Cannabinoid-Based Drugs: Potential Applications in Addiction and Other Mental Disorders
Abstract
The recreational and medicinal desirability of marijuana (Cannabis sativa), fomented by widespread and generationally shifted social acceptance, has created a perfect storm for the medical community. The emergence of this drug highlights the lack of scientific and clinical understanding of its effects, including those that are health adverse as well as medicinal. This review illustrates established findings to guide psychiatric practice (and nonpractice) and outlines open questions to motivate further study. This work provides a general introduction on the properties of marijuana, briefly describes the cannabinoid receptors (cell surface proteins that recognize the plant’s psychoactive constituent, Δ9-tetrahydrocannabinol), and delineates the potential utility of agents that activate such receptors. Current theories on the workings of the endogenous neurotransmitter system that is hijacked by Δ9-tetrahydrocannabinol are considered. Finally, this review proposes possible ways in which growing knowledge of this system might lead to the discovery of new medicines to treat mental illness.
The resin secreted by the flowers and leaves of marijuana (Cannabis sativa) contains a family of chemically related terpene-like molecules that are virtually unique to this plant (1). Δ9-Tetrahydrocannabinol (Δ9-THC) is the most studied and best understood member of this family (Figure 1). Δ9-THC has a complex set of pharmacologic properties that stem from its ability to combine with selective receptor proteins found on the surface of neurons and other cells throughout the human body. The binding of Δ9-THC to one such protein, called the CB1 cannabinoid receptor, causes the characteristic mental state that accompanies marijuana consumption. Experienced users describe this condition as a combination of enhanced sociability, quickened mental associations, increased appetite for sweet and fatty foods, alterations in the perception of time and space, and heightened sensitivity to certain sensory stimuli (e.g., sounds or colors) (2). These subjective feelings are why people like marijuana, but the sensations occur with and are outlasted by substantial impairments in cognition, judgment, and motor coordination. When Δ9-THC reaches high levels in the blood, some marijuana users even experience panic, paranoid thoughts, and hallucinations (2). In experimental animals, moderate doses of pure Δ9-THC produce a standard set of measurable behavioral and physiological responses, which include lowered motor activity and body temperature along with higher pain thresholds and increased food intake (3). The concomitant administration of drugs that block CB1 receptors blunts these effects (3) as well as those of smoked marijuana in human volunteers (4).
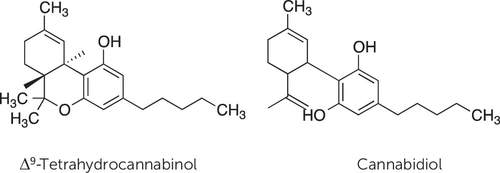
Figure 1. Chemical Structures of the Two Main Terpene-Like Chemicals Present in Cannabis Resina
a Δ9-Tetrahydrocannabinol is responsible for the majority of the psychotropic effects of the drug; it acts by binding to G protein–coupled cannabinoid receptors in the brain and other tissues of the body. Cannabidiol displays a distinct set of pharmacologic properties (e.g., antipsychotic and antiepileptic), which do not involve cannabinoid receptor activation.
As one would expect from its pharmacology, marijuana is primarily consumed for recreational purposes (www.drugabuse.gov), but its medicinal use is also widespread. In fact, since its partial legalization in the United States, marijuana has gained a great deal of credibility and popularity as a medicine. Physicians recommend it to relieve chronic pain resistant to standard analgesics, control muscle spasms caused by multiple sclerosis, improve appetite and alleviate nausea, prevent seizures, and reduce symptoms of inflammatory bowel disease (5, 6). Some of these indications are rooted in the history of Western medicine—Cannabis was listed in European and U.S. pharmacopeias of the 19th and early 20th centuries as an analgesic, anticonvulsant, and hypnotic (7)—and they are supported by clinical evidence. This is the case for chronic neuropathic pain (8–11) and, albeit partially, inflammatory bowel disease (12) and spasticity in multiple sclerosis (13). However, credible data are not yet available for many other claimed indications such as those advertised by some medical marijuana enthusiasts on popular Internet sites. Perhaps not surprisingly, potential treatments for mental disorders provide a particularly rich source of unsupported claims, and practicing psychiatrists are frequently faced with the challenge of managing questions from patients and their families about the risks and benefits associated with marijuana use in a diversity of medical conditions, including autism, depression, and bipolar disorders, among others. These are legitimate questions to which, unfortunately, we can rarely provide evidence-based answers. However, we do have a general framework of knowledge upon which we can build. In the coming sections, we outline known properties of cannabinoid receptors and describe possible therapeutic uses of agents that activate them. We then consider the endogenous neurotransmitter system that normally engages those receptors, and we discuss how our growing understanding of such a system might lead to the discovery of new medicines for psychiatry.
Cannabinoid Receptors
The existence of cannabinoid receptors was postulated more than 30 years ago, when it was discovered that human-made chemicals created to replicate the effects of Δ9-THC were able to bind a specific site in brain membranes and, by doing so, inhibit the enzyme that converts ATP into the intracellular second messenger cAMP (14). The subsequent mapping of cannabinoid binding sites in the rat brain (15) and the molecular cloning of the first cannabinoid receptor gene, now called CB1 (16), established the presence of unique cell surface receptors that recognize Δ9-THC and its synthetic mimics. A second such receptor, CB2, was identified 3 years later (17).
