Complementary and Alternative Medicine for Posttraumatic Stress Disorder Symptoms: A Systematic Review
Abstract
Objectives:
To (1) characterize complementary and alternative medicine studies for posttraumatic stress disorder symptoms, (2) evaluate the quality of these studies, and (3) systematically grade the scientific evidence for individual CAM modalities for posttraumatic stress disorder.
Design:
Systematic review. Eight data sources were searched. Selection criteria included any study design assessing posttraumatic stress disorder outcomes and any complementary and alternative medicine intervention. The body of evidence for each modality was assessed with the Natural Standard evidence-based, validated grading rationale.
Results and Conclusions:
Thirty-three studies (n = 1329) were reviewed. Scientific evidence of benefit for posttraumatic stress disorder was strong for repetitive transcranial magnetic stimulation and good for acupuncture, hypnotherapy, meditation, and visualization. Evidence was unclear or conflicting for biofeedback, relaxation, Emotional Freedom and Thought Field therapies, yoga, and natural products. Considerations for clinical applications and future research recommendations are discussed.
(Reprinted with permission from Journal of Evidence‐Based Complementary & Alternative Medicine, 19(3): 161–175, 2014)
Introduction
Posttraumatic stress disorder is a serious and growing health issue. Approximately 7.7 million American adults (3.5%) have posttraumatic stress disorder in a given year.1 Not only do people with posttraumatic stress disorder experience debilitating symptoms of posttraumatic stress disorder, but they also have a higher prevalence of other psychiatric and physical comorbid conditions such as depression. The annual economic burden of anxiety disorders in the United States is estimated at $42.3 to $46.6 billion.2 The personal and societal costs of posttraumatic stress disorder are high because of chronic symptoms, increased comorbidities, and marked functional impairment.3-5
Posttraumatic stress disorder may occur when a person has been exposed to a traumatic event that involves actual or threatened death, serious injury, or threat to the physical integrity of self or others. People who acquire posttraumatic stress disorder after a traumatic event experience a constellation of symptoms that were not present before the trauma. Symptoms fall into 4 diagnostic criteria: intrusion, avoidance, negative alterations in cognitions and mood, and alterations in arousal and reactivity. People with posttraumatic stress disorder persistently reexperience the trauma through recurrent and intrusive distressing recollections of the event, and they may experience severe distress when exposed to cues that symbolize or resemble aspects of the trauma. They also avoid thoughts, feelings, conversations, people, places, or activities that are reminiscent of the initial event. People with posttraumatic stress disorder can have negative alterations in cognition or mood criteria such as the inability to recall key features of the traumatic event, persistent negative beliefs and expectations about oneself or the world, and markedly diminished interest in significant activities. They also have altered arousal and reactivity symptoms such as hypervigilance, difficulty concentrating, difficulty falling or staying asleep, irritability or out-bursts of anger, or exaggerated startle response.6
The complex psychopathology and frequency of comorbid conditions often makes posttraumatic stress disorder difficult to treat. Trauma-focused psychotherapy has the strongest evidence for posttraumatic stress disorder treatment.7 Yet, a high percentage of individuals do not engage in or drop out prematurely from these treatments because of chronic patterns of avoidance and an inability to tolerate the intense emotions often experienced with these approaches.8 Avoidance behaviors may maintain posttraumatic stress disorder symptoms by interfering with the processing of traumatic memories and preventing habituation or relearning to conditioned stimuli.9 Selective serotonin and serotonin-norepinephrine reuptake inhibitors also have strong evidence for posttraumatic stress disorder treatment; however, medication refusal and noncompliance are quite high in this population.10 Thus, the evaluation of posttraumatic stress disorder treatments that could be used in conjunction with or as an alternative to existing therapies is warranted.
Complementary and alternative medicines may be beneficial for people with posttraumatic stress disorder. The National Institutes of Health, National Center for Complementary and Alternative Medicine defines complementary and alternative medicine as a group of diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine.11 Complementary and alternative medicine therapies are attractive because they use an integrative approach to healing and usually do not report side effects. Most complementary and alternative medicine modalities engage the healing process without trauma recall, and are thus, not trauma-focused.
Many people with posttraumatic stress disorder currently use complementary and alternative medicine for their symptoms despite a lack of definitive evidence for their benefit. Thirty-eight percent of the 23 393 U.S. adults polled in a 2007 National Health Interview Survey used complementary and alternative medicine. Of these users, 2.8% reported using complementary and alternative medicine for anxiety symptoms or anxiety-related conditions, including posttraumatic stress disorder.12 The most commonly used modalities were natural products, deep breathing, meditation, chiropractic, massage, yoga, diet-based therapies, progressive relaxation, guided imagery, and homeopathic treatment. Another survey of 1004 adults reported that 43% of respondents used complementary and alternative medicine for generalized anxiety disorder, panic disorder, social anxiety disorder, or posttraumatic stress disorder. Acupuncture, meditation/relaxation, biofeedback, chiropractic, massage, prayer, or spiritual practices were the most commonly used modalities, followed by dietary supplement and/or herbal medicine use.13 Additionally, nearly 40% people with posttraumatic stress disorder surveyed within the Veterans Administration used complementary and alternative medicine to address emotional and mental problems.14
Posttraumatic stress disorder is a serious and growing health concern without treatment that is acceptable to all people. Complementary and alternative medicine therapies may offer complementary and alternative therapies to existing treatments and people with posttraumatic stress disorder are already using them for their symptoms. However, the evidence of efficacy of complementary and alternative medicine for posttraumatic stress disorder is limited. To date, 2 systematic reviews have assessed the efficacy of complementary and alternative medicine for anxiety-related disorders, including posttraumatic stress disorder. One meta-analysis found that meditative therapies provided significant improvements in anxiety symptoms compared to controls.15 This study only included 1 complementary and alternative medicine modality and was not specific for posttraumatic stress disorder. Another systematic review found very few complementary and alternative medicine-based randomized controlled trials for posttraumatic stress disorder using stringent inclusion/exclusion criteria and the authors were unable to make any conclusions about efficacy.16
Building on this previous work, the purpose of this systematic review was to assess the state of the science of complementary and alternative medicine for posttraumatic stress disorder. The objectives of this systematic review were to (1) characterize complementary and alternative medicine studies where posttraumatic stress disorder outcomes were assessed, (2) evaluate the quality of these studies, and (3) systematically evaluate the evidence of complementary and alternative medicine for posttraumatic stress disorder symptoms. This study adds to the complementary and alternative medicine and posttraumatic stress disorder field by evaluating the efficacy of complementary and alternative medicine modalities for posttraumatic stress disorder with broader inclusion/exclusion criteria and therefore, describing a wider view of the research literature than previously done.
