the effects of one drug contradict or conflict with those of another? An example is simultaneous use of an anticholinergic drug (such as benztropine) and a procholinergic drug (such as donepezil).
Are current medications consistent with the patient’s present clinical state? Antidepressants would be illogical during acute mania, as would be the absence of one or more antimanic drugs.
Are adverse effects of one drug causing or mimicking psychiatric symptoms? Akathisia can be mistaken for agitation; anticholinergics impair cognitive function; and sleeplessness or loss of libido can be caused by a monoaminergic antidepressant or by depression. Adverse effects may also exacerbate a medical problem (e.g., beta blockers worsening asthma; noradrenergic drugs driving hypertension).
Are medications being retained if they have been deemed ineffective (and have all medications in a regimen received an adequate trial and been deemed effective or ineffective)?
Are medications being used and retained even when they have an abundance of negative controlled trial data for an intended purpose, such as topiramate or gabapentin in mania or paroxetine in bipolar depression, or an illogical rationale, such as use of modafinil or amphetamine for agitation or anxiety?
Have clinically important drug interactions gone unrecognized? For example, carbamazepine and primidone are potent inducers of cytochrome P450 enzymes and may effectively reduce serum levels and pharmacodynamic efficacy of drugs that undergo Phase I oxidative metabolism.
Do Guidelines Offer Guidance?
Practice guidelines convey only limited insight into the utility of complex combination therapy. Beyond specifying episode features such as with versus without psychosis or mixed versus pure affective polarity, they tend to say little about contexts and comorbid conditions that typically influence treatment decisions. Examples might include mania in someone with or without metabolic syndrome, in someone with or without substance misuse, or in a sexually active woman of child-bearing age or a person with bipolar depression with versus without prominent anxiety, with comorbid ADHD, or with suicidal features. For a patient with severe, acute mania, some guidelines advocate a combination of two drugs (usually lithium or divalproex plus an antipsychotic) as a first- (7–9) or second- (10) line intervention. Some make no specific mention of combining three or more agents in any phase of bipolar disorder so much as replacing an ineffective medication with an alternative (e.g., the 2018 Canadian Network for Mood and Anxiety Treatments Guidelines; 8). Others identify triple (or greater) therapy regimens as worthy of consideration only after the failure of multiple single- or dual-drug therapy efforts for manic or mixed episodes (e.g., 9, 10), and these regimens usually consist of two mood stabilizers plus a first-generation antipsychotic or a second-generation antipsychotic (SGA).
For acute bipolar depression, current guidelines have not suggested a role for more than two drugs in combination (generally a mood stabilizer or SGA plus an antidepressant). Guidelines usually list novel agents (such as pramipexole [11], inositol [12], anti-inflammatory or antioxidant drugs [13], or thyroid hormone [14]) collectively as last-step options, but without regard to the total net number of medications in a regimen. Most also seldom offer explicit recommendations about when and how to discontinue a drug once it has been introduced into a regimen, apart from often blanket discouragement of long-term use of antidepressants for people with bipolar disorder (despite a lack of consensus among experts and a limited evidence base from which to inform decisions about deprescribing antidepressants when they are acutely effective; 15).
No systematic studies have looked at combining two (or more) monoaminergic antidepressants that have complementary mechanisms as a strategy to treat bipolar depression, unlike for major depressive disorder. The relatively small handful of randomized trials that have involved the use of traditional antidepressants to treat bipolar depression have generally not demonstrated robust efficacy—not unlike for treatment-resistant unipolar depression. However, with no studies of the use of combination antidepressants in treating bipolar depression, the potential utility of novel or multiple antidepressant approaches remains unknown. Clinical characteristics associated with the use of combination therapy for bipolar disorder are summarized in Table 1 (2–4, 16–18).
| Characteristic | Findings |
|---|---|
| Sex | Female (2) |
| Race or ethnicity | White (2) |
| Age (years) | >50 (16) |
| Psychosis | Present (2) |
| Drug dosage | Typically low (16) |
| Depressive illness burden | High (4) |
| Comorbid personality disorders | Borderline personality disorder (2) |
| Comorbid psychiatric diagnoses | Posttraumatic stress disorder (2) Anxiety disorders (2, 3) |
| History of 1 or more suicide attempts | Present (3, 4) |
| Personality features | Low ratings on openness, extraversion, and conscientiousness (17, 18) |
TABLE 1. Factors associated with complex combination pharmacotherapy for patients with bipolar disorder
Polypharmacy has been identified as one of the biggest contributors to guideline-discordant treatment of bipolar disorder (18), yet herein lies a major dilemma for practitioners: Guideline recommendations are based on traditional efficacy trials for specific phases of an illness rather than on effectiveness studies with real-world populations. Snippets of guideline recommendations can be judged for concordance with prescribed medications for specific illness phases of bipolar disorder (e.g., in the STEP-BD, guideline-concordant treatments were prescribed for more than 80% of manic-hypomanic, mixed, or depressive episodes per se; 19), but most patients with bipolar disorder outside of clinical trials require treatment for more than simply a current affective phase of illness. Clinician surveys that examine guideline nonadherence in treating bipolar disorder have pointed to the failure of guidelines to address particular features of unique clinical populations (20). Indeed, it would be difficult to craft an effective guideline-driven drug regimen for a bipolar II depressed patient with prominent anxiety, attentional complaints, substance use comorbidity, chronic suicidal ideation, metabolic syndrome, obstructive sleep apnea, and chronic kidney disease.
Guidelines become less useful when diagnoses and illness phases do not conform neatly to DSM-5 criteria, patient priorities drive adherence, and prominent comorbid conditions and complex features (e.g., trauma histories) must be addressed. In addition, the clinical trials literature does not indicate whether and when mono- or dual pharmacotherapies yield better outcomes than more extensive but thoughtfully devised combination regimens is largely unknown. The shortcomings of current practice guidelines for bipolar disorder are summarized in Box 1.
Box 1. Limitations of current practice guidelines for bipolar disorder
Largely ignore the presence of psychiatric and medical comorbid conditions.
Do not account for patients whose bipolar spectrum symptoms may not fulfill traditional DSM-5 diagnostic criteria.
Often lump together recommendations for patients with bipolar I vs. bipolar II disorder, those with vs. without rapid cycling, or those with chronic vs. nonchronic presentations.
Do not account for risk-benefit situations (e.g., metabolic risk vs. gravity of psychiatric disability when considering clozapine).
Minimally consider incorporating specific medications for narrow intended purposes (e.g., lithium to reduce suicide risk; topiramate solely for weight loss).
Do not account for multipurpose drugs with greater value in unique situations (e.g., divalproex for patients with bipolar disorder who experience migraines; bupropion for patients with bipolar II depression and obesity, cigarette smoking, and concerns about iatrogenic sexual dysfunction).
May blur opinion-based with evidence-based recommendations.
Provide little if any guidance on when and how to deprescribe.