CB1 receptor expression is high in the regions of the human brain that are implicated in the psychological and physiological effects of marijuana. For example, substantial numbers of receptors are found in the structures involved in cognitive functions and reward processing as well as in the areas that control movement and activity of the autonomic nervous system (18). CB1 is also present in many cell types outside the brain, including pain-sensing neurons, innate-immune cells (e.g., macrophages), adipocytes, hepatocytes, and skeletal myocytes. This broad distribution reflects the importance of endogenous cannabinoid (endocannabinoid) messengers in the peripheral control of energy balance, pain, and inflammation (19, 20), among other functions. We revisit these substances later in this review. In addition to CB1, the brain contains a relatively small number of CB2 receptors (21). However, this subtype is expressed at much higher levels in cells of the immune system (e.g., B lymphocytes, macrophages, and microglia) as well as in osteoclasts and osteoblasts (21). The contributions of CB2 to endocannabinoid signaling in the brain appear to be important but are still the subject of unsettled debate (22).
Both CB1 and CB2 signal through G proteins, which transduce receptor activation into intracellular responses (23) (Figure 2). Binding of Δ9-THC to CB1 receptors results not only in the lowering of cAMP levels in the cell cytosol (as mentioned above) but also in the closing of voltage-gated calcium channels, opening of potassium channels, and stimulation of various protein kinases (23). In the brain, where CB1 is primarily localized to presynaptic nerve terminals, two important consequences of its activation are the suppression of neuronal excitability (by stimulation of potassium channels) and the reduction of neurotransmitter release (by inhibition of calcium channels) (23).
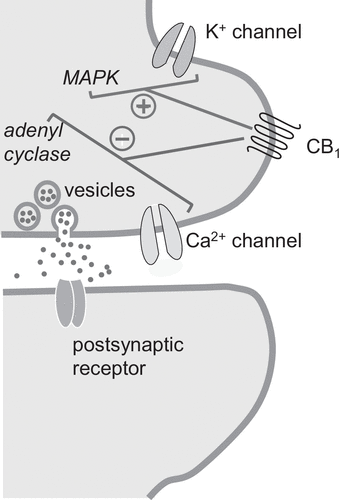
Figure 2. CB1 Cannabinoid Receptor Signaling in the Braina
a CB1 receptors are present in nerve terminals of excitatory (glutamatergic), inhibitory (GABAergic), and modulatory (serotonergic) neurons of the brain. By recruiting G proteins, CB1 increases the activity of potassium (K+) channels (reducing neuronal excitability) and decreases that of calcium (Ca2+) channels (inhibiting neurotransmitter release). In addition, CB1 inhibits adenyl cyclase (lowering intracellular cAMP levels) and stimulates various protein kinases, including MAPKs, leading to phosphorylation of synaptic proteins. MAPK, mitogen-activated protein kinase.
The persistent occupation of CB1 receptors by Δ9-THC starts a molecular process of desensitization that renders subjects tolerant to the effects of the drug (24). In animals, tolerance to Δ9-THC is associated with, and presumably caused by, a partially reversible decrease in the number of CB1 receptors, combined with impaired coupling of the receptors to their transducing G proteins (24, 25). Likewise, in humans, positron emission tomography (PET) imaging studies demonstrated that long-term marijuana use dampens CB1 levels in cortical structures and that abstinence reverses this effect (26). Despite lingering popular notions to the contrary, long-term exposure to the drug can also lead to physical dependence (6, 27). Epidemiological surveys indicate that 8%–9% of individuals who try marijuana become addicted (28), and the number of Americans who are now dependent on the drug—approximately 2.7 million according to recent estimates (6)—is close to that of people suffering from schizophrenia (approximately 2.2 million).
Cannabinoid Receptor Agonists
A variety of synthetic chemicals that bind to and activate cannabinoid receptors have been described in the scientific literature (3). Some of these agents have high potency and efficacy in animal models. However, although these properties are desirable in a research setting, they can be detrimental in the clinic because they translate into unacceptably high levels of CB1-mediated psychotropic activity. Because of these looming side effects, the three currently licensed drugs that target cannabinoid receptors do not stray much from plant-derived Δ9-THC. A synthetic version of the compound is marketed under the international nonproprietary name of dronabinol (Marinol), which is used clinically to increase appetite and decrease nausea in patients who have AIDS or are undergoing cancer chemotherapy. A close chemical analogue of Δ9-THC, nabilone (Cesamet), is prescribed for similar indications. Both dronabinol and nabilone are administered orally and have a slow onset of action, which reduces their attractiveness to recreational marijuana users who may often (albeit not always) seek the quicker “buzz” given by the smoked drug (29). In addition to these synthetic compounds, a standardized C. sativa extract called nabiximols (Sativex) has been approved in various parts of the world (but not yet in the United States) for the symptomatic relief of pain and muscle spasticity in multiple sclerosis and as an adjunctive analgesic in patients with cancer (3). Nabiximols is administered as an oromucosal spray, a formulation strategy that improves the bioavailability of its active constituents. A meta-analysis of 666 patients concluded that nabiximols reduces spasticity and is well tolerated (30).
Along with Δ9-THC, nabiximols contains approximately equal amounts of cannabidiol, the other main terpene-like molecule present in the Cannabis resin (Figure 1). Cannabidiol does not activate cannabinoid receptors, but it nonetheless displays an important pharmacological profile that may include antipsychotic (31–33) and antiepileptic (34) activities. Current theories attribute these effects to interactions with a diverse array of molecular targets, a nonexhaustive list of which comprises the 5-hydroxytryptamine 1 (serotonin) receptor, the transient receptor potential vanilloid type 1 channel, the α3 and α1 glycine receptors, the orphan G protein–coupled receptor GPR55, and the equilibrative nucleoside transporter (34). This plethora of mechanisms might reflect a true polypharmaceutical action or, more likely, the fact that we are still missing something fundamental about the molecular events triggered by cannabidiol. Because its properties do not appear to involve cannabinoid receptors, we will not further consider them here. The interested reader is referred to recent reviews with a focus on schizophrenia (33), epilepsy (34), neuropathic pain (35), and stroke (36).
