Overlay of Late-Life Depression and Cognitive Impairment
Abstract
This article appraises several facets of the linkage between depression and cognitive impairment, including dementia, mild cognitive impairment, and vascular dementia. Potential mechanisms for this association are examined. This review was crafted to be extensive but not exhaustive. The authors searched PubMed, using the terms depression, late-life depression, cognitive impairment, and dementia. Articles included are seminal articles from the field as well as representative, heuristic studies. A link between depression and cognitive impairment was found. Depression likely serves as both a risk factor and a prodromal symptom of dementia. Mechanisms whereby depression could induce cognitive impairment include hippocampal atrophy, alterations in glucocorticoid secretion, cerebrovascular compromise, deposition of β-amyloid plaques, chronic inflammation, apolipoprotein E status, and deficits of nerve growth factors. This article will benefit the practicing clinician by increasing awareness of the links between depression and dementia and encouraging greater emphasis on screening for cognitive impairment among individuals with depression or a history of depression.
Overview
Cognitive deficits are a core feature of depression among both adults and elders, which makes depression a “cognitive disorder” for many people (1). Cognitive deficits are consistently found in the domains of memory, verbal learning, motor speed, executive functioning, and processing speed (2). In the past, such deficits were thought to be temporary and isolated to periods of dysphoria. They were labeled “pseudodementia” or “reversible dementia.” Increasingly, there is evidence to suggest that cognitive deficits persist despite remission of depression. Continuing cognitive deficits may be related to underlying brain changes, including hippocampal atrophy, alterations in glucocorticoid secretion, and cerebrovascular compromise (3–5). Other biological mechanisms linking depression to cognitive impairment include increased deposition of β-amyloid plaques, chronic inflammation, and deficits of nerve growth factors, such as brain-derived neurotrophic factor (6).
Fifteen years ago, Jorm (7) proposed three hypotheses to explain the relationship between dementia and depression. Depression can, first, be an early prodromal sign of dementia; second, bring forward the clinical manifestations of dementing disease; and third, lead to hippocampal damage through a glucocorticoid cascade. He concluded that depression is likely a risk factor for dementia in general and Alzheimer’s disease specifically (7).
This article appraises several facets of the linkage between depression and cognitive impairment, including whether depression is a risk factor or a prodromal feature of dementia. Other areas of exploration include the impact of midlife, late-life, and vascular depression on cognition and possible mechanisms whereby depression may result in lasting cognitive impairment. This review was crafted to be extensive, although not exhaustive, because this is a rapidly expanding area of inquiry.
Description
Depression among older adults can have a variety of presentations. It may arise as recurrent disease stemming from earlier life. This has been termed early-onset or midlife depression. Late-onset or late-life depression is described as depression that initially occurs past the age of 60 to 65 years. Depression can also occur as a mood disorder secondary to another medical condition or as mood symptoms secondary to substance use or medication.
Symptomatic differences have been noted in early-onset depression and late-life depression. Early-onset depression has been characterized by greater personality abnormalities, a family history of psychiatric illness, and dysfunctional past maternal relationships. The mood is often depressed, hopeless, and accompanied by suicidal thoughts (8). Late-life depression has been described by fearfulness, poor appetite, weariness, hopelessness, worthlessness, guilt, and anger. People with late-life depression have fewer crying spells and less sadness (8, 9). Anxiety, somatic complaints, and thoughts of death, rather than outright suicide ideation, may occur more frequently (9).
