An Additive Mix? Acute Urinary Retention in a Patient With Benign Prostatic Hyperplasia Treated With Suboxone, Lurasidone, and Trazodone
Abstract
Treatment of psychiatric patients is frequently complicated by medical comorbidities, complex pharmacologic regimens, and side effects occurring secondarily to those regimens. Acute urinary retention is an infrequently discussed side effect of such regimens. This report describes the development of acute urinary retention (AUR) in a 60-year-old man with a history of benign prostatic hyperplasia. The patient developed AUR during treatment with combination buprenorphine/naloxone, trazodone, and lurasidone. We discuss the potential relationship of these agents to the development of this patient’s AUR, the complicated neurochemical dynamic of the voiding process, and the pathologic consequences that psychotropic agents can have on that process.
Psychiatric patients encounter a range of side effects when treated with psychopharmacological agents. Acute urinary retention (AUR) is an infrequently discussed yet relevant side effect. AUR, a urological emergency, presents with abdominal pain and acute inability to urinate (1, 2). This diagnosis can be split into three broad pathophysiologic categories: obstruction of the outflow tract, inefficient detrusor contraction, and interruption of spinal cord nerve pathways (2, 3). Pharmacologic agents can influence each of these categories, and their effects should be considered when patients complain of clinically relevant symptoms.
AUR is more prevalent in male patients than female patients, with a reported prevalence of 6.8 in every 1,000 male patients compared with 0.07 cases in every 1,000 female patients (1). Male-specific prostate illnesses bolster these rates; 130 in every 1,000 patients with benign prostatic hyperplasia (BPH) are reported to develop AUR (1).
Pharmaceuticals play a modest role in the development of urinary retention, with 2%−10% of AUR cases being linked to pharmaceutical agents (1, 4). In their 2008 article, Verhamme et al. reported a link between urinary retention and select psychopharmaceuticals, including antipsychotics (particularly ziprasidone), antidepressants (particularly tricyclics), benzodiazepines, and opioids (1). Some of these agents have strong links to urinary retention documented in the literature, but many, such as selective serotonin reuptake inhibitors (SSRIs), are linked only through case reports (5). Development of symptoms typically occurs in settings of polypharmacy in these reports, suggesting an additive role between agents (5).
Despite the clinical evidence linking many psychopharmacologic agents to AUR, there is little evidence linking orally administered buprenorphine and no evidence linking trazodone or lurasidone. Our group encountered a patient who developed AUR while being treated with these agents. The receptor profile of these agents theoretically links them to AUR, but scant literature exists on the topic.
Case
Mr. AB is a 60-year-old man with a history of bipolar disorder, as well as opioid, alcohol, benzodiazepine, and nicotine use disorders with multiple hospitalizations for bipolar disorder, substance abuse, and acute suicidality. The patient had been abusing alcohol, benzodiazepines, and oral opiates on and off for 30 years but had been sober, maintained on daily combination buprenorphine/naloxone 20 mg/5 mg, until he relapsed two months before admission at our facility. Since relapsing, he was drinking two pints of whiskey and taking a total of 150 mg oral oxycodone daily, with frequent but not daily use of one to three tablets of 3 mg clonazepam. The patient presented because of concern regarding his substance abuse and suicidal ideation. His medical history was significant for benign prostatic hyperplasia, coronary artery disease, hyperlipidemia, myocardial infarction, and chronic back pain. He had no past episodes of AUR, although the patient stated he usually urinated two to three times per night and often felt as if he did not completely void.