Rationales for Pharmacotherapy Combinations
From one perspective, combining diverse medications makes sense with a disease entity in which known drug mechanisms of action are complementary and related to the pathophysiology of the underlying disease process. In the case of bipolar disorder, such exactitude is unfortunately largely unknown and may at best be speculative. Although a systematic review of current theories about the pathogenesis of bipolar disorder is beyond the scope of this article, theories that have been reviewed elsewhere (e.g., Manji et al. [21]), ranging from the level of molecular-intracellular function to neuronal networks, include the following: monoaminergic dysregulation; elevated intracellular calcium and abnormal calcium signaling (e.g., calcium channel blockade); circadian dysrhythmias (e.g., light entrainment of the circadian pacemaker; sleep-wake cycle perturbations); dysfunction of purine metabolism (hyperuricemia in mania); endocrinopathies (e.g., glucocorticoid receptor dysfunction, hypothalamic-pituitary-adrenocortical dysregulation, hypothalamic-pituitary-thyroid axis dysfunction); abnormal signal transduction (e.g., overactive inositol phosphate signaling, elevated intracellular inositol or cyclic adenosine monophosphate); immuno-inflammatory mechanisms, impaired neuronal plasticity, regulation of oxidative stress, and neuroprotection against neuronal loss; and disrupted connectivity of neural circuits.
Table 2 summarizes presumed neuronal and molecular-cellular mechanisms of action for psychotropic drugs commonly used to target mania, depressive symptoms, or both in patients with bipolar disorder (22–55). The list of potential mechanisms is far from complete due to the limited knowledge about bipolar disorder’s basic pathophysiology, along with emerging novel hypotheses that do not yet clearly translate to any specific biological interventions (e.g., mitochondrial dysfunction, shortened telomere length).
| Mechanism | Lithium | DVPX | CBZ | LTG | SGAs | MADs |
|---|---|---|---|---|---|---|
| PKC inhibition | √ (22) | √ (23) | − (24) | − (25) | ? | ? |
| BDNF upregulation | √ (26) | √ (23) | √ (27) | √ (28, 29) | √ (30) | √ (31) |
| bcl–2 upregulation | √ (32) | √ (33) | √ (27) | √ (34) | ? b | ? c |
| GSK3β inhibition | √ (37) | √ (38) | — | — | ? d | ? e |
| MARCKS downregulation | √ (23) | √ (23) | — | — | — | — |
| GABA upregulation | √ (41) | √ (42) | √ (43) | — | — | ? |
| Glutamate downregulation | √ (44) | √ (42) | √ (45) | √ (46) | ? | ? f |
| Na+ channel blockade | √ (48) | √ (49) | √ (50) | √ (51) | ? | √ g |
| Ca++ channel blockade | — | √ | √ | √ | — | — |
| 5-HT2A antagonism | — | — | — | — | √ h | √ h |
| 5-HT1A partial agonism | ? | — | — | — | √ i | √ i |
| 5-HT1B antagonism, partial agonism or inverse agonism | ? j | — | — | — | √ k | ? k |
| D2 antagonism | √ l | √ l | √ lk | — | √ | — |
| D3 partial agonism | — | — | — | — | √ m | — |
| 5-HT7 antagonism | — | — | — | — | √ n | √ n |
TABLE 2. Putative mechanisms of action of antimanic and antidepressant drugsa
In addition, as noted in the table and its footnotes, in many instances only preliminary animal or in vitro data exist regarding the effects of specific psychotropic drugs on possible receptor targets; these effects may not be so straightforward or easily translated. For example, neither lithium nor anticonvulsant mood stabilizers exert known direct effects on specific serotonin receptors, but they might exert indirect modulating effects; possible in vitro effects of monoaminergic antidepressants on ion channels may not translate to pharmacodynamic effects in humans. Preclinical or in vitro receptor binding studies may not necessarily provide information about regional anatomical differences in drug effects. Existing treatments, for better or worse, are geared more practically toward altering observable psychopathology rather than modifying an identified neurobiological process.
Some proposed mechanisms are likely more specific to the treatment of depression than to that of mania (e.g., serotonin reuptake inhibition) or vice versa (e.g., protein kinase C inhibition or inositol depletion), and even identified neuronal or molecular mechanisms do not necessarily provide information about pharmacodynamic class effects (e.g., mania may respond to some drugs that inhibit protein kinase C inhibitors [e.g. tamoxifen; 56], but not all [e.g., omega-3 fatty acids; 57]). Existing knowledge about drug mechanisms of action also does not specifically address pharmacodynamic effects relevant to particular illness subcomponents (e.g., use of a drug for narrow purposes such as putative antisuicide effects, such as lithium [58]), procognitive effects (e.g., withania somnifera [59] or lurasidone [60]), anticraving effects (e.g., naltrexone), or targeting of impulsive aggression (e.g., divalproex; 61). It is also worth noting that nuanced differences exist within the broad mechanisms included in Table 2 whose depth is beyond the scope of this article. For example, some anticonvulsant drugs (divalproex, carbamazepine) are believed to exert antiglutamatergic effects only through N-methyl-D-aspartate receptor blockade, whereas others (lamotrigine) appear to also affect α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Calcium channel blocking effects of mood-stabilizing anticonvulsants may occur through L-type calcium channels (carbamazepine); L- and T-type calcium channels (divalproex); or L-, P/Q-, R-, and T-type calcium channels (lamotrigine). Among SGAs, individual agents vary in their specific receptor profiles and affinities, as well as in their unique targets (e.g., 5-HT7 antagonism or D3 partial agonism are not universally shared targets across SGAs, as noted in Table 2).
Complex Combination Pharmacotherapy for Bipolar Disorder and Clinical Outcomes
Almost no systematic studies have provided information about whether and when a more extensive pharmacotherapy regimen leads to a better or worse clinical outcome than a simpler regimen. Such questions may never be answerable because of the inherent complexities of controlled study designs and the matching of drug regimens to individual patient characteristics that in themselves influence medication choice. It is obviously also an oversimplification to count the sheer number of medications someone takes without regard to the appropriateness, responsivity, redundancies, and purposefulness of each drug on a treatment roster, as though counting instruments in an orchestra without distinguishing woodwinds from brass or strings or the composition of the overall ensemble. Specific medication choices in a given regimen, and their response likelihoods, are also often made not just on the basis of an overall Food and Drug Administration indication (e.g., acute mania) but more precisely according to other characteristics associated with more specific symptom domains (e.g., divalproex vs. lithium in mixed episodes; lamotrigine for maintenance therapy in patients more prone to depressive rather than manic recurrences) (62).