As noted, cognitive deficits are a core feature of depression for both adults and elders. Among younger adults, decreased concentration and indecisiveness are seen, whereas cognitive complaints of older adults may include selective attention, problems in working memory, impaired memory retrieval, difficulties in learning, slowed processing speed, and executive dysfunction (10–12). Table 1 summarizes these presentations. Cognitive deficits persist even during euthymic periods, and, compared with healthy others, euthymic patients postdepression have had significantly poorer cognitive function (11). Cognitive deficits seem to be more common among patients with late-onset depression, compared with those with early-onset disease. This supports theories suggesting that vascular and neurodegenerative factors, which are more common in late life, affect a substantial number of these patients (11) and that etiologically, late-life depression may be different from early-onset depression.
| Symptom Domain | Adult Presentation | Geriatric Presentation |
|---|---|---|
| Mood | Depressed mood, anhedonia, suicidal thoughts | Depressed, hopeless, feels worthless, psychic anxiety, thoughts of death |
| Somatic | Sleep changes, appetite changes, activity changes | Activity changes, general somatic symptoms |
| Cognitive | Decreased concentration, indecisiveness | Decreased processing speed, executive function, selective attention, working memory, verbal fluency, spatial planning |
Depression and Dementia
The crux of the association between depression and dementia has focused on whether depression is a risk factor for dementia, a prodrome of dementia, or some combination of both. The majority of published studies have highlighted an increased risk of dementia among people with previous diagnoses of depression. However, approximately one quarter of studies have not shown any statistically significant increase in this risk (15), which has led to continued uncertainty regarding this association. Other areas of inquiry have been whether there is differential risk for dementia among patients with early-onset, late-life, vascular, or recurrent depression.
Cognitive deficits can persist despite remission of depressive symptoms, which indicates that depression may have a longer term pernicious effect on cognition. In one study, conducted over three years, irreversible dementia developed significantly more frequently among depressed elders with reversible dementia or pseudodementia (43%) than among the group with depression alone (12%). The group with reversible dementia had a 4.69 times higher chance of developing dementia than patients with depression alone (16). Cognitive areas affected included visuoperception, processing speed, and memory function (13). Researchers have continued to find deficits up to 18 months and four years later postdepression, so that cognitive impairment among elderly patients with depression may be a trait characteristic, such that even when depression has remitted, cognitive impairment persists (3).
With respect to depression as a risk factor for dementia, data have been mixed (7, 17). A large systematic review and meta-analysis concluded that depression was a risk factor for dementia. The researchers appraised 20 studies involving 102,172 patients. They separated case-control studies, which are retrospective and subject to recall bias, from cohort studies, which are prospective. The authors found calculated pooled odds ratios (ORs) of 2.03 for case-control studies and 1.90 for cohort studies. The interval between diagnoses of depression and Alzheimer’s dementia (AD) was positively related to increased risk of developing AD, which suggests that rather than a prodrome, depression may be a risk factor for AD (18). Another meta-analysis involving older adults and elders over age 50 found that depression was also associated with a significant risk of all-cause dementia (OR=1.85), AD (OR=1.65), and vascular dementia (VaD; OR=2.52) (14).
The hypothesis that depression is a prodrome to dementia is most compatible with studies in which depression occurs near the onset of dementia. The MIRAGE study was a case-control study involving more than 4,000 individuals. A significant association was found between depression and AD, with an OR of 2.13. Where depression first occurred within one year before onset of AD, the OR was 4.47. Where depression first occurred more than one year before onset of AD, the OR was 1.38. Where depression first occurred more than 25 years before onset of AD, the OR was 1.71 (19). The data suggested that depression is a significant prognostic indicator for dementia, as well as a risk factor. Barnes et al. (20) conducted a retrospective cohort review of 13,535 Kaiser Permanente patients with an average age of 81.1 years. Depressive symptoms were present among 14.1% of patients in midlife only, 9.2% in late-life only, and 4.2% in both. During six years of follow-up, 22.5% of the patients received a diagnosis of dementia. The adjusted hazard ratio of dementia was increased by approximately 20% for midlife depressive symptoms only (OR=1.19), 70% for late-life symptoms only (OR=1.72), and 80% for both (OR=1.77). When AD and VaD were examined separately, patients with late-life depressive symptoms had a twofold increase in AD risk (OR=2.06), whereas patients with midlife and late-life symptoms had more than a threefold increase in VaD risk (OR=3.51). In this analysis, late-life depressive symptoms were more predictive of dementia than early-life depressive symptoms (20). Other studies have also found that a history of current depression and increasing depressive symptoms in late life increase the risk of cognitive impairment, whereas a history of past depressive symptoms or low depression is not associated with cognitive impairment. These data suggest that depression in later life may also represent a prodromal phase of cognitive impairment (21, 22).