The patient arrived at the emergency room with two months of nonadherence to his buprenorphine/naloxone, an alcohol level of 220 mg/dL, and a urinary drug screen positive for benzodiazepines. He also reported recent opiate use. Upon admission to the psychiatric unit, the patient was acutely stabilized, and by hospital day 4 was started on buprenorphine/naloxone. The initial dose was 4 mg/1 mg daily, but the dose was titrated to 32 mg/8 mg daily by day 14. Additionally, a daily oral dose of 20 mg lurasidone was started on day 4 for mood stabilization and titrated to a dose of 40 mg on day 16. Lurasidone was briefly discontinued because of the onset of pedal edema and concerns of medication-related side effects, though the edema was later determined to be chronic and lurasidone was restarted. Finally, by day 11, because of reports of insomnia, a dose of 100 mg trazodone orally at bedtime was started. After several days on this regimen, on the evening of day 17, the patient complained of a 24-hour history of an inability to urinate and suprapubic discomfort. Physical exam was remarkable for generalized abdominal pain and a palpable mass in the hypogastric region extending 10 cm suprapubically. The patient was suspected to have AUR and was immediately catheterized. Urine (1,600 mL) was drained, confirming the diagnosis. The patient’s lab results, including complete blood count, basic metabolic panel, and urinalysis were collected and were unremarkable.
In response to the diagnosis, the patient’s dose of buprenorphine/naloxone was reduced to 24 mg/6 mg, and both trazodone and lurasidone were discontinued. The patient reported no recurrence of urinary retention symptoms throughout his hospitalization, even with a later reintroduction of lurasidone that was titrated to a daily oral dose of 20 mg, and restoration of his buprenorphine/naloxone dose to 32 mg/8 mg daily.
Of note, historically, the patient had previous medication trials of 100 mg nightly oral trazodone as well as 20 mg/5 mg daily sublingual buprenorphine/naloxone. In addition, he had taken these medications concurrently at 100 mg and 16 mg/4 mg, respectively, in the past. Urinary retention had not occurred previously, neither when the medications were taken individually nor concurrently.
The patient’s outpatient medications for chronic conditions were restarted upon arriving at the hospital and remained constant throughout the admission. A precise schedule of the patient’s medication dosing is included in Figure 1.
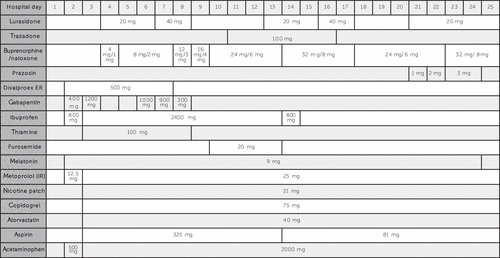
FIGURE 1. Patient Medication Schedule During Hospital Stay
Discussion
This case represents the complicated pharmacological dynamic that psychiatrists often face when treating patients with medical comorbidities. No single factor completely accounts for our patient’s development of AUR, but it is instead attributable to both the patient’s comorbidity (benign prostatic hyperplasia) and his medication regimen. Our discussion focuses on the three agents that, we suspect, contributed to our patient’s development of AUR: lurasidone, trazodone, and buprenorphine. A Naranjo scale (6) was used to assess the likelihood that these agents played a role in the development of Mr. AB’s AUR. Naloxone was not considered in our review, given its negligible oral bioavailability (1, 5, 7) and its previously established role in reversing urinary retention (8, 9).
We consider the pharmacodynamic activity of the agents, where relevant, on the D1, D2, 5HT2a/2c, alpha-1, M2, M3, nicotinic, beta-3, and mu receptors, given their role in micturition (1, 4, 10) (Table 1). We additionally consider pharmacokinetic interactions between these agents and other medications administered to the patient.