Existing randomized combination pharmacotherapy trials for bipolar disorder focus mainly on the potential utility of two medications versus one during either acute manic or depressive phases of illness or during maintenance and relapse prevention phases. This literature is summarized in Tables 3-5, respectively, (63–88) for treatment of acute bipolar manic or mixed episodes, acute bipolar depression, or bipolar maintenance and relapse prevention, respectively. For acute bipolar depression, Table 4 focuses on combinations of mood stabilizers or adjunctive SGAs with mood stabilizers; I did not include studies of traditional antidepressant or novel compounds (e.g., pramipexole, modafinil, psychostimulants) added to a mood stabilizer because such published studies generally do not account for variability in inherent potential antidepressant properties among mood stabilizers.
| Trial result and intervention | Duration | Outcome | Adverse effects |
|---|---|---|---|
| Positive trials | |||
| Allupurinol (N=218) or placebo (N=215) plus mood stabilizers (meta-analysis of five randomized trials; 63) | 4–8 weeks | Adjunctive allopurinol (dosed at 300–600 mg/day) was more effective than placebo in reducing baseline mania symptoms; greater effect in pure than in mixed mania | No significant differences from placebo |
| Aripiprazole (N=253) or placebo (N=131) plus lithium or divalproex (64) | 6 weeks | Adjunctive aripiprazole (mean dose=19 mg/day) was more effective than placebo; significantly greater reduction in mood elevation, hypersexuality, irritability, speech, aggression, insight); no significantly greater reduction in depression severity scores | More extrapyramidal symptoms with aripiprazole than with placebo, but no significant differences in weight changes or metabolic laboratory parameters |
| Asenapine (N=158) or placebo (N=166) plus lithium or divalproex (65) | 12 weeks (primary efficacy at week 3) | Adjunctive asenapine (mean dose=11.8 mg/day) was more effective than placebo in reducing mania symptoms; no between-groups differences in response or remission rates or changes from baseline in depression symptom severity | More sedation and somnolence, treatment-emergent depression, hypoesthesia, and weight gain with asenapine than with placebo |
| Celecoxib (N=23) or placebo (N=23) plus divalproex (66) | 6 weeks | Adjunctive celecoxib (400 mg/day) was more effective than placebo in reducing severity of mania symptoms | No significant differences from placebo |
| Olanzapine (N=51) or placebo (N=48) plus lithium or divalproex (67) | 6 weeks | Adjunctive olanzapine (mean modal dose=8.6 mg/day) was more effective than placebo in reducing both manic and depressive symptoms | Greater weight gain with combination than with monotherapy |
| Olanzapine (N=100) or placebo (N=101) plus lithium or divalproex (68) | 6 weeks | Adjunctive olanzapine was more effective than placebo in reducing both mania and depressive symptoms | More weight gain and higher fasting blood sugar with olanzapine than with placebo |
| Risperidone (N=52), haloperidol (N=53), or placebo (N=51) plus lithium or divalproex (69) | 3 weeks | Adjunctive risperidone (mean dose=3.8 mg/day) or haloperidol (mean dose 6.2 mg/day) was more effective than placebo in reduction in mania symptom severity | Greater weight gain with adjunctive risperidone than placebo |
| Quetiapine (N=91) or placebo (N=100) plus lithium or divalproex (70) | 3 weeks | Adjunctive quetiapine (mean dose=504 mg/day) was more effective than placebo in reduction in mania symptom severity reduction (greatest improvements in irritability, aggression, sleep, and motor activity) | Greater somnolence and dry mouth with adjunctive quetiapine than with placebo |
| Lithium (N=173) or placebo (N=182) plus quetiapine XR (71) | 6 weeks | Adjunctive lithium (mean modal dose=1,085.5 mg/day, mean serum [Li+]=0.72 mEq/L) was more effective than placebo in reduction in mania symptom severity, psychosis, agitation, aggression; no between-group differences in reduction in depressive symptom severity (mean quetiapine dose=623.1 mg/day) | More tremor, somnolence, dizziness, diarrhea, and vomiting with lithium than with placebo |
| Tamoxifen (N=20) or placebo (N=20) plus lithium (72) | 6 weeks | Adjunctive tamoxifen (fixed dose 80 mg twice daily) was more effective than placebo | More fatigue with tamoxifen than with placebo |
| Negative trials | |||
| Olanzapine (N=58) or placebo (N=60) plus carbamazepine (73) | 6 weeks | No significant between-groups differences in mania symptom severity or response rates with adjunctive olanzapine plus carbamazepine (mean dose=617.5 mg/day) vs. carbamazepine monotherapy (mean dose=717.3 mg/day) | Greater weight gain and higher triglyceride levels with olanzapine than with placebo; carbamazepine is known to reduce serum olanzapine levels by approximately 40%–50% |
| Paliperidone ER (N=150) or placebo (N=150) plus lithium or divalproex (74) | 6 weeks | Adjunctive paliperidone (mean dose=8.1 mg/day) no different from placebo | Greater somnolence, akathisia, weight gain, and increased appetite with paliperidone ER than with placebo |
| Topiramate (N=143) or placebo (N=144) plus divalproex or lithium (75) | 8 weeks | Adjunctive topiramate (mean dose 254.7 mg/day) no different from placebo | Paresthesias, diarrhea, and anorexia more common with topiramate than with placebo |
| Ziprasidone (N=439) or placebo (N=217) plus lithium or divalproex (76) | 3 weeks | Adjunctive ziprasidone (mean modal dose=60.1 mg/day in low-dose arm, 133.8 mg/day in high dose arm) no different from placebo in changes from baseline mania or levels of depression symptom severity | More sedation and somnolence with ziprasidone than with placebo |
TABLE 3. Randomized trials of mono- vs. dual pharmacotherapies in acute bipolar manic or mixed episodes
| Intervention | Duration | Outcome | Adverse effects |
|---|---|---|---|
| Lamotrigine (N=64) or placebo (N=60) plus lithium (77) | 8 weeks | Adjunctive lamotrigine (200 mg/day) was more effective than placebo | None greater with adjunctive lamotrigine than with lithium monotherapy |
| Lamotrigine (N=101) or placebo (N=101) plus quetiapine (78) | 12 weeks | Adjunctive lamotrigine (200 mg/day) > placebo | None greater with adjunctive lamotrigine than with placebo |
| Lurasidone (N=183) or placebo (N=165) plus lithium or divalproex (79) | 6 weeks | Adjunctive lurasidone (mean dose 66.3 mg/day) was more effective than placebo | Greater nausea, somnolence, tremor, akathisia, and insomnia with adjunctive lurasidone than with placebo |
| Ziprasidone (N=147) or placebo (N=147) plus lithium or divalproex or lamotrigine (80) | 6 weeks | Adjunctive ziprasidone (mean dose=89.8 mg/day) no different from placebo | More nausea, fatigue, dizziness, sedation, somnolence with adjunctive ziprasidone than with placebo |
TABLE 4. Randomized Trials of mono- versus dual pharmacotherapies for acute bipolar depression
| Trial result and intervention | Duration | Outcome | Adverse effects |
|---|---|---|---|
| Positive trials | |||
| Aripiprazole (N=168) or placebo (N=169) plus lithium or divalproex (81) | 12-week acute stabilization followed by 52-week maintenance | Lower 52-week relapse rate (any mood episode) with aripiprazole (mean dose 15.8–16.9 mg/day; 17%) than with placebo (29%); aripiprazole was more effective than placebo for mania but not depressive relapse | Comparable weight changes or metabolic parameters with aripiprazole or placebo |