Recurrent depression seems to present a greater risk for dementia than single-episode depression, such that patients with many prior hospitalizations for depression have an increased risk of developing subsequent dementia. On average, each depressive episode has been associated with a 13% increased rate of dementia (23). This result has been confirmed in a prospective study of 1,239 older adults from the Baltimore Longitudinal Study of Aging. In that study, each depressive episode was associated with a 14% increase in risk for all-cause dementia (24).
In sum, the proposals of depression as either a risk factor or a prodrome for dementia are not mutually exclusive. Data currently seem to reflect that depression is both a risk factor and a prodrome for dementia (15, 18–20, 25, 26), although there is evidence to the contrary (21, 27–29), likely because of heterogeneity in methodology, populations, and other factors. More data support early-onset depression’s serving as a risk factor for dementia (17, 30) than support late-life depression’s acting as a prodromal symptom of dementia (20, 21). However, in studies looking across age ranges, the magnitude of risk ratios decreases with the time between depression onset and dementia diagnosis (1, 19, 20), which makes it more likely that later onset of depression may be a more significant prodromal symptom or risk factor for later dementia (1). There also seems to be a dose-response relationship between depression and dementia, such that each depressive episode is associated with an increased rate of dementia (23, 24).
Depression and Mild Cognitive Impairment
The term mild cognitive impairment (MCI) is used to characterize nonnormal cognitive function that is not severe enough to meet diagnostic criteria for dementia (1). It has been conceptualized as a predementia state or a warning sign that the risk of dementia is high. Depression occurs commonly among people with MCI and has been found in 30%−45% of patients with MCI (31, 32). More than 20% of patients with MCI convert to dementia within a three-year period (33). However, the majority of MCI patients do not convert. Researchers have attempted to determine which factors lead to dementia conversion and have looked at depression, apolipoprotein E (APOE) genotypes, and amnestic MCI (aMCI).
Studies have shown that the presence of depressive symptoms increases the rate of conversion of MCI to dementia (34). In one study of MCI patients, after a mean period of three years, 85% of the patients with depression developed dementia, in comparison with 32% of the patients without depression. The relative risk (RR) was 2.6. Patients with MCI and depression had more than twice the risk of developing AD as those without depression (32). This finding was confirmed in a large meta-analysis. Eighteen studies were included, with a sample size of 10,861 patients with MCI. The pooled RR of progressing to dementia was 1.28 for the group of MCI patients with depressive symptoms, compared with nondepressed patients with MCI (35).
Other factors that might affect the role of depression in conversion of MCI to dementia include APOE status and the presence of aMCI. The risk of developing AD has been significantly higher among depressed aMCI groups than among depressed nonamnestic groups (36). The role of APOE status in conversion to dementia and the development of MCI has been more mixed. In one study, 21% of depressed elders met criteria for aMCI. Among depressed elders, aMCI and age were most associated with incident dementia and AD. Any APOE ε4 allele was not significant (37). Another study found significance only for depression and ε3/ε4 or ε4/ε4 genotypes in the development of MCI (hazard ratio=5.1) (38).
Researchers have used imaging studies to further explore the relationship between depression and MCI. A synergistic effect of depressive symptoms and smaller left hippocampal volume among MCI patients has been found to accelerate conversion to dementia (39). In the Alzheimer’s Disease Neuroimaging Initiative study, those with persistent depressive symptoms had higher conversion to AD, compared with those who remained without neuropsychiatric symptoms. Patients with MCI who had comorbid depressive symptoms showed more frontal, parietal, and temporal white matter atrophy than asymptomatic patients (40).