| Receptor | Effect | Result |
|---|---|---|
| Central | ||
| D1 | Facilitates storage | Bladder filling |
| D2 | Facilitates micturition | Promotes micturition |
| 5HT2a | Promotes external urethral sphincter contraction through activity at sacral plexus | Bladder filling |
| 5HT2c | Some inhibition of the central micturition reflex and promotion of external urethral sphincter contraction through activity at the sacral plexus | Bladder filling |
| Mu | Indirectly reduces afferent parasympathetic activity from the bladder, resulting in a reduced sensation of bladder fullness; indirectly increases efferent sympathetic activity on the internal urethral sphincter | Bladder filling |
| Peripheral | ||
| Beta-3 | Detrusor relaxation | Promotes micturition |
| Alpha-1 | Internal urethral sphincter contraction | Bladder filling |
| M2 | Detrusor contraction | Detrusor contraction |
| M3 | Main source of detrusor contraction | Promotes micturition |
| Nicotinic | Somatic contraction of external urethral sphincter | Bladder filling |
TABLE 1. Central and Peripheral Receptor Activity on Micturationa
This analysis included a review of the Embase, PubMed, and Medline databases using the MeSH search term “trazodone,” “lurasidone,” “buprenorphine,” or “suboxone” with the term “urinary retention” or “urinary hesitancy.” The search returned 107 total articles. Sixty-nine articles were excluded for not reporting on the agents of interest, 12 were excluded for having no English translation, and another 10 were excluded because they repeated articles. The remaining 16 articles addressed a link between AUR and buprenorphine (4, 7, 11–25). In particular, these sources focused on intrathecal (13, 15, 16, 26), epidural (17, 19, 25), direct nerve block (23), and intravenous (14, 18) administrations of buprenorphine. One retrospective analysis and several case reports addressed the link between buprenorphine and urinary hesitancy (6, 19) and urinary retention (4, 7, 22, 25). The database search did not return evidence linking trazodone or lurasidone to AUR.
A detailed discussion of the relationship between these agents and AUR follows.
Lurasidone
The Naranjo analysis score for lurasidone was 4, suggesting a possible link of this agent to the patient's development of AUR.
Lurasidone is a novel second-generation antipsychotic introduced to the U.S. market in 2010 (12). Treatment indications are for bipolar depression and schizophrenia (27). Genitourinary side effects are infrequent for this agent.
Several factors indicate that lurasidone may have contributed to Mr. AB’s symptoms. These include the chronological link between medication titration to symptom development and medication discontinuation to symptom remission. Although our review did not find reports linking lurasidone to AUR, a recent literature review did find reports linking the neuroleptics haloperidol, trebenzomine, ziprasidone, olanzapine, quetiapine, amisulpride, and aripiprazole to AUR (28). This review also reported on a large (N=200,000) Canadian matched-cohort study that found that use of the atypical antipsychotics risperidone, quetiapine, and olanzapine resulted in a statistically significant 0.16% increase in absolute risk of AUR (29). Sufficient pharmacodynamic similarities exist between these agents, particularly ziprasidone, and lurasidone to maintain suspicion of lurasidone’s role in our patient’s development of AUR. Lurasidone and ziprasidone both have a wide pharmacodynamic binding profile and share similar affinities and activities. These include strong antagonism at the 5HT2a, 5HT7, D2, and D3 receptors, low affinity for alpha-1 receptors (30, 31), and limited to no activity at muscarinic, nicotinic, and opioid receptors (32, 33). The agents do differ with respect to ziprasidone’s antagonistic activity at the 5HT1a, 5HT2c, and H1 receptors, as compared with lurasidone’s partial agonism at 5HT1a, and otherwise negligible activity at the 5HT2c and H1 receptors (31–33). Taking into consideration the receptors that are most relevant to the micturition process (Table 1) and the comparative affinity of lurasidone and ziprasidone on the D2 (binding affinity [Ki] of 1.68 [27] and 1.2–9.7 [34] for lurasidone and ziprasidone, respectively), 5HT2a (Ki of 2.03 [27] and 0.08–1.40 [34], respectively), and 5HT2c (Ki of 415 [27] and 0.55–68.0 [34], respectively) receptors, there appears to be a comparative profile that, if anything, favors lurasidone over ziprasidone in the risk of AUR development. That is to say, while both agents have similar D2 antagonism, promoting urinary storage, and 5H2a antagonism, promoting micturition, their difference in 5HT2c binding profiles suggests that ziprasidone’s 5HT2c antagonism may reduce its risk in contributing to urinary retention, as compared with lurasidone.