Applications of Cannabinoid Agents in Mental Illness
In addition to their uses in the treatment of pain, nausea, and appetite loss, dronabinol (oral synthetic Δ9-THC), nabilone (oral Δ9-THC analogue), and nabiximols (Cannabis extract spray) have been investigated, with some initial promising results, in three mental disorders that are currently lacking in good therapeutic options: cannabis use disorder, Tourette’s syndrome, and posttraumatic stress disorder (PTSD).
Cannabis Use Disorder
Treatment of cannabis use disorder should ideally address both dependence and withdrawal (DSM-5). However, no such treatment exists (37). On the basis of the relative success of agonist replacement therapy in other forms of addiction (tobacco and opiates), it stands to reason that cannabinoid receptor agonists should be considered beneficial in cannabis use disorder. This expectation was confirmed by a controlled human laboratory study, which showed that nabilone prolonged abstinence and decreased marijuana self-administration among relapsing participants (38). The same study also reported that nabilone ameliorated all primary symptoms of marijuana withdrawal: it lowered irritability scores, improved sleep quality, and normalized food intake and sociability (38). The usefulness of agonist replacement therapy in the treatment of marijuana withdrawal is also supported by human laboratory and clinical studies with dronabinol and nabiximols (27, 39, 40). Unlike nabilone, however, dronabinol and nabiximols do not significantly alleviate marijuana dependence (40, 41). A double-blind, placebo-controlled trial to further evaluate the efficacy of nabilone in cannabis use disorder is expected to report results in 2015 (ClinicalTrials.gov).
Tourette’s Syndrome
First- and second-generation antipsychotics reduce motor and phonic tics in many patients with Tourette’s syndrome but also cause intolerable side effects, whereas α2-adrenergic agonists are widely used but lack consistent support for their efficacy (42). Two double-blind, placebo-controlled trials suggest that oral Δ9-THC might offer an alternative to those drugs (43, 44). Both trials reported positive effects of the cannabinoid agent, which were accompanied by mild and transient adverse events. Nevertheless, the total number of patients (N=28) and the effect size were small in those studies (42). Research on this important topic has recently slowed, but a few case studies (45, 46) and many basic science findings provide good reasons to continue. CB1 receptors are expressed at high levels in dopamine-sensitive corticostriatal networks of the human brain (47), which have been implicated in Tourette’s pathology (48). Activation of CB1 receptors localized to these networks functionally counters the motor stimulation caused by dopamine D2 agonists (49, 50). These data, along with anecdotal reports of marijuana self-medication by people with Tourette’s syndrome (51), provide a plausible rationale to reexamine the usefulness of cannabinoid agonists in this disorder.
PTSD
It is estimated that 6.4%–7.8% of the U.S. population suffers from PTSD (52), and the prevalence of this disabling anxiety disorder rises to 23.6%−30.5% in combat veterans (53). The Food and Drug Administration (FDA) has approved antidepressant drugs that inhibit serotonin and norepinephrine uptake for the treatment of PTSD, but there is still a strong need for better therapies (54). Many afflicted war veterans report using marijuana as self-medication (55, 56); in fact, there are several clues that cannabinoid receptor activation might help alleviate the symptoms of PTSD. In an open-label trial of 47 persons diagnosed with PTSD, nabilone produced a significant reduction in the number and intensity of nightmares experienced by patients (57). These results are in line with those of a double-blind, placebo-controlled trial in 20 subjects suffering from anxiety, which reported marked effects of low-dose nabilone relative to placebo (58). Results of an earlier human laboratory study also suggested anxiolytic properties for low-dose nabilone (59). These findings can only be viewed as preliminary, but their heuristic value should not be underestimated. As we later discuss, a substantial set of preclinical and human laboratory data suggest that one of the main functions of the endocannabinoid system is to help orchestrate a successful response to stressful environmental stimuli (60–64). Evidence linking brain endocannabinoid transmission and PTSD is also emerging: for example, PET imaging studies have shown that CB1 receptors are elevated throughout the brain of persons with PTSD with noncombat trauma histories (65, 66). It is important to point out that cannabinoid agonists influence anxiety-like behaviors in animals with bimodal dose dependence: they reduce these behaviors at low doses but have an opposite effect at higher doses (67). Similarly in humans, marijuana can cause euphoria and relaxation or dysphoria and panic, depending on the dosage and the level of the user’s experience with the drug (6). This duplicity might reflect the engagement of noncannabinoid binding sites or, more likely, a process of differential recruitment or desensitization of CB1 receptors in areas of the brain that regulate effects in opposing ways.
Cannabinoid Receptor Antagonists
The first CB1 antagonist to be discovered, rimonabant (Acomplia), remains the most extensively studied member of this class (21). The compound selectively binds to CB1 receptors with nanomolar affinity and behaves as an inverse agonist (21)—that is, under certain circumstances it can produce effects that are opposite to those exerted by a cannabinoid agonist (by contrast, a neutral antagonist would simply block the access of an agonist to the receptor). In both rodents and humans, rimonabant prevents the acute behavioral/psychological and physiological effects of Δ9-THC (4, 21). More importantly, long-term treatment with rimonabant has a profoundly positive effect on energy balance in obese people (and animals), causing substantial improvements in lipid profiles and insulin resistance, along with a moderate but sustained weight loss (68). Laboratory experiments indicate that these antiobesity effects, which rimonabant shares with other CB1 antagonists, are the result of the agent’s ability to disable the regulatory control exerted by the endocannabinoid system on energy intake and expenditure: it is well recognized that an overarching function of central and peripheral endocannabinoid signaling is to promote food intake and maximize energy storage (19, 69). Overcoming such control can be therapeutically useful but comes at a price. As mentioned above, the endocannabinoids influence the activity of neuronal networks that help animals cope with environmental stress. Accordingly, in clinical trials aimed at assessing the effects of rimonabant in obesity, treatment was accompanied by a set of dose-dependent psychiatric events that included irritability, anxiety, depression, and suicidal thoughts (68). The anxiogenic properties of rimonabant, a major factor in its ultimate rejection by the FDA, have been confirmed with a human model of simulated public speaking (64). Similar anxiogenic-like effects have been also observed in most, albeit not all, animal experiments with rimonabant as well as in studies in which CB1 receptors were deleted via genetic techniques (70). Although the demise of rimonabant halted the clinical development of all CB1 antagonists, there are alternative ways of interfering with CB1 activity that might achieve metabolic improvements without a high risk for psychiatric liabilities. An emerging class of ligands that cannot cross the blood-brain barrier is particularly promising. These peripherally restricted antagonists effectively control body weight and insulin resistance in animal models, presumably by interrupting endocannabinoid signaling in the liver (71), adipose organs (72), and the sympathetic nervous system (73).
