Detection, Assessment, and Management of Schizophrenia in an Andean Population of South America: Parkinsonism Testing and Transcranial Ultrasound as Preventive Tools
Abstract
Schizophrenia is a debilitating psychiatric illness that is among the world's top 10 causes of long-term disability, affecting people who are just entering the peak of social, economic, and intellectual productivity. Such functional loss is particularly relevant in indigenous communities, which rely on change in functional status (rather than on the presence of symptoms) to identify mental illness. Particularly among the indigenous communities of Latin America, the gap between mental health need and availability of resources to reduce the burden has been judged “a case of outrageous exclusion.” For more than a decade, as part of the Investigation of Movement Abnormalities and Genetic of Schizophrenia study, the authors have been studying vulnerability markers (genetic, motor, imaging, and neuropsychological differences) for schizophrenia in a remote, indigenous population in rural northern Argentina. In this article, the authors discuss the implementation of a task-shifting paradigm resulting in more proficient identification and referral of individuals with untreated psychosis and a severalfold reduction in the duration of untreated psychosis, with very high retention rates (70%) and treatment adherence during a decade in a rural environment. The authors also propose to use transcranial ultrasound screening and testing for parkinsonism at illness onset before introduction of neuroleptics as potentially useful markers in determining illness severity, negative symptomatology, and tolerance to antipsychotic treatment/refractoriness.
Schizophrenia is a debilitating psychiatric illness that is among the world's top 10 causes of long-term disability. The symptoms of schizophrenia include psychosis, apathy and withdrawal, and cognitive impairment, which lead to problems in social and occupational functioning and self-care. Approximately 0.7% of the population is affected by schizophrenia, with similar rates across different countries and cultural groups (1, 2). Mental health care in indigenous populations in Latin America is markedly constrained by the scarcity of mental health resources, as well as by the gap in knowledge about their cultural health practices and the forms of expression of mental symptoms in their cultural context (3–5). Schizophrenia affects people who are just entering the peak of social, economic, and intellectual productivity. People affected by schizophrenia withdraw; fail to establish meaningful relationships with peers, relatives, or life partners; and fail to reach the expected level of social function. Such functional loss is particularly relevant in indigenous communities, which rely on change in functional status (rather than on the presence of symptoms) to identify mental illness (6). Particularly among the indigenous communities of Latin America, the gap between mental health need and availability of resources to reduce the burden has been judged “a case of outrageous exclusion” (3). The World Health Organization (WHO) proposed an action plan to scale up services giving priority to schizophrenia along with other burdensome disorders (7); the plan included a set of interventions including “task-shifting” strategies as a rational redistribution of tasks among health workforce teams (7).
For more than a decade, as part of the Investigation of Movement Abnormalities and Genetic of Schizophrenia (IMAGES) study, our group has been studying vulnerability markers (genetic, motor, imaging, and neuropsychological differences) for schizophrenia in a remote, indigenous population in rural northern Argentina (8–10). At the center of the IMAGES strategy was the implementation of a task-shifting paradigm in which recognition and engagement of “index” cases were accomplished by specifically providing training for primary care health workers, leading to effective interventions coordinated at local primary care delivery systems (9). The most striking success of this strategy was that because ongoing training of health agents resulted in more proficient identification and referral of individuals with untreated psychosis, a severalfold reduction in the duration of untreated psychosis was observed across the entire province (9). A longer duration of untreated psychosis is a well-established predictor of poorer outcomes (11, 12) and may result in reduced cortical thickness (13) and cognitive impairment (14), although the latter was disputed recently (15, 16). A recent meta-analysis confirmed that a longer duration of untreated psychosis predicts poor general symptomatic outcomes, more severe positive and negative symptoms, lesser likelihood of remission, and poor social functioning and global outcomes (17). Thus, reducing the duration of untreated psychosis severalfold, as our study shows is possible through a task-shifting strategy, has the potential to greatly affect the burden of disease in lower- to middle-income communities and improve the trajectory of psychosis.
Identification of Proxy Markers of Risk for Schizophrenia in a Primary Care Environment
Because of geographical isolation and a lack of distributed mental health services, people in the Andean region are unlikely to come into contact with mental health specialists during much of the course of their illness. Therefore, we reasoned that in addition to posing a public health deficit, their situation offered a unique scientific opportunity to study disease processes without interference from the effects of medications at the time of detection. One of the main findings from this investigational effort is that neuroleptic-naive patients with schizophrenia have increased hyperechogenicity of the substantia nigra (a marker for parkinsonism) and an associated increase in motor symptoms of parkinsonism (10). Furthermore, the same abnormalities, albeit to a less severe degree, were found in their unaffected first-degree relatives (10). Although it has been well established that antipsychotic drugs can lead to parkinsonism (18, 19), it is worth pointing out that the presence of parkinsonism in untreated schizophrenia is not a novel finding. Kraepelin (20) described identical movement abnormalities in patients with schizophrenia more than a century before the introduction of neuroleptics, and parkinsonism has been found repeatedly in diverse populations of neuroleptic-naive patients with schizophrenia, including first-episode (21–25) and chronically ill patients (10, 26). In high-risk populations, delayed motor development and impaired motor skills have been reported in individuals who went on to have schizophrenia (27–29), and an increased rate of parkinsonism in first-degree relatives of patients with schizophrenia has also been reported (10). These studies suggest that motor abnormalities may be an integral part of the pathogenic process of schizophrenia and may serve as indicators of vulnerability to the disorder.