Trazodone
The Naranjo analysis score was 4 for trazodone, suggesting a possible link between the agent and the patient’s development of AUR.
Trazodone is an antidepressant with serotonin antagonism and reuptake inhibition (35, 36). Trazodone’s previously reported genitourinary side effects include priapism and, infrequently, urinary incontinence (37). As with lurasidone, Mr. AB’s development of AUR upon trazodone administration and immediate improvement upon discontinuation suggests a possible correlation between the medication and the patient’s development of AUR. Apart from the aforementioned points, the evidence linking trazodone to the patient’s symptom development is not strong. Our literature review did not return any reports linking the agent to AUR. Furthermore, trazodone’s pharamacodynamic activity does not strongly suggest a role in the development of AUR. Trazodone is a serotonin reuptake inhibitor, a partial agonist at the 5HT1a receptor, and an antagonist at the 5HT2a, 5HT2b, 5HT2c, alpha-1, and alpha-2 receptors (36). The 5HT2a and alpha-1 receptors promote urinary storage and internal urethral sphincter contraction, respectively (Table 1), and trazodone’s antagonism would thus point away from a link between trazodone and AUR. However, the agent’s active metabolite, m-chlorophenylpiperazine (m-CPP) does have significant affinity and partial agonism at the 5-HT2c receptor (38). It is possible that m-CPP’s activity at the 5-HTc receptor alongside trazodone and m-CPPs’ serotonin reuptake inhibition (boosting central serotonergic drive) may have contributed to the promotion of retention in our patient.
Buprenorphine
The Naranjo analysis returned a score of 5 for buprenorphine, suggesting the agent was a probable cause of this patient’s AUR.
Mr. AB was administered buprenorphine in the combination medication comprising buprenorphine and naloxone. The combination of buprenorphine, a partial agonist on the mu receptor and antagonist on the kappa receptor, and naloxone, a complete mu antagonist (34) with negligible oral bioavailability (8), discourages parenteral abuse of buprenorphine due to the potent effects of naloxone when administered in such a fashion (7). Given the negligible oral bioavailability of naloxone (8) for this section, we focus only on the role of buprenorphine in the development of AUR.
Several factors link buprenorphine to the development of AUR in Mr. AB. The first of these are his development of symptoms and subsequent improvement upon dose titration and tapering, respectively. Second, buprenorphine has a clearly established pharmacodynamic mechanism, partial agonism at the mu receptor, that indirectly promotes urinary retention (7).
In addition to the aforementioned text, our review returned several articles linking buprenorphine to urinary hesitancy and AUR, in contrast to lurasidone and trazodone. Although orally administered buprenorphine, either alone or in combination with naloxone, seems to be clearly linked to urinary hesitancy (6, 19), the development of AUR secondary to the oral administration of the agent is infrequently described (4, 7, 11, 25). Our search returned four articles linking the agent to AUR (Table 2). Consistent with Verhamme et al.’s 2008 review (1) on the elevated rates of AUR in males (4, 34), all the AUR reports were of male patients, and one of the four AUR cases had a history of BPH (Table 2). The similarities between these patients and Mr. AB strongly point to the role of buprenorphine in his development of AUR.