| Lithium plus divalproex combination vs. either monotherapy (BALANCE; 82) | 24 months | Lithium plus divalproex (N=110) was more effective than divalproex monotherapy (N=110) but not lithium monotherapy (N=110); lithium was more effective than divalproex for depressive relapses | No significant differences in serious adverse events among the treatment arms |
| Lithium or divalproex plus aripiprazole of placebo (83) | 12-week acute stabilization followed by 52-week maintenance | Adjunctive aripiprazole (mean dose=16.1 mg/day if index episode manic, 16.8 mg/day if mixed) was more effective than placebo for prevention of manic but not mixed episodes | More tremor with aripiprazole than with placebo |
| Olanzapine (N=51) or placebo (N=48) plus lithium or divalproex (84) | 18 months | Adjunctive olanzapine (mean dose=8.6 mg/day) was more effective than placebo for symptomatic but not syndromal relapse for mania or depression (but no significant differences for time to mania relapse or time to depressive relapse) | More weight gain and less insomnia with adjunctive olanzapine than with placebo |
| Risperidone long-acting injectable (N=65) or placebo (N=59) plus treatment as usual (85) | 16-week open stabilization, then 52 weeks | Adjunctive risperidone long-acting injectable (modal dose 25 mg/day) was more effective than placebo for time to any mood episode | Tremor, insomnia, muscle rigidity, and mania more likely with risperidone long-acting injectable than with placebo |
| Ziprasidone (N=127) or placebo (N=113) plus lithium or divalproex (86) | 8-week open stabilization, then 16 weeks | Adjunctive ziprasidone (mean modal doses of 80, 119, and 160 mg/day across three strata) was more effective than placebo in time to any mood episode and, separately, time until a manic or until a depressive relapse | Incidence of tremor greater with ziprasidone than placebo |
| Negative trials | |||
| Lurasidone (N=246) or placebo (N=250) plus lithium or divalproex (87) | 28 weeks | No advantage for adjunctive lurasidone over placebo in time until recurrence of a depressive, manic, or mixed episode; if index episode polarity was depressed, lurasidone was more effective than placebo for time until recurrence of any mood episode | No clinically meaningful differences with lurasidone versus placebo |
| Oxcarbazepine (N=26) or placebo (N=29) plus lithium (88) | 52 weeks | No advantage for adjunctive oxcarbazepine (mean modal dose 1,200 mg/day) over placebo in time until recurrence of any mood episode | Drowsiness, diarrhea, and tremor more common with oxcarbazepine than placebo |
TABLE 5. Randomized trials of mono- versus dual pharmacotherapies for bipolar maintenance and relapse prevention
Some naturalistic studies have suggested that patients with bipolar disorder who take fewer medications (e.g., one) manifest more symptom stability (remain relapse-free) over time than those on two, three, or more medications (5, 89); still others have suggested that when medication regimens include certain core pharmacotherapies such as lithium, there may be less need to add additional medications (90). Open (nonrandomized) data suggest that inpatients with mania who begin pharmacotherapy with a combination of lithium and divalproex at the outset may incur a lesser dosage burden of antipsychotic cotherapy than those who begin on lithium without divalproex (91). The NIMH Lithium Treatment Moderate Dose Use Study (LiTMUS) trial found that cotherapy with subtherapeutic doses of lithium has not shown greater symptomatic benefit than placebo (although a post hoc analysis suggested that low-dose adjunctive lithium may be associated with lower dosages of concomitant SGAs; 92). By contrast, use of other medications (notably, chronic benzodiazepine use) in a regimen for treatment of bipolar disorder may be associated with higher recurrence rates and more severe overall illness (93)—not necessarily in a causal fashion, but more likely because of the stigmata of pathology.
The literature on extensive polypharmacotherapy of psychotropic drugs is largely more naturalistic and observational than randomized, which makes it hard to draw causal inferences about treatment outcomes. In such studies, medications are usually chosen on the basis of patients’ individual conditions, leading to the problem of confounding by indication. For example, some observational studies have attributed lower suicide attempts or completions to the inclusion of lithium in a treatment regimen over other mood stabilizers (94), but these studies have not accounted for the possibility that clinicians might avoid prescribing lithium to patients at a higher risk of suicide (e.g., in the aftermath of an overdose or other attempt), relegating prescription of lithium to a potential marker or artifact of a better prognosis at baseline. Similarly, some observational studies of rapid cycling have concluded that prescription of antidepressants may lead to more frequent episodes (95), without considering the reverse possibility that frequent depressive episodes may cause more prescription of antidepressants. In any nonrandomized pharmacotherapy study (but especially in those involving multidrug regimens), possible confounding by indication poses obstacles to inferring causal drug effects as a result of unrecognized and unaccounted-for moderators and mediators of outcome (62).
A further dilemma when trying to assess extensive combination pharmacotherapy regimens involves uncertainties about within-class differences versus generalizabilities among medications. For example, the relative magnitude of antimanic efficacy across individual SGAs appears comparable (96), although individual agents may vary in their antidepressant, anxiolytic, or tolerability profiles. Similarly, monoaminergic antidepressants are often lumped together as homogeneous entities, although the absence of placebo-controlled studies for most newer agents (vortioxetine, vilazodone, desvenlafaxine, levomilnacipran, mirtazapine) makes it difficult to generalize about within-class efficacy or the potential for synergy from particular antidepressant combinations.
What About Antidepressant Adjuncts to Mood Stabilizers for Bipolar Disorder?
Practice guidelines (7, 8, 10) and expert consensus statements (15) emphasize the importance of avoiding antidepressant monotherapies in bipolar I depression (with possibly greater leniency in bipolar II depression). Such recommendations mainly reflect concerns about the potential for antidepressants to destabilize mood via a treatment-emergent affective switch (TEAS). Consequently, guidelines have advised using antidepressants only in tandem with mood stabilizers in bipolar depression, mainly on the basis of assumptions that mood stabilizers help safeguard against TEAS outcomes rather than the perception that most mood stabilizers exert meaningful intrinsic antidepressant effects that synergize with antidepressant cotherapies. One often cited example, the NIMH STEP-BD study, found only a modest antidepressant response rate with mood stabilizer monotherapy (about 24%) and no added benefit with antidepressant cotherapy (97). Said differently, the current evidence base has suggested that neither mood stabilizers nor monoaminergic antidepressants, nor their combination, exert reliable and robust antidepressant effects on bipolar depression. Yet, contemporary observational studies have found that about one-third of patients with bipolar disorder take antidepressants on a long-term basis (>90 days) as part of their overall pharmacotherapy regimen (98). Such prescribing habits notwithstanding, the Florida Medicaid Guidelines (9) noted that “there is inadequate information (including negative trials) to recommend adjunctive antidepressants . . . for bipolar depression.”