However, neuromotor precursors are subtle and may lack sufficient specificity to be considered (at least by themselves) as proxy markers for primary prevention (30), which is unquestionably the goal to be strived for in resource-poor environments where treatment options may be severely limited and where the burden of disease is greatest. Neuroimaging techniques offer great promise to identify proxy markers of risk (31), but their cost and complexity generally rule out their use as screening instruments at the population level, particularly in rural environments such as the Andes. On the other hand, transcranial ultrasound screening is performed with a noninvasive, affordable, portable device with minimal support requirements and broad availability, which can be used in primary care rural environments. Given the well-established findings using this technique in the midbrain parenchyma of patients with idiopathic Parkinson’s disease, in which increased echogenicity of the substantia nigra is a marker of disease (32) and of disease risk (33), we reasoned that its use could lend itself as a biomarker of parkinsonian motor impairment in patients with schizophrenia and as a risk marker in their unaffected relatives. Indeed, hyperechogenicity of the substantia nigra correlates directly with the severity of antipsychotic-induced extrapyramidal side effects in patients with chronic schizophrenia (34, 35).The cause of hyperechogenicity of the substantia nigra has not been established, but it is associated with iron accumulation related to neuronal damage (36) and with reduced [18F]-dopa uptake in the striatum in patients with premotor Parkinson’s disease (37). Thus, increased echogenicity of the substantia nigra may be associated with an impairment of the dopaminergic nigrostriatal system. Notably, we recently reported that in our isolated, indigenous population of Argentina, hyperechogenicity was present and predicted parkinsonian motor impairment in patients with schizophrenia who were never exposed to neuroleptics and in their unaffected first-degree relatives (10), indicating that midbrain abnormalities along with movement abnormalities are inherent parts of the underlying pathophysiology and may serve as markers of risk.
Management of Chronic Schizophrenia in a Primary Care Environment
The IMAGES study is part of a larger multidisciplinary collaborative research effort designed to examine the complex etiology, assessment, and treatment outcomes of psychiatric and behavioral disorders, which contribute significantly to the burden of disease in Latin America. The original sample of Andean people with schizophrenia who were never exposed to neuroleptics was identified with a task-shifting strategy. Patients were successfully located and followed at various time points in their clinical course. Upon completion of the initial evaluation, patients were engaged by local mental health services, which are based in the regional neuropsychiatric hospital in the provincial capital, to initiate treatment free of charge under the provincial universal coverage system. Afterward, health agents in the patients’ local towns (9) were charged with ongoing monitoring of symptom severity, adhesion to pharmacotherapy, delivery of the medications to the patients or to their immediate families, and referral to the regional hospital-based psychiatrist when relapses occurred. Below we report our experience with a prospective follow-up of “index” patients to investigate treatment adherence, treatment response, predictors of therapeutic response, and levels of functioning in the Andean region of the province of Jujuy in Argentina. We hypothesized that substantia nigra hyperechogenicity and parkinsonian symptoms—measured before medication initiation—would negatively predict response to neuroleptics and would be markers of adverse functional outcome. In turn, these predictors may help to guide clinical treatment and management.
Methods
Study Population and Design
As part of the IMAGES study, health care agents in the province were instructed to notify the provincial epidemiology system upon detection of cases of untreated psychosis. From 2004 to 2015, a total of 62 patients with chronic untreated schizophrenia were evaluated and engaged in care by the public health system. Assessment consisted of semistructured interviews with the WHO Schedules of Clinical Assessment in Neuropsychiatry (SCAN); if criteria for a diagnosis of schizophrenia were met, motor assessment was obtained with the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-3) and a transcranial ultrasound. We only included participants who were aged 18–65 years, who had no comorbid neurological or psychiatric disorders or substance abuse disorders, and who were able to provide informed consent. All other participants who came into contact with the mental health specialists through health agent referrals were also engaged in treatment free of charge regardless of diagnosis or age, but they were not followed-up prospectively. Once engaged in care, participants were treated by nonstudy community providers using standard-of-care criteria. After varying lengths of treatment ranging from 3 to 11 years, all participants were invited to participate in the current follow-up study. We re-evaluated 43 participants (26 men and 17 women) using the Positive and Negative Syndrome Scale (PANSS), the Clinical Global Impressions Scale (CGI), and the UDPRS-3. In addition, we carried out chart reviews to evaluate medication history and to calculate dose-years (a measure of cumulative neuroleptic exposure). Three patients refused to participate, three patients were deceased, and the rest were unreachable. Thus, 70% of original patients were re-evaluated in the follow-up portion of the study. All protocols used in this study were approved by the Ethics Committee of the Province of Jujuy and by the Morsani College of Medicine Institutional Review Board at the University of South Florida.