Vascular Depression and Dementia
The vascular depression hypothesis postulates that cerebrovascular disease predisposes, precipitates, or perpetuates a depressive syndrome among some older adult patients (4). The hypothesis is supported by the known comorbidity of depression, vascular disease, vascular risk factors, and ischemic lesions with distinctive behavioral symptoms. Researchers have postulated that the central mechanisms are disruption of prefrontal systems and their limbic and hippocampal connections or their modulating pathways by single lesions or by an accumulation of lesions exceeding a threshold (39).
Patients with vascular depression have characteristic features. With respect to clinical symptoms, patients have significant psychomotor retardation, little psychomotor agitation, less guilt, poorer insight, and limited depressive symptoms, compared with healthy others (41). Individuals have tended to be older, have higher levels of hypertension and other cerebrovascular risk factors, and have less family history of depression (42, 43). They also have greater overall cognitive impairment and disability than those with nonvascular depression. Cognitively, fluency and naming are impaired (4, 41). However, the core cognitive deficits in late-life depression seem to be slowed processing speed, followed by executive dysfunction. Changes in processing speed most fully mediate the effect of age, depression severity, education, race, and vascular risk factors on other cognitive domains, including episodic memory and visuospatial performance. These declines persist even after remission of depression (44, 45).
Supporting the vascular depression hypothesis, a large proportion of elders with depression have had either a stroke or other evidence of cerebrovascular compromise (41, 46, 47). Pooled data from studies have found poststroke prevalence rates for major depression of 19.3% among hospitalized patients and 23.3% among outpatients (47). These numbers are higher than the prevalence of major depressive disorder in the general population (8.3%) (48).
High rates of cerebrovascular disease, along with white matter hyperintensities (WMHs) on MRIs, have also been found among depressed elders (49). WMHs are thought to be caused by small, silent cerebral infarctions. They are characterized by arteriosclerosis, perivascular demyelination, dilated periventricular spaces, and ischemia (50, 51). They can predispose individuals to depression (49, 52) by disrupting the fiber tracts connecting cortical and subcortical structures (52) in areas such as the dorsolateral prefrontal cortex and the anterior cingulate cortex (50). In one study involving patients with late-life depression, silent cerebral infarctions were observed among 65.9% of those with early-onset depression and among 93.7% of those with late-life depression (53). A systematic review (54) confirmed this finding. The authors found that late-life depression was characterized by more frequent and intense white matter abnormalities than early-onset depression. The odds of having white matter changes were over 4 for late-onset illness, compared with early-onset illness. These differences suggest different etiological mechanisms for late-life depression, in keeping with the vascular depression hypothesis (54). Last, considering structural brain changes, Kramer-Ginsberg et al. (55) found that among elders with depression, hyperintensity location/severity and the presence or absence of depression had a significant interaction with cognitive performance. Depressed patients with moderate-to-severe deep WMH had worse performance on general and delayed recall memory, executive function, and language testing than depressed patients without WMH and normal elders with or without deep white matter changes. (54).
Regarding vascular depression and vascular dementia, the similarity of risk factors and findings for the two conditions implies a connection. Both conditions encompass cerebrovascular compromise through vascular risk factors, ischemic lesions, and the detection of WMHs, among other potential findings. However, the path from vascular disease to vascular depression to vascular dementia has not been elucidated. Furthermore, the road may be reciprocal and not direct or sequential, given that depression can also increase the risk of vascular disease and vice versa (4). Unfortunately, research linking vascular depression to dementia, including vascular dementia, is limited (27, 56). However, it is likely that a connection exists between these conditions and that structural brain changes may be a distinctive feature of late-life depression linking it to cognitive impairment.