It is also worth noting that clinicians in real-world practice settings may sometimes use selective serotonin reuptake inhibitors (SSRIs) or other antidepressants for their putative anxiolytic properties (in addition to, or possible entirely apart from, depression as an intended treatment target). However, apart from one secondary analysis from a negative randomized controlled trial of paroxetine for bipolar depression (99), no prospective randomized trials have demonstrated anxiolytic efficacy for monoaminergic antidepressants in bipolar disorder. Although this absence of evidence is not evidence of absence and does not negate the possibility of benefit, prescribers must recognize that such assumptions have not been empirically tested.
Antipsychotic Combination Pharmacotherapy
The sustained use of two or more antipsychotic drugs, although more often seen with patients with schizophrenia or schizoaffective disorder, has been observed in 15%−20% of patients with bipolar disorder, often at prescribed daily doses higher than those seen during monotherapy (100). On entry into the STEP-BD, about 10% of patients with bipolar disorder had been taking two or more antipsychotics (101). Psychosocial functioning and symptom status were no different from those who were taking only one antipsychotic, but side effect burden and service utilization were both significantly more extensive. Practice guidelines, such as the Texas Implementation of Medication Algorithms or the Florida Medicaid Guidelines, explicitly advise against the use of two (or more) SGAs, mirroring the generally minimal, if any, benefit seen with the use of antipsychotic combination pharmacotherapy with people with schizophrenia.
Triple Therapy
Very few randomized trials have examined the use of three (or more) psychotropic medications in any phase of bipolar disorder. The existing literature is limited by methodological limitations such as small sample sizes, high dropout, and poor tolerability. For example, among rapid cyclers whose symptoms had not stabilized after 16 weeks of lithium plus divalproex, Kemp et al. (102) found no advantage of the addition of lamotrigine over placebo but suggested that the failure to demonstrate an advantage may have been a Type II error because of the small number of randomized participants. Other short-term open trials with small sample sizes (i.e., N<10) or case series have shown improvement among outpatients with bipolar disorder whose inadequate responses to dual-agent mood stabilizers (lithium plus divalproex) improved with the addition of a third (e.g., carbamazepine), but the lack of larger trials or randomized study designs limits the ability to distinguish multidrug interventions from possible outcomes with alternative strategies, including longer trial durations on existing regimens.
Deprescribing and Maintenance of Pharmacological Hygiene
Surprisingly few randomized pharmacotherapy discontinuation trials exist in research on bipolar disorder. Although such studies provide the most rigorous data about the optimal treatment duration for any medication, that information is especially important during combination therapies, in which unnecessarily prolonged inclusion of a drug jeopardizes overall treatment adherence and imposes an additive side effect burden, cost, and risk for drug-drug interactions. To the extent that randomized clinical trials nowadays fall mainly under the auspices of pharmaceutical industry sponsorship, there is little economic incentive for commercial manufacturers to drive the deprescribing of their products.
Give the scarcity of empirical information about when a particular cotherapy may no longer be beneficial or necessary, the components of a pharmacology regimen can potentially accrue in a manner sometimes akin to hoarding. Because pharmacotherapy per se is for most patients with bipolar disorder an indefinite, open-ended undertaking, it can be difficult to fathom specified end points for stopping certain medications within a regimen. One conspicuous exception is the frequent admonition against long-term antidepressant use espoused in some practice guidelines, based on theoretical concerns about accelerated cycling frequency. In fact, however, randomized discontinuation trial data would suggest that, contrary to popular belief, cessation of an antidepressant after an initial robust response may incur a greater risk for depression relapse (103, 104), except in patients with past-year rapid cycling, for whom long-term antidepressant use has been associated with more depressive episodes (104).
Rapid cycling may represent a particular instance in which monotherapy may rarely produce improvement. A notable example is a randomized trial specifically with patients with rapid-cycling bipolar disorder (105) in which open-label dual therapy with lithium plus divalproex was then converted to monotherapy with either agent. Only one-quarter of 254 rapid-cycling patients initially stabilized during combination therapy with lithium plus divalproex (meaning that most rapid-cycling patients either poorly tolerated or did not benefit from this combination); after subsequent randomization to either agent as monotherapy, only about half of patients remained relapse free over a 20-month period. Unfortunately, this study did not examine whether the continued combination of lithium plus divalproex might have prolonged the time until relapse more than occurred with either pharmacotherapy. It also did not examine whether the total number of relapses per year among rapid-cycling patients was less after randomization to either monotherapy than before.
In the case of adjunctive SGAs, Yatham and colleagues (106) studied patients with manic or mixed bipolar episodes who were stabilized on lithium or divalproex plus an SGA (olanzapine or risperidone) and then compared time until relapse among those randomized to continued combination therapy versus mood stabilizer monotherapy for one year. Relapse rates were significantly lower for those in the combination condition than in the monotherapy condition (hazard ratio=0.53, 95% confidence interval=0.33–0.86) up to 24 weeks, but beyond that time frame the combination provided no continued advantage in preventing relapse over monotherapy. However, weight gain was significantly greater with a continued adjunctive SGA beyond this time (mean 3.2 kg gain vs. ≤0.1 kg gain, respectively).
A basic principle of psychopharmacological hygiene involves discontinuing a psychotropic drug that exerts no benefit after an adequate trial has elapsed. More ambiguous can be knowing whether and when to stop ancillary pharmacotherapies for bipolar disorder, such as benzodiazepines or sleep aids, nonbenzodiazepine anxiolytics (buspirone, hydroxyzine, anxiolytic anticonvulsants), psychostimulants (particularly if used off label to counteract sedation, obesity, or slowed attentional processing), beta blockers for social anxiety or tremor, thyroid hormone in the absence of intrinsic thyroid disease (or safety concerns related to cardiac arrhythmias or osteoporosis), and vitamins or nutritional supplements.
Other tenets of basic pharmacological hygiene include avoiding mechanistic redundancies, contradictory mechanisms, and irrational strategies to avoid future events (e.g., maintaining antidepressants during an acute manic episode on the basis of the notion that doing so may ward off a subsequent depression); optimizing dosing of a first drug before adding additional agents aimed at the same intended symptom target; not maintaining sleep aids when hypersomnia is present; minimizing the number of new or additive drug exposures during pregnancy, to the extent feasible; and knowing why a prescribed drug was originally introduced to a regimen and addressing its utility and ongoing relevance for a given patient.
As a practical matter, it can be helpful (as well as rapport building) for patients and clinicians to conduct periodic “performance evaluations” of a pharmacotherapy regimen and review and mutually decide what value and cost each drug brings to the patient’s treatment. Medications whose relevance is uncertain can then be further assessed one by one (thus changing only one variable at a time), either by tracking intended target symptoms more closely and formally or by dosage reduction or cautious elimination to determine whether a unique benefit indeed exists.