Transcranial Ultrasound Screening
Protocols for a transcranial ultrasound examination were published previously (10). Briefly, we utilized a color-coded, phased-array ultrasound system, equipped with a 2.5-MHz transducer (Micromaxx; Sonosite Inc., Bothell, WA). Examinations were performed through a preauricular acoustic bone window (penetration depth=16 cm, dynamic range=45 dB) by an expert sonographer with more than 20 years of experience with the technique who was blinded to the patient’s condition. Less than 3% of cases were not suitable for transcranial ultrasound screening because of skull thickness (a much lower percentage of that reported in the Parkinson’s disease population, most likely because our patients are much younger). The substantia nigra was identified within the butterfly-shaped structure of the brainstem, scanning from each temporal bone window. Because the signal brightness (echogenicity) is not quantifiable by an ultrasound, the area of hyperechogenic signals in the substantia nigra region was measured (in centimeters squared) from each ipsilateral side separately, and unbiased quantification of the echogenic area was carried out post hoc on saved images by two different evaluators blinded to the patient’s condition.
Clinical Assessments
Diagnostic ascertainment was carried out with the SCAN 2.1 (WHO, Geneva) scored blindly by two independent raters at the initiation of the study. The SCAN is a set of instruments and manuals aimed at assessing, measuring, and classifying psychopathology and behavior associated with major psychiatric disorders in adult life. It can be used for clinical, research, and training purposes and was developed within the framework of WHO. The SCAN has a bottom-up approach in which no diagnosis-driven frames are applied in grouping the symptoms. Each symptom is assessed in its own right. It has a proven stability and robustness to differentially assess psychotic states. Interview data are entered into the program and are fed into algorithms for ICD-10 and DSM-IV diagnoses. These algorithms produce a diagnostic classification for both systems. To meet selection criteria, all participants met the criteria for DSM-IV schizophrenia when scored by two independent raters.
Motor assessment of parkinsonism in the initial phase of the study was scored blindly using the UPDRS-3 (38, 39) on videotaped examinations by two independent raters certified in its use. Thus, the diagnosis of parkinsonism was not based on the UK Brain Bank criteria but on an arbitrary cutoff point of the UPDRS-3. Parkinsonism at the time of the follow-up phase of the study was also scored blindly using the UPDRS-3 by raters certified in its use.
Clinical assessments used to measure psychopathology were the PANSS for schizophrenia (40) and the CGI severity scale (41). The PANSS assesses 30 different symptoms on a scale from 1 to 7 based on a clinical interview. For this study, total scores as well as positive and negative subscores were examined. The CGI severity scale rates the severity of the patient’s mental illness from 1 (normal, not at all ill) to 7 (extremely ill).
To measure cumulative neuroleptic exposure, we computed the dose-years (42), after conversion of all antipsychotic agents to chlorpromazine equivalents using published tables for conventional and atypical antipsychotic agents (43, 44). A dose-year is defined as the product of the chlorpromazine equivalents and the time on each dose expressed in years (42).
Statistical Analyses
Independent-measures t tests were used to measure the effect of gender on baseline characteristics (age, length of treatment, percentage treated with clozapine) as well as experimental measures (UPDRS-3, CGI, PANSS, right and left substantia nigra ultrasound, and dose-years). The independent-measures t test was also used to measure differences in clinical and motor outcomes in patients treated with clozapine versus those treated with other antipsychotic agents. To determine predictors of outcome (baseline transcranial ultrasound measurements, baseline parkinsonism, and age), multiple backward correlation analyses were calculated for follow-up measurements (PANSS, CGI, dose-years, and postmedication UPDRS-3 score), each as dependent variables. All analyses were performed with the IBM SPSS statistical package, version 23.