The Endocannabinoids
Δ9-THC imitates two lipid-derived molecules that are normally produced by the body: anandamide and 2-arachidonoyl-sn-glycerol (2-AG). These endocannabinoid substances obey only some of the classical rules of neural transmission. Like other neurotransmitters, they engage synaptic receptors and are rapidly deactivated. Unlike their more conventional counterparts, however, the endocannabinoids are not stored in vesicles and do not transmit information in a standard presynaptic-to-postsynaptic direction.
Anandamide
Figure 3 shows the canonical route of anandamide biosynthesis, which postulates that the compound is generated through enzyme-mediated hydrolysis of a relatively rare phospholipid present in cell membranes, called N-arachidonoyl-phosphatidylethanolamine (74). The enzyme responsible for this reaction is N-acylphosphatidylethanolamine-hydrolyzing phospholipase D, a unique phospholipase D whose molecular structure has been elucidated (75, 76). Two variants of this route have been proposed, both of which use N-arachidonoyl-phosphatidylethanolamine as a starting point but replace N-acylphosphatidylethanolamine-hydrolyzing phospholipase D with different lipid hydrolases (Figure 3) (77, 78). Irrespective of the mechanism involved, neurons produce anandamide rather slowly and release it into the extracellular space for a period of many minutes (49, 79). This timing is incompatible with classical point-to-point neurotransmission—of the type seen, for example, with glutamic or γ-aminobutyric acid—but it is similar to that of volume neurotransmitters such as adenosine and the neuropeptides (80). Anandamide appears to share with these unconventional messengers the ability to exert modulatory effects that go beyond the close boundaries of the synapse to involve nearby neurons and glial cells.
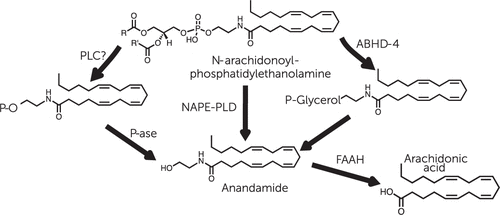
Figure 3. Formation and Deactivation of Anandamide in Brain Neuronsa
a The canonical route of anandamide biosynthesis is shown in the center. In this route, anandamide is released by hydrolysis of the phospholipid precursor, N-arachidonoyl-phosphatidylethanolamine, catalyzed by a selective phospholipase D. Two additional routes of anandamide biosynthesis have been described. (Left) An as-yet-uncharacterized phospholipase C converts N-arachidonoyl-phosphatidylethanolamine into phosphoanandamide, which is then dephosphorylated by a phosphatase-forming anandamide. (Right) N-arachidonoyl-phosphatidylethanolamine is hydrolyzed by α/β hydrolase domain–containing protein 4, forming glycerophosphoanandamide (P-glycerol), which is then converted to anandamide. ABHD-4, α/β hydrolase domain–containing protein 4; FAAH, fatty-acid amide hydrolase; NAPE-PLD, N-arachidonoyl-phosphatidylethanolamine phospholipase D; P-ase, phosphatase; P-O, phosphate; PLC, phospholipase C.
Like all brain neurotransmitters, anandamide must be deactivated. This process occurs in two steps. An enzyme called fatty-acid amide hydrolase (FAAH) cleaves anandamide into its two basic chemical fragments: arachidonic acid and ethanolamine (81) (Figure 3). The functional importance of this intracellular reaction is demonstrated by the fact that experimental interventions that disrupt FAAH activity (e.g., genetic deletion or pharmacological blockade) enhance anandamide-mediated signaling (60). As we later show, this effect of FAAH inhibition may be therapeutically valuable because many beneficial consequences of CB1 receptor activation (e.g., relief of anxiety, mood elevation) might be achieved with equal efficacy and fewer undesired effects by protecting anandamide from degradation.
In neurons, FAAH resides on membrane structures found within the dendritic spine (82). This localization raises the question of how anandamide, a molecule that is almost insoluble in water, is able to cover the water-filled space between its site of action (CB1 receptors on axon terminals) and its site of degradation (organelles inside postsynaptic spines). There is evidence that carrier proteins help bridge this gap (83, 84). Although the identities of such proteins are still very much debated, several small molecule inhibitors of anandamide transport have been identified. Like FAAH inhibitors, these agents hold therapeutic promise as enhancers of intrinsic endocannabinoid signaling, provided of course that their molecular mechanism of action is fully elucidated (83, 85).