Another useful practical rule of thumb involves the concept of making no changes to a drug regimen for at least four to six months once unequivocal wellness has been established. This approach may also sometimes include prolonging or suspending a cross-taper in progress. Although “stalled” cross-tapers can leave patients on potentially unnecessary or duplicative medications, and as such are less than elegant, greater gains are sometimes accomplished by changing nothing once wellness is achieved and letting patients accrue lengthier periods of euthymia before embarking on efforts to prune a drug regimen.
The four- to six-month time frame corresponds to the conventional time period that defines recovery, as noted by nomenclature task forces of both the American College of Neuropsychopharmacology (107) and the MacArthur Foundation (108). Symptoms that arise within this time frame after remission from an acute episode are generally recognized as signs of relapse (i.e., the index episode itself reignites), and symptoms that emerge beyond four to six months of wellness are considered signs of a recurrence (i.e., a new episode). Statistically, survivorship of four to six months of wellness after an index episode categorically places someone at a lesser risk for clinical deterioration and consequently represents a logical and potentially safer time to ask whether a particular component of a regimen is or is not actually necessary to sustain wellness.
Future Directions
Several important unmet needs exist for which further studies and guidelines can advance knowledge and clinical practice about complex combination pharmacotherapy for patients with bipolar disorder. These needs include examining sequential pharmacotherapies and specific augmentation-versus-replacement strategies among patients whose responses to core mood stabilizer monotherapies are inadequate; undertaking randomized discontinuation trials to determine whether and when a particular medication has a finite optimal duration of benefit (and at what point diminishing returns might be expected); and conducting formal studies designed specifically to treat common conditions that are comorbid with bipolar disorder, including anxiety disorders; alcohol and substance use disorders; and adult ADD, ADHD, and cognitive dysfunction. Such comorbid conditions contribute to combination pharmacotherapy, but the dearth of dedicated studies targeting such presentations severely limits the evidence base for differential therapeutics.
Other needs are identification of factors and clinical circumstances in which augmentation strategies versus switching from one drug to another produces better outcomes and testing hierarchical treatment approaches as a means to ultimately simplify pharmacotherapy regimens in complex or comorbid presentations. For example, in the case of bipolar disorder with comorbid obsessive-compulsive disorder, some authors have argued that optimization of mood stabilizers can adequately treat both conditions, obviating the necessity of SSRI cotherapy (109); in the case of mood disorder with concurrent alcohol use disorder, further studies are needed to determine whether and when pharmacotherapy of mood symptoms alone may simultaneously reduce excessive drinking (110).
Conclusions
Complex pharmacotherapy regimens can be elegant amalgams of complementary and synergistic elements in which the pharmacodynamic whole becomes greater than the sum of its parts, or they can represent more haphazard collections of medications united by more arbitrariness than purposeful intent. Good clinical practice involves following a logical rationale and combining appropriate, nonredundant drugs that serve identifiable functions and avoid pharmacokinetic or pharmacodynamic hazards. With diligent outcome tracking and a systematic approach to medication changes, logical and strategic medication combinations can be devised to target key symptom domains and minimize unnecessary exposure to redundant, ineffective, or otherwise superfluous psychotropic agents.
1 : Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry 2001; 158:420–426 Crossref, Google Scholar
2 : Complex psychotropic polypharmacy in bipolar disorder across varying mood polarities: a prospective cohort study of 2712 inpatients. J Affect Disord 2017; 221:6–10 Crossref, Google Scholar
3 : Medication burden in bipolar disorder: a chart review of patients at psychiatric hospital admission. Psychiatry Res 2014; 216:24–30 Crossref, Google Scholar
4 : Depressive illness burden associated with complex polypharmacy in patients with bipolar disorder: findings from the STEP-BD. J Clin Psychiatry 2009; 70:155–162 Crossref, Google Scholar
5 : Use of polypharmacy and self-reported mood in outpatients with bipolar disorder. Int J Psychiatry Clin Pract 2005; 9:251–256 Crossref, Google Scholar
6 : Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995–2009. PLoS One 2011; 6:e28725Crossref, Google Scholar
7
8 : Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord 2018; 20:97–170Crossref, Google Scholar
9 2015 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults. Tampa, University of South Florida, Florida Medicaid Drug Therapy Management Program for Behavioral Health, 2015.Google Scholar
10 : The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry 2005; 66:870–886 Crossref, Google Scholar
11 : Assessing efficacy/effectiveness and safety/tolerability profiles of adjunctive pramipexole in bipolar depression: acute versus long-term data. Int Clin Psychopharmacol 2013; 28:297–304 Crossref, Google Scholar
12 : Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry 2006; 163:210–216 Crossref, Google Scholar
13 : A double-blind, randomized, placebo-controlled study of aspirin and N-acetylcysteine as adjunctive treatments for bipolar depression. J Clin Psychiatry 2018; 80:18m12200. doi:
14 : The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord 2018; 229:410–414 Crossref, Google Scholar
15 : The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry 2013; 170:1249–1262 Crossref, Google Scholar
16 : Psychotropic polypharmacy for the treatment of bipolar disorder in Taiwan. Psychiatr Serv 2014; 65:125–128Link, Google Scholar
17 : Polypharmacy and bipolar disorder: what’s personality got to do with it? Int J Neuropsychopharmacol 2014; 17:1053–1061 Crossref, Google Scholar
18 : Pattern of pharmacotherapy by episode types for patients with bipolar disorders and its concordance with treatment guidelines. J Clin Psychopharmacol 2014; 34:577–587 Crossref, Google Scholar
19 : Concordance with treatment guidelines for bipolar disorder: data from the Systematic Treatment Enhancement Program for Bipolar Disorder. Psychopharmacol Bull 2007; 40:72–84 Google Scholar
20 : Use of treatment guidelines in clinical decision making in bipolar disorder: a pilot survey of clinicians. Curr Med Res Opin 2007; 23:467–475 Crossref, Google Scholar
21 : The underlying neurobiology of bipolar disorder. World Psychiatry 2003; 2:136–146 Google Scholar
22 : Potential mechanisms of action of lithium in bipolar disorder: current understanding. CNS Drugs 2013; 27:135–153 Crossref, Google Scholar