Results
Sample Population
The general characteristics of the sample are described in Table 1. There were 27 men and 16 women. Both men and women were similar in age at the follow-up assessment and at the initial evaluation, as well as in terms of length of treatment. A similar proportion of men (45%) and women (38%) were treated with clozapine. Other antipsychotic agents used were risperidone, olanzapine, aripiprazole, chlorpromazine, haloperidol, levomepromazine, and zuclopenthixol. Patients had a mean total educational score of 1.9±0.7 years using the following scale: 1, completed elementary school; 2, completed high school; and 3, additional education at trade school or college level. There was not a significant effect of gender on substantia nigra hyperechogenicity, UPDRS-3 scores, PANSS scores, dose-years, or CGI scores (not shown).
| Characteristic | Men (N=27) | Range | Women (N=16) | Range | p |
|---|---|---|---|---|---|
| Age (years) | 34±8.4 | 24–27 | 40±15.2 | 21–68 | 0.166 |
| Age of treatment initiation (years) | 25±8.4 | 17–50 | 32±13.1 | 16–58 | 0.130 |
| Length of treatment (years) | 8.4±2.1 | 4–12 | 8.7±3.2 | 3–15 | 0.822 |
| Percentage taking clozapine | 45 | 38 | 0.612 |
Table 1. Demographic Characteristics of the Study Populationa
Effect of Clozapine on Clinical and Motor Outcomes
Treatment was based on standard of care in the province, without reference to specific guidelines. As a rule, patients were initially prescribed an atypical antipsychotic (risperidone and aripiprazole were the two most common). If clinical improvement was judged insufficient (maximum tolerated dose for at least 12 weeks) or if intolerable side effects developed, a second antipsychotic trial was carried out, and if this failed, clozapine was started. Thus, treatment with clozapine can be taken as a measure of treatment refractoriness. Independent-samples t tests were conducted to compare baseline and posttreatment clinical and motor outcomes in patients treated with clozapine and those treated with other antipsychotic medications (no clozapine) (Figure 1). Patients who were treated with clozapine were younger (mean age=31.48±8.47 years) at the time of follow-up than those treated with other antipsychotic drugs (mean age=39.1±12.5 years, t=−0.22, df=1, p=0.035). Those treated with clozapine were also younger (mean age=23.1±7.0 years) at the time of treatment initiation (a proxy to the age of illness onset) than those treated with other antipsychotics (mean age =30.2±11.4 years, t=−2.26, df=1, p=0.03). There was no significant difference in the length of treatment with clozapine (mean=8.3±2.7 years) versus other antipsychotics (mean=8.9±2.3 years, t=−0.73, df=1, p=0.47).
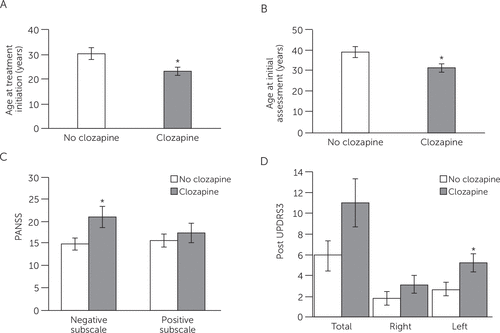
Figure 1. Clinical and Motor Measurements in Patients Treated With Clozapine or With Other Antipsychotic Agentsa
a Top: average age (±SEM) of patients during the study and at treatment initiation. Patients treated with clozapine were significantly younger when treatment was initiated and at the time of study than those who were treated with other antipsychotic agents. C: PANSS-negative scores, but not PANSS-positive scores, were significantly higher among patients treated with clozapine than among those treated with other antipsychotic agents. D: motor impairment on the left after treatment initiation was significantly worse among participants treated with clozapine than among those treated with other antipsychotic agents. PANSS, Positive and Negative Syndrome Scale. Possible scores range from 30 to 210, with higher scores indicating more symptoms. UPDRS3, Unified Parkinson’s Disease Rating Scale Part III. Possible scores range from 0 to 108, with higher scores indicating more severe parkinsonism.
Independent-samples t tests were conducted to compare pretreatment measures including substantia nigra hyperechogenicity and parkinsonism (UPDRS-3) in patients treated with clozapine versus those treated with other antipsychotics. There were no significant differences in right hyperechogenicity (mean=0.28±0.21 versus mean=0.24±0.22, t=1.10, df=1, p=0.29) or left hyperechogenicity (mean=0.27±0.21 versus mean=0.19±0.19, t=1.07, df=1, p=0.30) in patients treated with clozapine compared with those treated with other antipsychotic drugs. Likewise, there were no significant differences in pre–UPDRS-3 total scores (t=0.420, df=1, p=0.68), right scores (t=0.36, df=1, p=0.67), or left scores (t=0.57, df=1, p=0.57) in clozapine-treated patients versus patients treated with other medications.
Likewise, when posttreatment outcomes including CGI scores, PANSS scores, and parkinsonism (UPDRS-3) were compared in those patients treated with clozapine versus patients treated with other antipsychotic agents (Figure 1), we found no significant differences in CGI scores (mean for clozapine=4.47±1.70 versus mean for nonclozapine=3.65±1.55, t=1.58, df=1, p=0.12) or PANSS-positive scores. On the other hand, PANSS-negative scores were significantly higher in those treated with clozapine (mean=21.12±9.70 versus mean=15.0±6.53 for nonclozapine, t=2.40, df=1, p=0.02). Similarly, posttreatment UPDRS-3 total and right-sided scores were similar in both groups, but patients treated with clozapine had significantly higher UPDRS-3 scores on the left side (mean=5.27±3.28 versus mean=2.71±3.22 in nonclozapine-treated patients, t=2.37, df=1, p=0.03).