Depression in Dementia
Symptoms of depression are often present in dementia and may constitute one of the many behavioral and psychological symptoms of dementia. A considerable minority of AD patients, during the course of their illness, exhibit depressed mood (40%−50%), whereas depressive disorders are encountered in 10%−20% of patients (57). Depression may occur more commonly as a symptom in VaD than in AD (58). It is also common among patients with other forms of dementia, such as dementia with Lewy bodies, Parkinson’s disease, and Huntington’s disease (1). The high prevalence of depressive symptoms in dementia seems to indicate an ongoing association between these conditions.
Depression and Cerebral Disruption
Depression is associated with disruption in a number of brain regions. Some described areas include the hippocampus, prefrontal systems and their limbic-hippocampal connections, the orbitofrontal cortex (OFC), the dorsolateral prefrontal cortex (DLPFC), the anterior cingulate gyrus, and the amygdala (1, 59).
The hippocampus plays a critical role in learning, recall, and affect regulation (1). Depression serves as a neurobiological stressor. Overall, it leads to excess circulating glucocorticoids, which result in hippocampal neurotoxicity and volume loss (60). Decreased hippocampal volume may result from many factors. First, it has been demonstrated in animal studies that stress induces decreased cell proliferation in the hippocampal region, which leads to reduced neurogenesis (61). Second, the glucocorticoid hypothesis (62) postulates that a prolonged physical reaction to stress elicits sustained glucocorticoid secretion. There are glucocorticoid receptors in the hippocampus. Over time, glucocorticoid hypersecretion leads to excessive activation of these receptors. This overstimulation is eventually toxic and leads to neuronal cell death and atrophy. Such damage has been recorded in imaging studies (63), has been associated with cognitive decline and dementia (64, 65), and has been demonstrated in clinical studies (5, 66).
Sheline et al. (66) found that females, ages 23–86, with a history of depression (postdepression) had smaller hippocampal volumes bilaterally than did case-matched others without depression but with similar age and education. They scored lower on verbal memory, a neuropsychological measure of hippocampal function, which suggested that the volume loss was related to cognitive functioning. There was also a significant correlation with hippocampal volume loss and total lifetime duration of depression. This suggested a dose-response relationship between depression and cognitive impairment, which indicated that repeated stress from recurrent depressive episodes may result in cumulative hippocampal injury, reflected in volume loss (66).
A later prospective study of elders found that over two years, the depressed group showed a greater reduction in left hippocampal volume than the nondepressed group. The study found that hippocampal change from baseline to two years was associated with subsequent change in Mini–Mental State Examination scores from two years to two and a half years in the depressed group. Thus, depressed elders with hippocampal volume loss were at greater risk of cognitive decline (5). Age of depression onset seems to correlate negatively with hippocampal volume, so that patients with late-life depression have smaller hippocampal volumes compared with controls and those with early-onset depression (59, 63). However, others have found different results and have connected reductions in hippocampal volume with earlier onset depression (66) and longer duration of untreated depression (67).
Last, with respect to other brain areas involving depression and cognition, the OFC seems to have a role in selectively processing affective stimuli. This makes depressed individuals predisposed to cognitive errors if they have received negative feedback on preceding tasks (68). Increased lesion density has been found in the OFC among patients with late-life depression (69). The anterior cingulate gyrus seems to be involved in initiation and suppression of behavior through conflict monitoring and appraisal of motivational content (1). It is also involved in spatial planning and working memory. Individuals with depression have demonstrated lower activation of this region on the Tower of London task, which is a test of planning (70). The DLPFC is involved in working memory, retrieval of episodic memory, planning, and response monitoring (1). Late-life depression has also been associated with microstructural changes in the white matter of the DLPFC (71).