23 : Myristoylated alanine-rich C kinase substrate (MARCKS): a molecular target for the therapeutic action of mood stabilizers in the brain? J Clin Psychiatry 1996; 57(Suppl 13):23–33 Google Scholar
24 : The direct effect of lithium and carbamazepine on protein kinase C in rat brain. Jpn J Psychiatry Neurol 1994; 48:123–126 Google Scholar
25 : The current understanding of lamotrigine as a mood stabilizer. J Clin Psychiatry 2004; 65:791–804 Crossref, Google Scholar
26 : Brain-derived neurotrophic factor serum levels before and after treatment for acute mania. Neurosci Lett 2009; 452:111–113Crossref, Google Scholar
27 : Chronic administration of mood stabilizers upregulates BDNF and bcl-2 expression levels in rat frontal cortex. Neurochem Res 2009; 34:536–541 Crossref, Google Scholar
28 : Brain-derived neurotrophic factor signalling mediates antidepressant effects of lamotrigine. Int J Neuropsychopharmacol 2011; 14:1091–1098Crossref, Google Scholar
29 : Effects of acute and chronic treatment elicited by lamotrigine on behavior, energy metabolism, neurotrophins and signaling cascades in rats. Neurochem Int 2011; 59:1163–1174 Crossref, Google Scholar
30 : Psychopharmacology of atypical antipsychotic drugs: from the receptor binding profile to neuroprotection and neurogenesis. Psychiatry Clin Neurosci 2015; 69:243–258 Crossref, Google Scholar
31 : Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PLoS One 2017; 12:e0172270. doi:
32 : Lithium up-regulates the cytoprotective protein Bcl-2 in the CNS in vivo: a role for neurotrophic and neuroprotective effects in manic depressive illness. J Clin Psychiatry 2000; 61(Suppl 9):82–96 Google Scholar
33 : The use of mood stabilizers as plasticity enhancers in the treatment of neuropsychiatric disorders. J Clin Psychiatry 2003; 64(Suppl 5):3–17 Google Scholar
34 : Neuroprotective effects of the mood stabilizer lamotrigine against glutamate excitotoxicity: roles of chromatin remodelling and Bcl-2 induction. Int J Neuropsychopharmacol 2013; 16:607–620 Crossref, Google Scholar
35 : Antipsychotic drugs clozapine and olanzapine upregulate bcl-2 mRNA and protein in rat frontal cortex and hippocampus. Brain Res 2004; 1010:81–86Crossref, Google Scholar
36 : Repeated unpredictable stress and antidepressants differentially regulate expression of the bcl-2 family of apoptotic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology 2008; 33:1545–1558 Crossref, Google Scholar
37 : A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA 1996; 93:8455–8459 Crossref, Google Scholar
38 : Search for a common mechanism of mood stabilizers. Biochem Pharmacol 2003; 66:179–189 Crossref, Google Scholar
39 : Effects of antipsychotic drugs on BDNF, GSK-3β, and β-catenin expression in rats subjected to immobilization stress. Neurosci Res 2011; 71:335–340 Crossref, Google Scholar
40 : Fluoxetine regulates neurogenesis in vitro through modulation of GSK-3β/β-catenin signaling. Int J Neuropsychopharmacol 2014; 18:pyu099. doi:
41 : Brain gabaergic and dopaminergic systems following lithium treatment and withdrawal. Prog Neuropsychopharmacol 1981; 5:527–530Crossref, Google Scholar
42 : Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol 1999; 58:31–59 Crossref, Google Scholar
43 : Effects of some anticonvulsant drugs on brain GABA level and GAD and GABA-T activities. Neurochem Res 1984; 9:225–231 Crossref, Google Scholar
44 : Chronic carbamazepine administration reduces N-methyl-D-aspartate receptor-initiated signaling via arachidonic acid in rat brain. Biol Psychiatry 2007; 62:934–943 Crossref, Google Scholar
45 : The NMDA receptor/nitric oxide pathway: a target for the therapeutic and toxic effects of lithium. Trends Pharmacol Sci 2011; 32:420–434 Crossref, Google Scholar
46 : Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrus. Epilepsia 2008; 49:888–897 Crossref, Google Scholar
47 : Fluoxetine and citalopram decrease microglial release of glutamate and D-serine to promote cortical neuronal viability following ischemic insult. Mol Cell Neurosci 2013; 56:365–374 Crossref, Google Scholar
48 : Lithium inhibits function of voltage-dependent sodium channels and catecholamine secretion independent of glycogen synthase kinase-3 in adrenal chromaffin cells. Neuropharmacology 2007; 53:881–889 Crossref, Google Scholar
49 : Valproate and sodium currents in cultured hippocampal neurons. Exp Brain Res 1993; 93:279–287Crossref, Google Scholar
50 : Carbamazepine inhibits L-type Ca2+ channels in cultured rat hippocampal neurons stimulated with glutamate receptor agonists. Neuropharmacology 1999; 38:1349–1359 Crossref, Google Scholar
51 : Lamotrigine reduces voltage-gated sodium currents in rat central neurons in culture. Epilepsia 1997; 38:522–525 Crossref, Google Scholar
52 : The mechanism of activity-dependent sodium channel inhibition by the antidepressants fluoxetine and desipramine. Mol Pharmacol 2006; 70:2052–2063 Crossref, Google Scholar
53 : 5-HT1B receptors: a novel target for lithium. Possible involvement in mood disorders. Neuropsychopharmacology 1999; 21:530–541Crossref, Google Scholar
54 : Chronic valproate treatment blocks D2-like receptor-mediated brain signaling via arachidonic acid in rats. Neuropharmacology 2011; 61:1256–1264 Crossref, Google Scholar
55 : Chronic carbamazepine administration attenuates dopamine D2-like receptor-initiated signaling via arachidonic acid in rat brain. Neurochem Res 2008; 33:1373–1383 Crossref, Google Scholar
56 : Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord 2007; 9:561–570Crossref, Google Scholar
57 : Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry 2012; 73:81–86 Crossref, Google Scholar
58 : Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ 2013; 346:f3646. doi:
59 : Randomized placebo-controlled adjunctive study of an extract of Withania somnifera for cognitive dysfunction in bipolar disorder. J Clin Psychiatry 2013; 74:1076–1083 Crossref, Google Scholar
60 : Lurasidone versus treatment as usual for cognitive impairment in euthymic patients with bipolar I disorder: a randomised, open-label, pilot study. Lancet Psychiatry 2017; 4:208–217 Crossref, Google Scholar
61 : Treatment of aggression in patients with bipolar disorder. J Clin Psychiatry 1999; 60(Suppl 15):25–28 Google Scholar
62 : Personalized pharmacotherapy for bipolar disorder: how to tailor findings from randomized trials to individual patient-level outcomes. Focus 2019; 17:206–217Google Scholar
63 : Allopurinol as add-on treatment for mania symptoms in bipolar disorder: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry 2017; 210:10–15 Crossref, Google Scholar
64 : Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study. Am J Psychiatry 2008; 165:1316–1325 Crossref, Google Scholar
65 : Asenapine as adjunctive treatment for acute mania associated with bipolar disorder: results of a 12-week core study and 40-week extension. J Clin Psychopharmacol 2012; 32:46–55 Crossref, Google Scholar
66 : Celecoxib adjunctive therapy for acute bipolar mania: a randomized, double-blind, placebo-controlled trial. Bipolar Disord 2015; 17:606–614 Crossref, Google Scholar
67 : Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002; 59:62–69 Crossref, Google Scholar