Predictors of Clinical and Motor Outcomes at Follow-Up
Variables assessed before onset of medication treatment (age, substantia nigra hyperechogenicity, and UPDRS-3 scores) were entered into a multiple linear regression analysis as possible predictors of clinical and motor measures obtained at follow-up (PANSS, CGI, and UPDRS-3 scores, and dose-years) (Table 2). Pretreatment left substantia nigra hyperechogenicity significantly predicted the following outcomes at follow-up: PANSS-negative scores (β weight=15.7, t=2.265, df=1, p=0.031), UPDRS-3 total score (β weight=18.6, t=3.138, df=1, p<0.01), UPDRS-3 left score (β weight=9.6, t=3.95, df=1, p<0.01), and UPDRS-3 right score (β weight=5.520, t=2.18, df=1, p=0.04). On the other hand, substantia nigra hyperechogenicity on the right was not a predictor for any measure at follow-up. The baseline right UPDRS-3 score was predictive of CGI (β weight=0.223, t=2.52, df=1, p=0.02), and there was a strong trend toward prediction for dose-years (β weight=49.79, t=1.93, df=1, p=0.06). Baseline left UPDRS-3 scores were not a predictor for any measure at follow-up. None of the clinical measures obtained before treatment were significant predictors of the PANSS-positive or PANSS total score. Age was also not a significant predictor of motor or clinical variables at follow-up.
| Outcome/Predictor | Coefficient | Standard Coefficient | SE | tb | p | R | R2 | Adjusted R2 | SEM |
|---|---|---|---|---|---|---|---|---|---|
| CGI score | |||||||||
| Pre–UPDRS-3 R | 0.223 | 0.088 | 0.403 | 2.524 | 0.017 | 0.515 | 0.265 | 0.214 | 1.463 |
| L SN | 2.421 | 1.295 | 0.298 | 1.869 | 0.072 | ||||
| PANSS-negative score | |||||||||
| L SN | 15.770 | 0.382 | 6.963 | 2.265 | 0.031 | 0.382 | 0.146 | 0.118 | 7.877 |
| Post–UPDRS-3 total score | |||||||||
| Pre–UPDRS-3 L | 0.766 | 0.272 | 0.430 | 1.779 | 0.086 | 0.620 | 0.385 | 0.342 | 6.384 |
| L SN | 18.598 | 0.480 | 5.927 | 3.138 | 0.004 | ||||
| Post–UPDRS-3 L | |||||||||
| L SN | 9.628 | 0.585 | 2.438 | 3.949 | <.001 | 0.585 | 0.342 | 0.320 | 2.758 |
| Post–UPDRS-3 R | |||||||||
| Pre–UPDRS-3 L | 0.323 | 0.291 | 0.184 | 1.757 | 0.089 | 0.529 | 0.279 | 0.230 | 2.725 |
| L SN | 5.520 | 0.361 | 2.531 | 2.181 | 0.037 | ||||
| Dose-years | |||||||||
| Pre–UPDRS-3 R | 49.789 | 0.367 | 25.795 | 1.930 | 0.065 | 0.367 | 0.134 | 0.098 | 384 |
Table 2. Linear Regression Analysis of Clinical and Motor Outcomesa
Discussion
This study is part of a larger multidisciplinary collaborative effort designed to tackle the burden of severe mental illness in Latin America. Participants were recruited, monitored, and followed-up through a task-shifting paradigm that relies on community health agents as screeners and ongoing evaluators, with psychiatrists and mental health specialists functioning only as supervisors and consultants in designing and providing training for the interventions. This approach resulted in a severalfold decrease in the duration of untreated psychosis (9) and in very high retention rates (70%) and treatment adherence over a decade. Indeed, although formalized outcome measurements were not carried out until now, the majority of patients have had regular home visits by health agents for medication management and periodic follow-up visits at the regional hospital for medication adjustments. Blood draws for safety monitoring of clozapine are also carried out at the local health posts by the health agents, and these tests are submitted to the regional hospital for analysis. Our retention rate of 70% through a decade (43 of 62 patients) is acceptable and consistent with published data in population-based studies with much shorter follow-up times in developed countries (45). With this collective effort involving health care workers and a primary psychiatrist, patients were successfully followed in the clinical setting, and treatment adherence, treatment response, predictors of therapeutic response, and levels of functioning were all obtained many years after initial recruitment into the study.