2-AG
The concentration of 2-AG in brain tissue is approximately 200 times higher than that of anandamide (86). This quantitative difference may reflect a fundamental distinction in the roles played by these two molecules. Whereas anandamide may primarily act as a volume transmitter, 2-AG may instead serve as a point-to-point messenger that carries signals emerging from the dendritic spine back to the axon terminal. How does this process work? Current theories postulate that the excitatory neurotransmitter glutamate stimulates 2-AG formation by combining with a group of postsynaptic G protein–coupled receptors called mGlu5 metabotropic receptors (87). Newly formed 2-AG travels across the synaptic cleft in a direction opposite to that of glutamate, activates CB1 receptors on excitatory terminals, and, by doing so, inhibits glutamate release (88).
This retrograde feedback mechanism is made possible by the existence of an anatomically defined structure that is dedicated to the production and release of 2-AG (the 2-AG signalosome) (89) (Figure 4). Selectively localized to a zone of the dendritic spine that borders the postsynaptic density, the 2-AG signalosome is a multiprotein complex that physically connects mGlu5 receptors to two enzymes that are necessary for 2-AG biosynthesis: phospholipase C-β and diacylglycerol lipase-α (86, 89). Facilitated by its physical proximity to phospholipase C-β and diacylglycerol lipase-α, activated mGlu5 stimulates these enzymes and triggers a rapid and concentrated spike in 2-AG from a phospholipid precursor present in spine membranes. Upon reaching nerve terminals, 2-AG is hydrolyzed by monoacylglycerol lipase (90), whereas the compound that remains in the spine is eliminated by another lipase called α/β hydrolase domain–containing protein 6 (89, 91). Similarly to FAAH, inhibitors of monoacylglycerol lipase activity stop the degradation of 2-AG, causing the messenger to accumulate and persistently activate cannabinoid receptors (79, 89, 90). These inhibitors have demonstrated a wide array of pharmacological activities in animal models of pain, anxiety, and Alzheimer’s disease, among others (79, 92).
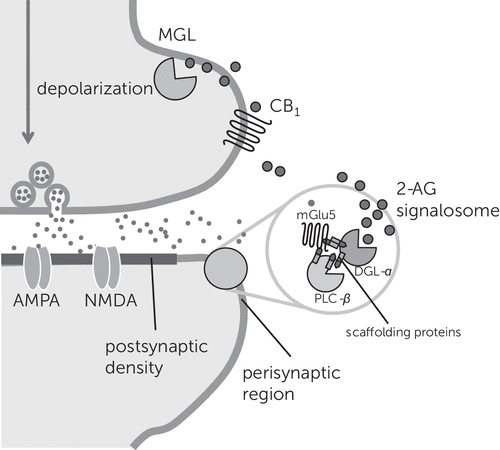
Figure 4. 2-AG–Mediated Signaling at Excitatory Synapses of the Braina
a A supramolecular complex selectively localized to the perisynaptic zone of the dendritic spine, the 2-AG signalosome, joins three key proteins involved in 2-AG production (mGlu5 metabotropic glutamate receptors, phospholipase C-β, and diacylglycerol lipase-α) in a single functional unit. The proteins may be held together by scaffolding Homer proteins. This physical proximity allows for the rapid local accumulation of 2-AG, which travels across the synaptic cleft to activate CB1 receptors on nerve terminals. The 2-AG that reaches axon terminals may be quickly hydrolyzed by monoacylglycerol lipase, whereas the 2-AG that remains in the spine may be degraded by α/β hydrolase domain–containing protein 6. 2-AG, 2-arachidonoyl-sn-glycerol; CB1, cannabinoid receptor protein; DGL-α, diacylglycerol lipase-α; MGL, monoacylglycerol lipase; NMDA, N-methyl-d-aspartic acid; PLC-β, phospholipase C-β.
Applications of Endocannabinoid-Based Agents in Mental Health
The psychiatric side effects exerted by Δ9-THC and its synthetic mimics represent a serious obstacle to broader clinical development, although they do not preclude its therapeutic use in areas of medical need (e.g., Tourette’s syndrome or cannabis use disorder). However, the endocannabinoid signaling system offers several opportunities to circumvent such effects and possibly achieve adequate therapeutic efficacy. Small molecule allosteric receptor modulators may be used to improve the sensitivity of CB1 and make endocannabinoid signals more effective (23). A similar goal may be achieved by shielding anandamide and 2-AG from deactivation via protein-mediated uptake into cells and intracellular degradation. Although the majority of these strategies are still experimental, FAAH inhibitors have reached clinical testing and are currently being considered for the treatment of anxiety and cannabis use disorder. There are also some intriguing hints that they might be useful in treating schizophrenia.
FAAH Inhibitors in Anxiety Disorders
The idea that anandamide may be an important regulator of stress-coping behaviors was first suggested by animal experiments, which showed that the FAAH inhibitor URB597 decreases isolation-induced ultrasonic vocalizations in rat pups and increases the time spent by adult rats in the open arms of an elevated maze (60). Subsequent studies showed that URB597 also enhances active stress-coping behaviors in mouse and rat models of acute and chronic stress (61, 62). Other FAAH inhibitors were later shown to exert anxiolytic-like effects that were similar to those of URB597 (63, 93). These effects are prevented by the administration of a CB1 antagonist, which is an indication that they are the result of enhanced anandamide-mediated transmission at CB1 receptors. A study in healthy volunteers showed that the circulating levels of anandamide were elevated after the subjects were exposed to a psychosocial stress test, further implicating anandamide in the response to stressful stimuli (94).
The amygdala is considered to be a crucial node in the anxiety-reducing network recruited by FAAH-regulated anandamide signaling (70). A laboratory study showed that people who carry a FAAH variant that results in low enzyme expression exhibit quicker habituation of amygdala reactivity to threats and have lower scores on the personality trait of stress reactivity (63). Moreover, PET imaging experiments in trauma survivors demonstrated that increased attentional bias to threat (an endophenotype linked to the transition of trauma-related physiopathology to chronicity) is associated with elevated CB1 levels in the amygdala (95). These findings, together with the previously mentioned upregulation of CB1 receptors in the brain and downregulation of anandamide in the serum of subjects with PTSD (65, 66), formed the basis for a programmed clinical trial that will examine the efficacy of URB597 in PTSD (A. Neumeister, personal communication, May 10, 2014). In this context, it is important to point out that URB597 has no rewarding effects in rodents (61) and is not self-administered by squirrel monkeys (96), marking a clear mechanistic distinction with Δ9-THC and suggesting that this FAAH inhibitor might be used in the clinic without an overt risk for abuse.