68 : Olanzapine-divalproex combination versus divalproex monotherapy in the treatment of bipolar mixed episodes: a double-blind, placebo-controlled study. J Clin Psychiatry 2009; 70:1540–1547 Crossref, Google Scholar
69 : Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 2002; 159:1146–1154 Crossref, Google Scholar
70 : Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord 2004; 6:213–223 Crossref, Google Scholar
71 : Lithium as add-on to quetiapine XR in adult patients with acute mania: a 6-week, multicenter, double-blind, randomized, placebo-controlled study. Int J Bipolar Disord 2014; 2:14. doi:
72 : Double-blind, randomized, placebo-controlled 6-week study on the efficacy and safety of the tamoxifen adjunctive to lithium in acute bipolar mania. J Affect Disord 2011; 129:327–331 Crossref, Google Scholar
73 : Olanzapine plus carbamazepine v carbamazepine alone in treating manic episodes. Br J Psychiatry 2008; 192:135–143Crossref, Google Scholar
74 : Paliperidone extended-release as adjunctive therapy to lithium or valproate in the treatment of acute mania: a randomized, placebo-controlled study. J Affect Disord 2011; 129:252–260 Crossref, Google Scholar
75 : Adjunctive topiramate therapy in patients receiving a mood stabilizer for bipolar I disorder: a randomized, placebo-controlled trial. J Clin Psychiatry 2006; 67:1698–1706 Crossref, Google Scholar
76 : Adjunctive oral ziprasidone in patients with acute mania treated with lithium or divalproex, part 1: results of a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2012; 73:1412–1419 Crossref, Google Scholar
77 : Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. J Clin Psychiatry 2009; 70:223–231 Crossref, Google Scholar
78 : Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry 2016; 3:31–39 Crossref, Google Scholar
79 : Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 2014; 171:169–177 Crossref, Google Scholar
80 : Efficacy and safety of adjunctive oral ziprasidone for acute treatment of depression in patients with bipolar I disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2011; 72:1413–1422 Crossref, Google Scholar
81 : Efficacy of aripiprazole adjunctive to lithium or valproate in the long-term treatment of patients with bipolar I disorder with an inadequate response to lithium or valproate monotherapy: a multicenter, double-blind, randomized study. Bipolar Disord 2011; 13:133–144 Crossref, Google Scholar
82 : Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet 2010; 375:385–395 Crossref, Google Scholar
83 : Efficacy of aripiprazole versus placebo as adjuncts to lithium or valproate in relapse prevention of manic or mixed episodes in bipolar I patients stratified by index manic or mixed episode. J Affect Disord 2013; 147:365–372Crossref, Google Scholar
84 : Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v mood stabiliser alone. Br J Psychiatry 2004; 184:337–345 Crossref, Google Scholar
85 : A randomized, double-blind, placebo-controlled study of maintenance treatment with adjunctive risperidone long-acting therapy in patients with bipolar I disorder who relapse frequently. Bipolar Disord 2009; 11:827–839Crossref, Google Scholar
86 : Ziprasidone plus a mood stabilizer in subjects with bipolar I disorder: a 6-month, randomized, placebo-controlled, double-blind trial. J Clin Psychiatry 2010; 71:130–137 Crossref, Google Scholar
87 : Lurasidone in combination with lithium or valproate for the maintenance treatment of bipolar I disorder. Eur Neuropsychopharmacol 2017; 27:865–876 Crossref, Google Scholar
88 : A double-blind, randomized, placebo-controlled prophylaxis trial of oxcarbazepine as adjunctive treatment to lithium in the long-term treatment of bipolar I and II disorder. Int J Neuropsychopharmacol 2008; 11:445–452Crossref, Google Scholar
89 : Polypharmacy in maintenance of bipolar disorder. Clin Neuropharmacol 2016; 39:132–134 Crossref, Google Scholar
90 : Prevalence and clinical features associated with bipolar disorder polypharmacy: a systematic review. Neuropsychiatr Dis Treat 2016; 12:719–735 Crossref, Google Scholar
91 : Initial triple therapy of acute mania, adding lithium and valproate to neuroleptics. Pharmacopsychiatry 2002; 35:244–246Crossref, Google Scholar
92 : Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry 2013; 170:102–110Crossref, Google Scholar
93 : Benzodiazepine use and risk of recurrence in bipolar disorder: a STEP-BD report. J Clin Psychiatry 2010; 71:194–200 Crossref, Google Scholar
94 : Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA 2003; 290:1467–1473 Crossref, Google Scholar
95 : The prospective course of rapid-cycling bipolar disorder: findings from the STEP-BD. Am J Psychiatry 2008; 165:370–377, quiz 410Crossref, Google Scholar
96 : Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry 2006; 67:509–516 Crossref, Google Scholar
97 : Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356:1711–1722Crossref, Google Scholar
98 : Antidepressant prescription patterns in bipolar disorder: a nationwide, register-based study in Korea. J Korean Med Sci 2018; 33:e290Crossref, Google Scholar
99 : A double-blind, placebo-controlled study of quetiapine and paroxetine as monotherapy in adults with bipolar depression (EMBOLDEN II). J Clin Psychiatry 2010; 71:163–174 Crossref, Google Scholar
100 : Persistent antipsychotic polypharmacy and excessive dosing in the community psychiatric treatment setting: a review of medication profiles in 435 Canadian outpatients. J Clin Psychiatry 2010; 71:566–573 Crossref, Google Scholar
101 : Safety and tolerability associated with second-generation antipsychotic polytherapy in bipolar disorder: findings from the Systematic Treatment Enhancement Program for Bipolar Disorder. J Clin Psychiatry 2011; 72:240–247Crossref, Google Scholar
102 : Lamotrigine as add-on treatment to lithium and divalproex: lessons learned from a double-blind, placebo-controlled trial in rapid-cycling bipolar disorder. Bipolar Disord 2012; 14:780–789Crossref, Google Scholar
103 : Impact of antidepressant continuation after acute positive or partial treatment response for bipolar depression: a blinded, randomized study. J Clin Psychiatry 2009; 70:450–457 Crossref, Google Scholar
104 : Antidepressant discontinuation in bipolar depression: a Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry 2010; 71:372–380 Crossref, Google Scholar
105 : A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry 2005; 162:2152–2161 Crossref, Google Scholar
106 : Optimal duration of risperidone or olanzapine adjunctive therapy to mood stabilizer following remission of a manic episode: a CANMAT randomized double-blind trial. Mol Psychiatry 2016; 21:1050–1056 Crossref, Google Scholar
107 : Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006; 31:1841–1853 Crossref, Google Scholar
108 : Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry 1991; 48:851–855 Crossref, Google Scholar
109 : Treatment of comorbid bipolar disorder and obsessive-compulsive disorder: a systematic review. J Affect Disord 2014; 166:258–263Crossref, Google Scholar
110 : Current status of co-occurring mood and substance use disorders: a new therapeutic target. Am J Psychiatry 2013; 170:23–30Crossref, Google Scholar