| Case | Age (Years) | Comorbid Outflow Tract Obstruction | Coadministration of Additional Pharmacologic Agent | Suboxone Maximum Dose | Time to Symptom Development | Treatment | Subsequent Restart and Success |
|---|---|---|---|---|---|---|---|
| Altunsoy et al. (8) | 20 | No | No | 8 mg/2 mg | 2 days | Indwelling catheterization for 3 days + drug discontinuation | No, patient refused |
| Edwards et al. (4) | 49 | No | Unknown | 12 mg/3 mg | 3 days | Indwelling catheterization for 1 week + drug discontinuation | No, patient refused |
| Rai (26) | 58 | BPH | Yes | 12 mg/3 mg | 3 days | Intermittent catheterization + eventual drug discontinuation | Attempts at continuing suboxone failed with recurrence of symptoms |
| Murray (12) | 66 | No | Unknown | 6× 200-μg tablets in 18 hours (buprenorphine alone) | 1 day | Drug withheld | Further use of buprenorphine resulted in recurrent, though self-remitting, anuria |
TABLE 2. Cases Linking Suboxone or Buprenorphine Alone to the Development of AURa
Notably, while buprenorphine is most likely to have played a role in Mr. AB’s development of AUR, we believe that his stability on final retitration of the agent speaks to the multifactorial nature of his development of AUR. The use of prazosin, an alpha-1 antagonist, may have contributed to the patients’ stability during buprenorphine retitration. The patient’s continued stability despite prazosin discontinuation, however, suggests alternate etiologies for his stability, original development of symptoms, or both. These include the titration of lurasidone and trazodone; the only other agents titrated incident to symptom development.
Pharmacokinetics.
It appears on analysis that two important pharmacokinetic factors may have contributed to Mr. AB’s development of AUR. These include the possible saturation of the CYP3A4 enzyme system and inhibition of glucuronyl transferase. While each of lurasidone, trazodone, and buprenorphine vary in their metabolism, they all have in common the CYP3A4 enzyme system (30, 38, 39). Besides these agents, Mr. AB was additionally treated with clopidogrel, metoprolol, and atorvastatin, which are also metabolized by the CYP3A4 system (40–42). Given the shared metabolism between all these agents, it is possible that in the case presented, the CYP3A4 system was saturated, exposing Mr. AB to higher concentrations of each of the aforementioned agents and their consequent side effects. Additionally, buprenorphine’s metabolism through glucuronidation (43) warrants consideration. As the patient’s complicated pharmaceutical regimen included valproic acid, a glucuronyl transferase inhibitor (44), it is likely that buprenorphine concentrations were consequently elevated due to reduced glucuronidation.
Treatment.
Treatment for AUR generally involves catheterization and treatment of the underlying cause (1). In Mr. AB’s case, catheterization and taper/discontinuation of the suspected agents were adequate for symptom resolution. Three of the four literature-reported cases on this topic used similar treatment (drug discontinuation and catheterization), with one simply utilizing drug discontinuation (Table 2). While two of the three catheterization cases represent patients who were treated with indwelling catheters, we advocate for treatment using intermittent catheterization, given the success of the approach in Mr. AB’s case as well as the case described by Altunsoy et al. (8). In addition, the literature suggests that intermittent catheterization results in improved rates of spontaneous voiding and reduced risk of urinary tract infections (1).
Finally, Mr. AB was successfully continued on buprenorphine/naloxone without redeveloping symptoms; however, the literature is lacking on the matter of drug restart or continuation once patients develop this reaction. Certainly, in the case of urinary hesitancy, successful drug rechallenge or continuation is well documented (6, 19). Two of the four reviewed AUR cases reattempted oral buprenorphine/naloxone or buprenorphine alone, and in both cases, the patients redeveloped AUR (11, 25). Mr. AB’s medications were not discontinued, but tapered and then retitrated to the previous dose, and he did not redevelop AUR during the time of hospitalization. As the patient was lost to follow-up after his hospital stay, we cannot report on his long-term outcome on the agent. Considering the limited literature, clinicians should be mindful of urinary retention in patients rechallenged on oral buprenorphine/naloxone or buprenorphine alone after having previously developed AUR on the agent. Patients who are treated on an outpatient basis should be provided with catheters in case of symptomatic recurrence and should be advised to seek immediate emergency evaluation if symptoms redevelop.