FAAH Inhibitors in Cannabis Use Disorder
The preclinical pharmacological profile of FAAH inhibitors predicts that they should attenuate several of the symptoms experienced by marijuana-dependent individuals who are trying to remain abstinent, including anxiety, depression, and deterioration of sleep quality (37, 97). Animal studies support this prediction. In a model of cannabinoid withdrawal in which mice were first rendered dependent on Δ9-THC and then were given a fully active dose of the CB1 antagonist rimonabant, previous treatment with URB597 prevented somatic signs of precipitated withdrawal (98). A randomized, double-blind clinical trial aimed at determining the safety and efficacy of the compound PF-04457845, a FAAH inhibitor that is structurally different from URB597, is ongoing (ClinicalTrials.gov). The outcome of this study will likely influence future research directions in other areas of addiction medicine such as tobacco (99) and cocaine use disorder (100).
FAAH Inhibitors in Schizophrenia
Prolonged marijuana use has been linked to psychosis and schizophrenia. In a cohort of more than 45,000 Swedish male conscripts, intense marijuana usage in the teenage years increased schizophrenia risk later in life by sixfold (101). It is likely that marijuana exposure heightens psychosis susceptibility, rather than the reverse possibility of psychosis leading to self-medication with the drug: meta-analyses estimated that marijuana consumption roughly doubles the overall schizophrenia risk, whereas psychosis liability does not predict marijuana usage (102, 103). However, the same analyses also indicate that only a minute percentage of heavy marijuana users (3%) progress to schizophrenia, suggesting that exposure to the drug is neither necessary nor sufficient for psychosis but increases vulnerability within a complex interplay of genetic and developmental factors.
Does the connection of marijuana with an increased risk for psychosis reflect an implication of brain endocannabinoid signaling in schizophrenia? Autoradiography and PET imaging studies have consistently reported elevations in cortical and subcortical CB1 receptor densities among persons with schizophrenia (102, 104–110). Although these findings are generally interpreted as implying that excessive endocannabinoid transmission is a causative factor in psychosis (111), data from other studies offer a radically different perspective. First, a simplistic “endocannabinoid hypothesis of schizophrenia” is negated by the fact that the CB1 antagonist rimonabant did not significantly improve disease symptoms in a placebo-controlled clinical trial of subjects with schizophrenia or schizoaffective disorder (112) or in a subsequent double-blind, placebo-controlled trial aimed at assessing the effect of the drug on cognitive function in subjects with schizophrenia (113). Second, a study of persons with nonmedicated first-episode psychosis showed that cerebrospinal levels of anandamide correlated inversely with positive and negative symptoms of schizophrenia (114, 115). Third, in a double-blind randomized clinical trial of cannabidiol in acute psychosis, treatment with the drug was accompanied by a substantial increase in circulating anandamide levels, which was significantly associated with clinical improvement (31). Finally, in patients in the prodromal states of schizophrenia, lower cerebrospinal levels of anandamide were linked with a higher risk for an earlier transition to psychosis (116). In addition to being internally consistent, these results also tally well with preclinical experiments indicating that anandamide release in the basal ganglia may be part of a negative feedback loop that attenuates the consequences of excessive dopaminergic activity (49, 50). Together, the results summarized above identify anandamide as a homeostatic controller of dopamine neurotransmission and a protective signal in schizophrenia. A corollary of this hypothesis, which is currently being tested (ClinicalTrials.gov), is that FAAH inhibitors may be beneficial in psychosis and possibly in other mental disorders in which hyperactive dopamine transmission might be implicated (e.g., Tourette’s syndrome). How does marijuana fit into this picture? We do not yet know, but an intriguing possibility is that the drug might actually suppress anandamide signaling and, by doing so, remove its tempering influence on dopaminergic activity. This model requires testing but is tentatively supported by a study of patients with schizophrenia who were experiencing their first episode of psychosis; the study reported an association between exposure to cannabis and decreased anandamide content in cerebrospinal fluid (117).
Conclusions
The past two decades of research on endocannabinoids and their receptors have greatly expanded our understanding of these unconventional signaling molecules and the roles they play in mental health. Information from the synapse to the patient has illuminated how Δ9-THC and other exogenous cannabinoids hijack the endocannabinoid signaling system, leading to serious side effects but at the same time providing promising opportunities for therapeutic intervention. Although important questions remain, it is clear that the medical potential of endocannabinoid modulation cannot be ignored. The social emergence of marijuana accentuates this call for further investigation.