Conclusion
Summary of the Literature on Cognitive Deficits With Late-Life Depression
Cognitive deficits are a core feature of depression among both adults and elders, which makes depression a cognitive disorder (1). Deficits are consistently found in the domains of memory, verbal learning, motor speed, executive function, and processing speed (2). Depression may be associated with cognitive impairment through a variety of mechanisms, including hippocampal volume loss from excess secretion of glucocorticoids, resulting in neurotoxicity; inhibition of hippocampal neurogenesis (61, 62); cerebrovascular compromise, including disruption of prefrontal systems and their limbic-hippocampal connections (4); increased deposition of β-amyloid plaques; chronic inflammation; and deficits of nerve growth factors, such as brain-derived neurotrophic factor (6). Studies of late-life depression have demonstrated a reduction in hippocampal volume (63, 72), and this has been linked to impaired cognition (5, 66). Late-life depression and total lifetime duration of depression may have the greatest overall effects on volume loss (59, 66). Depression could also be an important marker for increased risk of conversion from depression to dementia among patients with MCI and especially aMCI. A partial model depicting some of these relationships is presented in Figure 1.
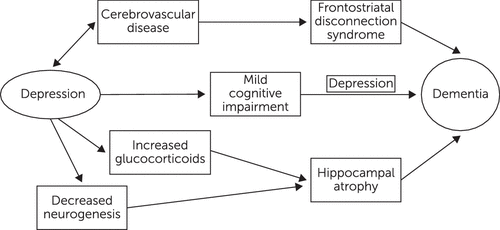
FIGURE 1. Some Pathways Linking Depression With Dementia
It also seems that depression is a both a risk factor and a prodrome for dementia (15, 18–20, 25, 26). The quantity of data is greater for early-onset depression’s serving as a risk factor for dementia (17, 30) than for late-life depression’s serving as a prodromal symptom of dementia (20, 21). However, the strength of the linkage decreases with increasing time between depression onset and dementia diagnosis (1, 19, 20). This suggests that older age at depression onset may be more important as a prodromal symptom or a risk factor for dementia than earlier onset illness (1). There also seems to be a dose-response relationship between depression and dementia, such that each depressive episode is associated with an increased rate of dementia (23, 24). Future studies will probably continue to suggest one relationship, both relationships, or neither. This is likely a reflection of the heterogeneous presentations and diverse pathologies of depression and dementia. Perhaps the more vital issues are identifying factors that cause depression to become a risk factor for dementia and determining which factors cause depression to develop into prodromal dementia (1).
Clinical Relevance
The evidence that late-life depression may represent a prodrome for dementia and that midlife depression may be a risk factor for dementia should be concerning for clinicians. Increased vigilance in screening for signs and symptoms of depression and cognitive impairment is imperative, especially among elders (60). Researchers have estimated that 10% of dementia cases could be attributed to depression. A 10% reduction in depression prevalence, through aggressive screening and management, could result in more than 325,000 fewer AD cases worldwide and 67,000 fewer cases in the United States (73).
Although strategies for identifying depression and cognitive impairment are beyond the scope of this review, common methods include obtaining a patient history and a functional assessment; collecting collateral information; excluding reversible causes of dementia; using standardized assessment measures, such as the Montreal Cognitive Assessment (74) and the Geriatric Depression Scale (75); considering neuropsychological testing; and ordering neuroimaging, such as a brain MRI or head CT. Future tools that are not currently the standard of care may include fluorodeoxyglucose–PET imaging (76), PiB-PET imaging for amyloid deposition (77), and APOEε4 allele screening (78), among many others. Biomarkers being investigated comprise soluble Abeta, tau, synuclein, and others for brain inflammation (77). Clinicians caring for older adults with depression should also emphasize vascular risk factor reduction: “A healthy brain starts with a healthy heart.” As a consequence, they could collaborate with primary care providers and support patient adherence with medications, dietary and lifestyle modifications, follow-up visits, and other recommendations.