Conclusion
Mr. AB’s presentation represents the only case in the reviewed literature of a patient for whom oral buprenorphine was successfully tapered and retitrated after the development of AUR on the agent. Additionally, this case raises to clinical consideration the possible causal role of lurasidone and trazodone in the development of AUR. The case speaks to the importance of psychiatrists obtaining thorough medical assessments prior to treating patients with pharmacological agents. Patients with risk factors for AUR, such as male gender, advanced age, a history of obstructive conditions (BPH), and complex pharmacologic regimens, should trigger clinicians to be especially cautious with the addition of further agents to the patient’s treatment. Clinicians should attempt to slowly titrate medication while continuing to screen actively for this serious reaction in their medical review of systems.
1 : Drug-induced urinary retention: incidence, management and prevention. Drug Saf 2008; 31:373–388 Crossref, Google Scholar
2 : Acute urinary retention: causes, clinical features and patient care. Nurs Stand 2007; 21:42–46 Crossref, Google Scholar
3 : Acute urinary retention. BJU Int 2000; 85:186–201Crossref, Google Scholar
4 : Acute urinary retention secondary to buprenorphine administration. Am J Emerg Med 2014; 32:109.e1–109.e2 Crossref, Google Scholar
5 : The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom 2016; 85:270–288 Crossref, Google Scholar
6 : A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30:239–245 Crossref, Google Scholar
7 : Bethanechol for buprenorphine-related urinary hesitancy: a case series. J Addict Med 2011; 5:227–228 Crossref, Google Scholar
8 : Acute urinary retention related with sublingual buprenorphine administration. Klinik Psikofarmakol Bulteni 2016;26.Crossref, Google Scholar
9 : Naloxone reversal of urinary retention after epidural morphine. Lancet 1981; 2:1411 Crossref, Google Scholar
10 : Investigation of the role of 5-HT2 receptor subtypes in the control of the bladder and the urethra in the anaesthetized female rat. Br J Pharmacol 2008; 155:343–356 Crossref, Google Scholar
11 : Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther 2007; 82:48–53 Crossref, Google Scholar
12 : Acute urinary retention associated with sublingual buprenorphine. Br Med J (Clin Res Ed) 1983; 286:763–764 Crossref, Google Scholar
13 : Effect of route of buprenorphine on recovery and postoperative analgesic requirement in paediatric patients. Paediatr Anaesth 2002; 12:786–790Crossref, Google Scholar
14 : Hemorrhoidectomy with posterior perineal block: experience with 400 cases. Dis Colon Rectum 2000; 43:809–812 Crossref, Google Scholar
15 : Progressive systemic sclerosis: intrathecal pain management. Reg Anesth Pain Med 1999; 24:89–93 Google Scholar
16 : Continuous intrathecal infusion of opioid and bupivacaine in the treatment of refractory pain due to postherpetic neuralgia: a case report. Neuromodulation 1998; 1:85–89 Crossref, Google Scholar
17 : Comparison of extradural buprenorphine and extradural morphine after caesarean section. Br J Anaesth 1988; 60:627–631Crossref, Google Scholar
18 : Comparison of buprenorphine with morphine in the treatment of postoperative pain in children. Anesth Analg 1988; 67:233–239Crossref, Google Scholar
19 : Epidural buprenorphine for postoperative analgesia. A controlled comparison with epidural morphine. Anaesthesia 1986; 41:76–79 Crossref, Google Scholar
20 . Buprenorphine-induced urinary hesitancy is common and managed with ease: a retrospective chart review. J Alcohol Drug Depend 2017; 5:258Crossref, Google Scholar