1 : Cannabis - from cultivar to chemovar. Drug Test Anal 2012; 4:660–667Crossref, Google Scholar
2 Iversen LL: The Science of Marijuana. New York, Oxford University Press, 2001Google Scholar
3 : Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond B Biol Sci 2012; 367:3353–3363Crossref, Google Scholar
4 , et al: Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 2001; 58:322–328Crossref, Google Scholar
5 , et al: Medicinal use of cannabis in the United States: historical perspectives, current trends, and future directions. J Opioid Manag 2009; 5:153–168Google Scholar
6 , et al: Adverse health effects of marijuana use. N Engl J Med 2014; 370:2219–2227Crossref, Google Scholar
7 : History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodivers 2007; 4:1614–1648Google Scholar
8 , et al: Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 2007; 68:515–521Crossref, Google Scholar
9 , et al: Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34:672–680Crossref, Google Scholar
10 , et al: A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9:506–521Crossref, Google Scholar
11 , et al: Medical marijuana: clearing away the smoke. Open Neurol J 2012; 6:18–25Google Scholar
12 , et al: Cannabis for inflammatory bowel disease. Dig Dis 2014; 32:468–474Crossref, Google Scholar
13 , et al: Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ 2012; 184:1143–1150Google Scholar
14 , et al: Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 1988; 34:605–613Google Scholar
15 , et al: Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 1990; 87:1932–1936Crossref, Google Scholar
16 , et al: Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990; 346:561–564Crossref, Google Scholar
17 Munro S, Thomas KL, Abu-Shaar M: Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365:61–65Google Scholar
18 : Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 1997; 77:299–318Crossref, Google Scholar
19 , et al: Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. FASEB J 2013; 27:2513–2520Crossref, Google Scholar
20 : Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci 2014; 17:164–174Crossref, Google Scholar
21 : Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol 2006; 46:101–122Crossref, Google Scholar
22 : CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 2010; 160:467–479Crossref, Google Scholar
23 : CB(1) cannabinoid receptors and their associated proteins. Curr Med Chem 2010; 17:1382–1393Crossref, Google Scholar
24 : Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav 2005; 81:300–318Crossref, Google Scholar
25 , et al: Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat Neurosci 2015; 18:75–86Crossref, Google Scholar
26 , et al: Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 2012; 17:642–649Crossref, Google Scholar
27 , et al: Marijuana dependence and its treatment. Addict Sci Clin Pract 2007; 4:4–16Google Scholar
28 , et al: Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 2011; 115:120–130Crossref, Google Scholar
29 : The abuse potential of the synthetic cannabinoid nabilone. Addiction 2010; 105:494–503Crossref, Google Scholar
30 , et al: Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler 2010; 16:707–714Crossref, Google Scholar
31 , et al: Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatr 2012; 2:e94Crossref, Google Scholar
32 , et al: A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des 2012; 18:5131–5140Crossref, Google Scholar
33 : A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res 2015; 162:153–161Crossref, Google Scholar
34 , et al: Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014; 55:791–802Crossref, Google Scholar
35 , et al: The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache 2015; 29:7–14Google Scholar
36 , et al: Cannabinoids in experimental stroke: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2015; 35:348–358Crossref, Google Scholar
37 : Pharmacological treatment of cannabis dependence. Curr Pharm Des 2011; 17:1351–1358Crossref, Google Scholar
38 , et al: Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology 2013; 38:1557–1565Crossref, Google Scholar
39 , et al: Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology 2004; 29:158–170Crossref, Google Scholar
40 , et al: Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry 2014; 71:281–291Crossref, Google Scholar
41 , et al: Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008; 197:157–168Crossref, Google Scholar
42 : Cannabinoids for Tourette’s syndrome. Cochrane Database Syst Rev 2009; (4):CD006565Google Scholar
43 , et al: Treatment of Tourette’s syndrome with delta 9-tetrahydrocannabinol (THC): a randomized crossover trial. Pharmacopsychiatry 2002; 35:57–61Crossref, Google Scholar
44 , et al: Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry 2003; 64:459–465Google Scholar
45 , et al: Oral delta 9-tetrahydrocannabinol improved refractory Gilles de la Tourette syndrome in an adolescent by increasing intracortical inhibition: a case report. J Clin Psychopharmacol 2010; 30:190–192Crossref, Google Scholar
46 , et al: Cannabinoids improve driving ability in a Tourette’s patient. Psychiatry Res 2011; 190:382Crossref, Google Scholar
47 : Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci 1997; 17:5327–5333Crossref, Google Scholar
48 : Tourette syndrome: update. Brain Dev (Epub ahead of print, Jan. 17, 2015)Google Scholar
49 , et al: Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 1999; 2:358–363Crossref, Google Scholar
50 , et al: Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci 2000; 20:3401–3407Crossref, Google Scholar
51 , et al: Cannabinoids: possible role in patho-physiology and therapy of Gilles de la Tourette syndrome. Acta Psychiatr Scand 1998; 98:502–506Crossref, Google Scholar
52 , et al: Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord 2011; 25:456–465Crossref, Google Scholar
53 , et al: Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry 2010; 67:614–623Crossref, Google Scholar
54 : Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2006; (1):CD002795Google Scholar
55 , et al: Posttraumatic stress disorder and cannabis use characteristics among military veterans with cannabis dependence. Am J Addict 2013; 22:277–284Crossref, Google Scholar
56 : Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend 2014; 136:162–165Crossref, Google Scholar
57 : The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther 2009; 15:84–88Crossref, Google Scholar
58 : The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. J Clin Pharmacol 1981; 21(Suppl):377S–382SCrossref, Google Scholar
59 , et al: A single dose study of nabilone, a synthetic cannabinoid. Psychopharmacology (Berl) 1980; 71:137–142Crossref, Google Scholar
60 , et al: Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 2003; 9:76–81Crossref, Google Scholar
61 , et al: Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA 2005; 102:18620–18625Crossref, Google Scholar
62 , et al: Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry 2007; 62:1103–1110Crossref, Google Scholar