1 : Geriatric depression and cognitive impairment. Psychol Med 2008; 38:163–175Crossref, Google Scholar
2 : A comparison of neurocognitive impairment in younger and older adults with major depression. Psychol Med 2009; 39:725–733Crossref, Google Scholar
3 : The pattern and course of cognitive impairment in late-life depression. Psychol Med 2010; 40:591–602Crossref, Google Scholar
4 : Vascular disease, depression, and dementia. J Am Geriatr Soc 2003; 51:1178–1180Crossref, Google Scholar
5 : Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry 2011; 19:4–12Crossref, Google Scholar
6 : Depression and risk of developing dementia. Nat Rev Neurol 2011; 7:323–331Crossref, Google Scholar
7 : History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry 2001; 35:776–781Crossref, Google Scholar
8 : Age differences in symptom expression in patients with major depression. Int J Geriatr Psychiatry 2012; 27:601–611Crossref, Google Scholar
9 : Is dysthymia a different disorder in the elderly? Am J Psychiatry 1994; 151:1592–1599Crossref, Google Scholar
10 : Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003; 58:249–265Crossref, Google Scholar
11 : Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med 2013; 43:2017–2026Crossref, Google Scholar
12 : Differential diagnoses and assessment of depression in elderly patients. J Clin Psychiatry 2009; 70:e47Crossref, Google Scholar
13 : Residual cognitive impairment in late-life depression after a 12-month period follow-up. Int J Geriatr Psychiatry 2003; 18:571–576Crossref, Google Scholar
14 : Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013; 202:329–335Crossref, Google Scholar
15 : Affective disorders and risk of developing dementia: systematic review. Br J Psychiatry 2013; 202:177–186Crossref, Google Scholar
16 : The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry 1993; 150:1693–1699Crossref, Google Scholar
17 : Depression and the risk for dementia. Curr Opin Psychiatry 2012; 25:457–461Crossref, Google Scholar
18 : Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 2006; 63:530–538Crossref, Google Scholar
19 : Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol 2003; 60:753–759Crossref, Google Scholar
20 : Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012; 69:493–498Crossref, Google Scholar
21 : Depression as a risk factor for cognitive impairment in later life: the Health In Men cohort study. Int J Geriatr Psychiatry 2016; 31:412–420Crossref, Google Scholar
22 : 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry 2016; 3:628–635Crossref, Google Scholar
23 : Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? J Neurol Neurosurg Psychiatry 2004; 75:1662–1666Crossref, Google Scholar
24 : Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 2010; 75:27–34Crossref, Google Scholar
25 : Depression history, depressive symptoms, and incident dementia: the 3C Study. J Alzheimers Dis 2011; 26:27–38Crossref, Google Scholar
26 : Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses. J Affect Disord 2003; 73:261–269Crossref, Google Scholar
27 ;
28 : Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch Gen Psychiatry 2006; 63:153–160Crossref, Google Scholar
29 : Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ 2004; 329:881–882 Crossref, Google Scholar
30 :
31 : Prevalence of major and minor depression in elderly persons with mild cognitive impairment--MADRS factor analysis. Int J Geriatr Psychiatry 2004; 19:1168–1172Crossref, Google Scholar
32 : Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol 2004; 61:1290–1293Crossref, Google Scholar
33 : The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry 2007; 22:563–567Crossref, Google Scholar
34 : Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry 2006; 63:273–279Crossref, Google Scholar
35 : Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: systematic review and meta-analysis. Int J Geriatr Psychiatry 2016; 31:905–911Crossref, Google Scholar
36 : Impact of Depressive Symptoms on Conversion from Mild Cognitive Impairment Subtypes to Alzheimer’s Disease: A Community-Based Longitudinal Study. J Alzheimers Dis 2016; 51:405–415Crossref, Google Scholar
37 : Amnestic mild cognitive impairment and incident dementia and Alzheimer’s disease in geriatric depression. Int Psychogeriatr 2014; 26:2029–2036Crossref, Google Scholar
38 : Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol 2006; 63:435–440Crossref, Google Scholar
39 : Depressive Symptoms and Small Hippocampal Volume Accelerate the Progression to Dementia from Mild Cognitive Impairment. J Alzheimers Dis 2015; 49:743–754Crossref, Google Scholar