Several limitations must be pointed out, including the skewed gender distribution (i.e., twice as many male than female index cases), overall small sample size (N=43), as well as a varying amount of years in study participation (from 3 to 11 years). Another topic to consider is the possible influence of culture on symptom profiles, course, and outcome of schizophrenic disorders. Two international collaborative research projects undertaken by the Mental Health Division of WHO, namely the International Pilot Study of Schizophrenia and the study of the Determinants of Outcome of Severe Mental Disorders, surveyed patients and outcomes at 12 research sites in diverse sociocultural settings (Colombia, Czechoslovakia, Denmark, India, Ireland, Japan, Nigeria, Russia, the United Kingdom, and the United States) and revealed that syndromes of schizophrenia occur in all cultures and geographical areas investigated and that their rate of incidence is very similar in the different populations (quite a narrow range between 0.1 and 0.4 per 1,000 population) (46, 47). In WHO international studies of schizophrenia, patients in Western developed countries showed a higher frequency of depressive symptoms, primary delusions, thought insertion, and thought broadcasting than those in non-Western developing countries, who instead endorsed visual and directed auditory hallucinations more frequently (46, 47). Remarkably, however, although the content of delusions and hallucinations varies from culture to culture, the form of the illness remained very similar around the globe (47). We used the same standardized instrument as the two large-scale WHO studies: the present state examination (SCAN), which has shown strong reliability in this Andean population (9). The reliability of the SCAN is well established, and the instrument has been used extensively in cross-cultural studies. It has been translated into approximately 20 languages and field tested in a dozen countries (whoscan.org). Thus, although subtle differences were indeed noticeable regarding the specific content of delusions, these are unlikely to affect the results of diagnoses based on the Present State Examination–SCAN, because the overall symptom profile of the disease was indistinguishable from descriptions of chronic schizophrenia throughout the world.
The prospective analysis of outcome predictors highlights two main findings: namely, that left substantia nigra hyperechogenicity at the time of diagnosis predicts negative symptoms and parkinsonism at follow-up, and that right-sided parkinsonism at diagnosis significantly predicts a more severe course of illness (Table 2). With respect to the predictive value of ultrasound findings for motor deficits, this is in line with several correlational studies reporting an association with hyperechogenicity of the substantia nigra and parkinsonism in nonmedicated patients with schizophrenia (10) and in acutely and chronically treated patients with schizophrenia (34, 35). Of note, the transcranial ultrasound findings and motor deficits in treatment-naive patients with schizophrenia were not identical to ours, but the overall implications were very similar. Specifically, participants with untreated schizophrenia have more left-sided motor deficits and simultaneous contralateral right substantia nigra abnormalities on an ultrasound at the initial diagnosis. After treatment, these asymmetries become less apparent. Nonetheless, patients with schizophrenia before and after treatment have significant parkinsonism and more substantia nigra hyperechogenicity, which correlate with course severity. Indeed, to our knowledge, this study is the first to show that substantia nigra hyperechogenicity can serve as a marker for adverse motor and clinical outcomes many years later.
With respect to the predictive value of hyperechogenicity for ongoing negative symptoms and parkinsonism in our sample of patients, our findings are again consistent with previously published reports of parkinsonism in treated patients and are associated with negative symptoms (4), a combination described as a ‘‘negative movement disorder’’ (48). The presence of extrapyramidal side effects correlated with more negative symptoms and poorer treatment outcomes that were reflected in a longer time to, and lower level of, remission (48). On the other hand, in one study of patients who were not medicated, the relationship between psychopathology and extrapyramidal side effects could not be established (26).
Neither age of illness onset nor premedication parkinsonism in our sample was predictive of postmedication parkinsonism, psychopathology, or cumulative drug exposure. Indeed, the relationship between age and extrapyramidal side effects is controversial in the literature. In nonmedicated patients, no correlation between extrapyramidal symptoms and age at assessment or at onset of psychotic symptoms was found (23, 26). Conversely, in medicated patients, the risk for dyskinesia increased with age (49) and duration of illness (50). In chronically treated patients with schizophrenia, hyperechogenicity correlates with age and severity of parkinsonian symptoms but not with dose or type of antipsychotic agent (35). In our study, the majority of patients were younger than 25 years at illness onset, and the narrow distribution of ages would make it difficult to detect an effect of age on multiple outcome variables, given our relatively young population.
Clozapine improves outcome in patients with schizophrenia in whom typical antipsychotic medications are ineffective or are not tolerable (51) and results in lower incidence of extrapyramidal symptoms, which are the best predictor of quality of life (52). Roughly one-half of our patients were treated with risperidone, aripiprazole, chlorpromazine, levomepromazine, or haloperidol, whereas the rest were switched to clozapine after two failed medication trials with other antipsychotic agents. We examined differences in age of illness onset, treatment duration, substantia nigra hyperechogenicity, and parkinsonism in clozapine-treated patients versus those treated with other antipsychotic drugs. Patients treated with clozapine were younger at onset, had more negative symptoms, and had more left parkinsonian motor impairment at follow-up. There are several explanations for increased parkinsonism in those treated with clozapine, including clozapine side effects, fluctuations of extrapyramidal symptoms with time (53), or more parkinsonism overall in patients refractory to treatment, independent of medication side effects. These findings support the regression analysis data showing that worse parkinsonism and negative symptomatology are predicted by left substantia nigra abnormalities, which may be an indicator of overall severity of illness and eventual treatment with clozapine.