21 : Epidural buprenorphine for postoperative analgesia in spinal surgery. J Anaesthesiol Clin Pharmacol 2005; 21:385–388Google Scholar
22 : Major laparoscopic surgery under regional anesthesia: A prospective feasibility study. Med J Armed Forces India 2015; 71:126–131Crossref, Google Scholar
23 : P.; Rani, Shobha; Kumaresan, C.; Murthy, Hanuman K. Comparison of clonidine and buprenorphine as adjuvants to bupivacaine for continuous postoperative epidural analgesia. Br J Anaesth 2012; 108:432–433Google Scholar
24 : Psychotropic drugs and their effects on lower urinary tract function: an update. Curr Bladder Dysfunct Rep 2016; 11:258–265Crossref, Google Scholar
25 N Vidhya YS. Comparison of low dose hyperbaric bupivacaine combined with fentanyl, clonidine or buprenorphine for spinal anaesthesia in anorectal surgeries. Res J Pharm Biol Chem Sci 2017; 8:1381Google Scholar
26 : Sublingual suboxone (buprenorphine/naloxone) leading to acute urinary retention requiring emergent attention. J Addict Res Ther 2014; 5:104Google Scholar
27 : Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 2010; 334:171–181 Crossref, Google Scholar
28 : Linking the evidence between urinary retention and antipsychotic or antidepressant drugs: a systematic review. Neurourol Urodyn 2016; 35:866–874 Crossref, Google Scholar
29 : Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study. Ann Intern Med 2014; 161:242–248 Crossref, Google Scholar
30 : Clinical pharmacology of atypical antipsychotics: an update. EXCLI J 2014; 13:1163–1191 Google Scholar
31 : The psychopharmacology of ziprasidone: receptor-binding properties and real-world psychiatric practice. J Clin Psychiatry 2003; 64(Suppl 19):6–12Google Scholar
32 : Ziprasidone mesylate injection. Cleveland, OH, Cleveland Clinic Center for Continuing Education, 2003. https://www.clevelandclinicmeded.com/medicalpubs/pharmacy/janfeb2003/ziprasidone.htm. Accessed Jan 20, 2003Google Scholar
33 : An update of the preclinical profile of lurasidone. Evidence-Based Pyschiatric Care 2015; 1:67–72Google Scholar
34 : The multiplicity of serotonin receptors: uselessly diverse molecules or an embarrassment of riches? Neuroscientist 2000; 6:252–262Crossref, Google Scholar
35 Latuda prescribing information. Marlborough, MA, Sunovion Pharmaceuticals, 2013. http://www.latuda.com/LatudaPrescribingInformation.pdf. Accessed May 15, 2018Google Scholar
36 Trazodone and urinary incontinence. s’Hertogenbosch, the Netherlands, Netherlands Pharmacovigilance Centre Lareb 2013. https://databankws.lareb.nl/Downloads/KWB_2013_3_Trazodone_and_urinary_incontinence.pdf. Accessed May 15, 2018Google Scholar
37 : Mechanism of action of trazodone: a multifunctional drug. CNS Spectr 2009; 14:536–546 Crossref, Google Scholar
38 : Trazodone is metabolized to m-chlorophenylpiperazine by CYP3A4 from human sources. Drug Metab Dispos 1998; 26:572–575Google Scholar
39 : Buprenorphine: a (relatively) new treatment for opioid dependence. Psychiatry (Edgmont Pa) 2005; 2:29–39 Google Scholar
40 : Clopidogrel pathway. Pharmacogenet Genomics 2010; 20:463–465 Google Scholar
41 : An evaluation of the CYP2D6 and CYP3A4 inhibition potential of metoprolol metabolites and their contribution to drug-drug and drug-herb interaction by LC-ESI/MS/MS. Biomed Chromatogr 2016; 30:1556–1572Crossref, Google Scholar
42 : Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors. Drug Saf 2008; 31:587–596 Crossref, Google Scholar
43 : Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology 2011; 115:1251–1260 Google Scholar
44 : The effect of valproic acid on drug and steroid glucuronidation by expressed human UDP-glucuronosyltransferases. Biochem Pharmacol 2003; 65:1441–1449 Crossref, Google Scholar