40 : Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease–related regions. Biol Psychiatry 2012; 71:814–821Crossref, Google Scholar
41 : Clinically defined vascular depression. Am J Psychiatry 1997; 154:562–565Crossref, Google Scholar
42 : Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry 2004; 55:390–397Crossref, Google Scholar
43 : Psychiatric disease in the twenty-first century: The case for subcortical ischemic depression. Biol Psychiatry 2006; 60:1299–1303Crossref, Google Scholar
44 : Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006; 60:58–65Crossref, Google Scholar
45 : Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med 2000; 30:679–691Crossref, Google Scholar
46 : Mood disorder as a specific complication of stroke. J Neurol Neurosurg Psychiatry 1977; 40:1018–1020Crossref, Google Scholar
47 : Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry 2003; 54:376–387Crossref, Google Scholar
48 : Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R). Psychol Med 2010; 40:225–237Crossref, Google Scholar
49 : MRI-defined vascular depression: a review of the construct. Int J Geriatr Psychiatry 2011; 26:1101–1108Google Scholar
50 : Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry 2002; 59:785–792Crossref, Google Scholar
51 : Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993; 43:1683–1689Crossref, Google Scholar
52 : ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997; 54:915–922Crossref, Google Scholar
53 : Incidence of silent cerebral infarction in patients with major depression. Stroke 1993; 24:1631–1634Crossref, Google Scholar
54 : White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry 2008; 79:619–624Crossref, Google Scholar
55 : Neuropsychological functioning and MRI signal hyperintensities in geriatric depression. Am J Psychiatry 1999;156:438–444Google Scholar
56 : Progression of subcortical ischemic disease from vascular depression to vascular dementia. Am J Psychiatry 2003; 160:1751–1756Crossref, Google Scholar
57 : Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 1989; 146:577–587Crossref, Google Scholar
58 : The prevalence of depression in Alzheimer’s disease and vascular dementia in a population sample. J Affect Disord 1999; 52:169–176Crossref, Google Scholar
59 : Hippocampal volume change in depression: late- and early-onset illness compared. Br J Psychiatry 2004; 184:488–495Crossref, Google Scholar
60 : J’accuse! depression as a likely culprit in cases of AD. Int Psychogeriatr 2016; 28:1407–1408Crossref, Google Scholar
61 : Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 1998; 95:3168–3171Crossref, Google Scholar
62 : The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Sci SAGE KE 2002; 2002:284–301Google Scholar
63 : Hippocampal volume in geriatric depression. Biol Psychiatry 2000; 48:301–309Crossref, Google Scholar
64 : Quantitative NMR measurements of hippocampal atrophy in Alzheimer’s disease. Magn Reson Med 1988; 8:200–208Crossref, Google Scholar
65 : A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry 2004; 161:2081–2090Crossref, Google Scholar
66 : Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999; 19:5034–5043Crossref, Google Scholar
67 : Untreated depression and hippocampal volume loss. Am J Psychiatry 2003; 160:1516–1518Crossref, Google Scholar
68 : Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med 2003; 33:455–467Crossref, Google Scholar
69 : Medial orbital frontal lesions in late-onset depression. Biol Psychiatry 2001; 49:803–806Crossref, Google Scholar
70 : Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med 1997; 27:931–942Crossref, Google Scholar
71 : Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry 2004; 161:1293–1296Crossref, Google Scholar
72 : Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 2009; 30:3719–3735Crossref, Google Scholar
73 : The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011; 10:819–828Crossref, Google Scholar
74 : The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry 2007; 52:329–332Crossref, Google Scholar
75 : Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982-1983; 17:37–49Crossref, Google Scholar
76 : FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 2007; 130:2616–2635Crossref, Google Scholar
77 : PET imaging of brain amyloid in dementia: a review. Int J Geriatr Psychiatry 2011; 26:991–999Crossref, Google Scholar
78 : Neuropsychiatric symptoms, APOE ε4, and the risk of incident dementia: a population-based study. Neurology 2015; 84:935–943Crossref, Google Scholar