In summary, parkinsonism is common in neuroleptic-naive patients with schizophrenia, and patients with more severe motor impairment are likely to be more susceptible to extrapyramidal side effects from antipsychotics, leading to treatment refractoriness (54) and to a higher likelihood of clozapine use (52, 55). Thus, we propose transcranial ultrasound screening and testing for parkinsonism at illness onset before the introduction of neuroleptics as potentially useful markers in determining illness severity, negative symptomatology, and tolerance to antipsychotic treatment/refractoriness. Transcranial ultrasound screening may help to identify a subset of patients with worse clinical course and outcome. This subset could be considered for clozapine earlier in the course of illness, eliminating the costly process of determining resistance, treatment withdrawals, hospital readmissions, and severe side effects.
Our experience in a rural indigenous population of the Andean region has shown the feasibility of building teams of local researchers. These teams, by working on a rigorous state-of-the-science research program, have also had an immediate, lasting, and measurable impact on decreasing barriers of the most vulnerable and underserved populations to mental health care, and they have modified local practices by incorporating mental health surveillance practices into primary health strategies as well as by fostering early detection of severe mental health disorders. The regional teams for multidisciplinary collaborative research reflect the necessary range of expertise required for tackling research and treatment of the complex etiology of psychiatric and behavioral disorders.
1 : Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 2008; 30:67–76Crossref, Google Scholar
2 : Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res 2008; 102:1–18Crossref, Google Scholar
3 : The forsaken mental health of the Indigenous Peoples - a moral case of outrageous exclusion in Latin America. BMC Int Health Hum Rights 2009; 9:27Crossref, Google Scholar
4 : Community mental health services in Latin America for people with severe mental disorders. Public Health Rev 2012; 34Crossref, Google Scholar
5 : Global focus, local acts: providing mental health services to indigenous people. Arch Psychiatr Nurs 2011; 25:311–319Crossref, Google Scholar
6 : Psychiatric implications of health and illness in a Maya Indian group: a preliminary statement. Soc Sci Med 1970; 3:609–626Crossref, Google Scholar
7 : Mental Health Gap Action Programme: Scaling Up Care for Mental, Neurological, and Substance Use Disorders. Geneva, WHO Press, 2008Google Scholar
8 : Temperament traits associated with risk of schizophrenia in an indigenous population of Argentina. Schizophr Res 2006; 83:299–302Crossref, Google Scholar
9 : The efficacy of targeted health agents education to reduce the duration of untreated psychosis in a rural population. Schizophr Res 2015; 161:184–187Crossref, Google Scholar
10 : Sex and laterality differences in parkinsonian impairment and transcranial ultrasound in never-treated schizophrenics and their first degree relatives in an Andean population. Schizophr Res 2015; 164:250–255Crossref, Google Scholar
11 : Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry 2005; 62:975–983Crossref, Google Scholar
12 : Review: longer duration of untreated psychosis is associated with worse outcome in people with first episode psychosis. Evid Based Ment Health 2006; 9:36Crossref, Google Scholar
13 : Duration of untreated psychosis is associated with temporal and occipitotemporal gray matter volume decrease in treatment naïve schizophrenia. PLoS One 2013; 8:e83679Crossref, Google Scholar
14 : Duration of untreated psychosis and cognitive functioning. Schizophr Res 2013; 145:43–49Crossref, Google Scholar
15 : Neurocognition and duration of psychosis: a 10-year follow-up of first-episode patients. Schizophr Bull 2015; sbv083Crossref, Google Scholar
16 : Minimal evidence that untreated psychosis damages brain structures: a systematic review. Schizophr Res 2015; 162:222–233Crossref, Google Scholar
17 : Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2014; 205:88–94Crossref, Google Scholar
18 : Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res 1999; 35:51–68Crossref, Google Scholar
19 : A review and Bayesian meta-analysis of clinical efficacy and adverse effects of 4 atypical neuroleptic drugs compared with haloperidol and placebo. J Clin Psychopharmacol 2011; 31:698–704Crossref, Google Scholar
20 : Dementia Praecox and Paraphrenia. Miami, HardPress Publishing, 2013Google Scholar
21 : Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry 1993; 150:1343–1348Crossref, Google Scholar
22 : Incidence and correlates of acute extrapyramidal symptoms in first episode of schizophrenia. Psychopharmacol Bull 1992; 28:81–86Google Scholar
23 : Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry 1995; 152:1724–1729Crossref, Google Scholar