63 , et al: Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry 2013; 18:813–823Crossref, Google Scholar
64 , et al: Rimonabant effects on anxiety induced by simulated public speaking in healthy humans: a preliminary report. Hum Psychopharmacol 2014; 29:94–99Crossref, Google Scholar
65 , et al: Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry 2013; 18:1034–1040Crossref, Google Scholar
66 , et al: Translational evidence for a role of endocannabinoids in the etiology and treatment of posttraumatic stress disorder. Psychoneuroendocrinology 2015; 51:577–584Crossref, Google Scholar
67 , et al: The endocannabinoid system and psychiatric disorders. Exp Neurol 2010; 224:3–14Crossref, Google Scholar
68 Engeli S: Central and peripheral cannabinoid receptors as therapeutic targets in the control of food intake and body weight. Handb Exp Pharmacol 2012; 209:357–381Google Scholar
69 : Intestinal lipid-derived signals that sense dietary fat. J Clin Invest 2015; 125:891–898Crossref, Google Scholar
70 : The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res 2007; 56:367–381Crossref, Google Scholar
71 : The case for peripheral CB₁ receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol 2011; 163:1423–1431Crossref, Google Scholar
72 : CB1 antagonists for obesity—what lessons have we learned from rimonabant? Nat Rev Endocrinol 2009; 5:633–638Crossref, Google Scholar
73 , et al: Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB₁ receptor blockade. Proc Natl Acad Sci USA 2013; 110:4786–4791Crossref, Google Scholar
74 : The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 2003; 4:873–884Crossref, Google Scholar
75 , et al: Biosynthetic pathways of bioactive N-acylethanolamines in brain. CNS Neurol Disord Drug Targets 2013; 12:7–16Crossref, Google Scholar
76 , et al: Structure of human N-acylphosphatidylethanolamine-hydrolyzing phospholipase D: regulation of fatty acid ethanolamide biosynthesis by bile acids. Structure 2015; 23:598–604Crossref, Google Scholar
77 , et al: Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology 2008; 54:1–7Crossref, Google Scholar
78 : Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem 2008; 283:9341–9349Crossref, Google Scholar
79 , et al: An endocannabinoid mechanism for stress-induced analgesia. Nature 2005; 435:1108–1112Crossref, Google Scholar
80 , et al: Understanding wiring and volume transmission. Brain Res Brain Res Rev 2010; 64:137–159Crossref, Google Scholar
81 : Chemical probes of endocannabinoid metabolism. Pharmacol Rev 2013; 65:849–871Crossref, Google Scholar
82 , et al: Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 2004; 20:441–458Crossref, Google Scholar
83 , et al: Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 1997; 277:1094–1097Crossref, Google Scholar
84 : More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology 2014; 76(Pt B):228–234Crossref, Google Scholar
85 : Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov 2008; 7:438–455Crossref, Google Scholar
86 : A second endogenous cannabinoid that modulates long-term potentiation. Nature 1997; 388:773–778Crossref, Google Scholar
87 , et al: Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol 2005; 68:1196–1202Crossref, Google Scholar
88 , et al: Endocannabinoid signaling and synaptic function. Neuron 2012; 76:70–81Crossref, Google Scholar
89 , et al: Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun 2012; 3:1080Crossref, Google Scholar
90 , et al: Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA 2002; 99:10819–10824Crossref, Google Scholar
91 , et al: The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci 2010; 13:951–957Crossref, Google Scholar
92 , et al: Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Reports 2012; 2:1329–1339Crossref, Google Scholar
93 , et al: Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatr 2014; 4:e408Crossref, Google Scholar
94 , et al: Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology 2012; 37:2416–2427Crossref, Google Scholar
95 , et al: Cannabinoid type 1 receptor availability in the amygdala mediates threat processing in trauma survivors. Neuropsychopharmacology 2014; 39:2519–2528Crossref, Google Scholar
96 , et al: Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry 2008; 64:930–937Crossref, Google Scholar
97 : The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology 2009; 56(Suppl 1):235–243Crossref, Google Scholar
98 , et al: Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J 2009; 11:342–352Crossref, Google Scholar
99 , et al: Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacology 2013; 38:1198–1208Crossref, Google Scholar
100 : Modulation of the endocannabinoid system: therapeutic potential against cocaine dependence. Pharmacol Res 2007; 56:406–417Crossref, Google Scholar
101 , et al: Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet 1987; 2:1483–1486Crossref, Google Scholar
102 , et al: The environment and schizophrenia: the role of cannabis use. Schizophr Bull 2005; 31:608–612Crossref, Google Scholar
103 , et al: Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry 2004; 184:110–117Crossref, Google Scholar
104 , et al: Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 2001; 103:9–15Crossref, Google Scholar
105 : Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:355–360Crossref, Google Scholar
106 : Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res 2006; 172:556–560Crossref, Google Scholar
107 , et al: Paranoid schizophrenia is characterized by increased CB1 receptor binding in the dorsolateral prefrontal cortex. Neuropsychopharmacology 2011; 36:1620–1630Crossref, Google Scholar
108 , et al: Binding of a tritiated inverse agonist to cannabinoid CB1 receptors is increased in patients with schizophrenia. Schizophr Res 2012; 141:185–188Crossref, Google Scholar
109 , et al: [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol 2015; 20:357–367Crossref, Google Scholar
110 , et al: Reciprocal alterations in cortical cannabinoid receptor 1 binding relative to protein immunoreactivity and transcript levels in schizophrenia. Schizophr Res 2014; 159:124–129Crossref, Google Scholar
111 : Gone to pot - a review of the association between cannabis and psychosis. Front Psychiatry 2014; 5:54Crossref, Google Scholar
112 , et al; : Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry 2004; 161:975–984Crossref, Google Scholar
113 , et al: Rimonabant for neurocognition in schizophrenia: a 16-week double blind randomized placebo controlled trial. Schizophr Res 2012; 134:207–210Crossref, Google Scholar
114 , et al: Elevated endogenous cannabinoids in schizophrenia. Neuroreport 1999; 10:1665–1669Crossref, Google Scholar
115 , et al: Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology 2004; 29:2108–2114Crossref, Google Scholar
116 , et al: Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry 2009; 194:371–372Crossref, Google Scholar
117 , et al: Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr Res 2007; 94:29–36Crossref, Google Scholar