24 : Extrapyramidal symptoms and signs in first-episode, antipsychotic exposed and non-exposed patients with schizophrenia or related psychotic illness. J Psychopharmacol 2005; 19:277–285Crossref, Google Scholar
25 : Motor deficits and schizophrenia: the evidence from neuroleptic-naïve patients and populations at risk. J Psychiatry Neurosci 1999; 24:304–314Google Scholar
26 : Extrapyramidal symptoms in unmedicated schizophrenia. J Psychiatr Res 2005; 39:261–266Crossref, Google Scholar
27 : Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull 2000; 26:367–378Crossref, Google Scholar
28 : Neuromotor precursors of schizophrenia. Schizophr Bull 1994; 20:441–451Crossref, Google Scholar
29 : Childhood videotaped social and neuromotor precursors of schizophrenia: a prospective investigation. Am J Psychiatry 2004; 161:2021–2027Crossref, Google Scholar
30 : Developmental precursors of psychosis. Curr Psychiatry Rep 2004; 6:168–175Crossref, Google Scholar
31 : A multimodal approach to investigate biomarkers for psychosis in a clinical setting: the integrative neuroimaging studies in schizophrenia targeting for early intervention and prevention (IN-STEP) project. Schizophr Res 2013; 143:116–124Crossref, Google Scholar
32 : Specificity and sensitivity of transcranial sonography of the substantia nigra in the diagnosis of Parkinson’s disease: prospective cohort study in 196 patients. BMJ Open 2013; 3:e002613Crossref, Google Scholar
33 : Substantia nigra hyperechogenicity is a risk marker of Parkinson’s disease: yes. J Neural Transm 2011; 118:613–619Crossref, Google Scholar
34 : Echogenicity of substantia nigra determined by transcranial ultrasound correlates with severity of parkinsonian symptoms induced by neuroleptic therapy. Biol Psychiatry 2001; 50:463–467Crossref, Google Scholar
35 : Susceptibility to neuroleptic-induced parkinsonism--age and increased substantia nigra echogenicity as putative risk factors. Eur Psychiatry 2003; 18:177–181Crossref, Google Scholar
36 : Iron accumulation in the substantia nigra in rats visualized by ultrasound. Ultrasound Med Biol 1999; 25:901–904Crossref, Google Scholar
37 : Transcranial sonography in the premotor diagnosis of Parkinson’s disease. Int Rev Neurobiol 2010; 90:93–106Crossref, Google Scholar
38 : Expanded and independent validation of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). J Neurol 2013; 260:228–236Crossref, Google Scholar
39 : Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism Relat Disord 2015; 21:50–54Crossref, Google Scholar
40 : The Positive and Negative Syndrome Scale--Spanish adaptation. J Nerv Ment Dis 1990; 178:510–517Crossref, Google Scholar
41 : The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007; 4:28–37Google Scholar
42 : Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 2010; 67:255–262Crossref, Google Scholar
43 : Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003; 64:663–667Crossref, Google Scholar
44 : The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 2004; 30:193–217Crossref, Google Scholar
45 : Feasibility and effectiveness of a multi-element psychosocial intervention for first-episode psychosis: results from the cluster-randomized controlled GET UP PIANO Trial in a catchment area of 10 million inhabitants. Schizophr Bull 2015; 41:1192–1203Google Scholar
46 : Early manifestations and first-contact incidence of schizophrenia in different cultures. A preliminary report on the initial evaluation phase of the WHO Collaborative Study on determinants of outcome of severe mental disorders. Psychol Med 1986; 16:909–928Crossref, Google Scholar
47 : Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl 1992; 20:1–97Crossref, Google Scholar
48 : Relationship of neuroleptic-induced akathisia to drug-induced parkinsonism. Ital J Neurol Sci 1990; 11:439–442Crossref, Google Scholar
49 : Identifying risk factors for tardive dyskinesia among long-term outpatients maintained with neuroleptic medications. Results of the Yale Tardive Dyskinesia Study. Arch Gen Psychiatry 1993; 50:723–733Crossref, Google Scholar
50 : Predicting the long-term risk of tardive dyskinesia in outpatients maintained on neuroleptic medications. J Clin Psychiatry 1993; 54:133–139Google Scholar
51 : Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry 1999; 156:990–999Google Scholar
52 : Lack of extrapyramidal side effects predicts quality of life in outpatients treated with clozapine or with typical antipsychotics. Psychiatry Res 2005; 133:277–280Crossref, Google Scholar
53 : Spontaneous dyskinesia and parkinsonism in never-medicated, chronically ill patients with schizophrenia: 18-month follow-up. Br J Psychiatry 2002; 181:135–137Crossref, Google Scholar
54 : Nonresponding schizophrenia: differentiation by neurological soft signs and neuropsychological tests. Schizophr Bull 1999; 25:813–825Crossref, Google Scholar
55 : Prevalence of extrapyramidal syndromes in psychiatric inpatients and the relationship of clozapine treatment to tardive dyskinesia. Schizophr Res 2000; 42:223–230Crossref, Google Scholar