Methods
Literature Search Methods
Comprehensive searches were conducted by a research librarian using MEDLINE (1950 to March 12, 2013), PsycINFO (1967 to March 12, 2013), CINAHL (1982 to March 12, 2013), Alt HealthWatch (1984 to March 12, 2012), AMED (1980 to March 12, 2013), Cochrane Library: CENTRAL (March 12, 2013), Cochrane Database of Systematic Reviews (March 12, 2013), Database of Abstracts of Reviews of Effects (March 12, 2013), and Health Technology Assessment Database (March 12, 2013). Search terms included complementary and alternative medicine modalities and posttraumatic stress disorder terms dependent on the search strategy required for each database (the search strategy for MEDLINE is included as Supplemental Data). All peer-reviewed studies in any language were included.
Study Eligibility
Two reviewers independently screened titles and abstracts of all publications retrieved by the search strategies. Studies meeting the following inclusion criteria, and those with insufficient information to determine eligibility from the abstract, were selected for further review.
Study Design.
Randomized controlled trials, nonrandomized controlled trials, crossover trials, prospective and retrospective observational studies with controls, case-control studies, and uncontrolled pre-post studies where the sample size was greater or equal to 5. Studies with intention-to-treat or completer analyses were included.
Types of Participants.
Adults diagnosed with posttraumatic stress disorder and/or adult participants who were administered a measure assessing posttraumatic stress disorder symptoms.
Interventions.
Any complementary and alternative medicine modality as described on the National Institutes of Health, National Center for Complementary and Alternative Medicine Web site at the time this review was planned (March 1, 2012),11 including Natural Products (herbal medicines, botanical medicine, botanicals, vitamins, minerals, other ‘‘natural products,’’ dietary supplements, probiotics, fish oil); Mind-Body Medicine (meditation, yoga, deep-breathing exercises, guided imagery, hypnotherapy, progressive relaxation, qi gong, tai chi, biofeedback); Whole Medical Systems (acupuncture or traditional Chinese medicine, homeopathy, naturopathy, ayurvedic medicine); Manipulative and Body-Based Practices (spinal manipulation, chiropractic, osteopathy, massage); Movement Therapies (Feldenkrais method, Alexander technique, pilates, Rolfing Structural Integration, Trager psychophysical integration); Traditional/Spiritual Healing (shamanic healing, curandero); and Energy Medicine (magnet therapy, light therapy, biofields, applied qi gong, Reiki, healing touch, therapeutic touch). The interventions included were based solely on the National Center for Complementary and Alternative Medicine Web site at the time of study design rather than on the presence of a rationale or mechanism for each included complementary and alternative medicine modality and why it may or may not be appropriate for posttraumatic stress disorder. Cognitive behavioral therapy, prolonged exposure therapy, eye movement desensitization and reprocessing, imagery rehearsal and restructuring, journaling, or expressive writing studies were not included because they are used as evidenced-based standard care for posttraumatic stress disorder treatment. If one of these therapies was compared with a complementary and alternative medicine therapy directly, such as eye movement desensitization and reprocessing versus relaxation therapy, the study was included. If the therapy was multimodal, where it included cognitive behavioral therapy or prolonged exposure therapy in addition to some complementary and alternative medicine modalities such as relaxation or creative arts, it was excluded. Repetitive transcranial magnetic stimulation was included as a magnet therapy, and hypnotherapy and biofeedback were included as mind-body medicine therapies.
Outcome Measures.
Each study had to include at least one measure assessing posttraumatic stress disorder symptoms such as intrusion, avoidance, negative alterations in cognitions and mood, and alterations in arousal and reactivity. Outcomes from any version of the Diagnostic and Statistical Manual of Mental Disorders were allowed.
Data Extraction and Management
The following data were collected: study design, number of treatment arms, setting, participant description, inclusion and exclusion criteria, number of subjects, mean age, intervention type and description, attrition rate, home practice details (if any), group or individual practice, outcome, time points at which outcomes were measured, P values for each measure, and adverse events. A single reviewer extracted data and another independent reviewer verified the accuracy and completeness of the data extraction. Any discrepancies were resolved by consensus. All study data were managed with Microsoft Excel and an Access relational database (Microsoft Corporation, Redmond, WA).
Assessment of Methodological Quality
Each study was evaluated for risk of bias and methodological quality. Randomized controlled trials were evaluated with the Cochrane Risk of Bias Tool17 and the Quality Assessment Tool.17,18 Nonrandomized controlled trials studies were assessed for quality with the Quality Assessment Tool only since the Cochrane Risk of Bias Tool is specifically designed for randomized controlled trials. Two reviewers assessed the methodological quality of studies independently. A third reviewer resolved any disagreements through consensus.
Instruments Used to Assess Risk of Bias and Methodological Quality.
| • | The Cochrane Risk of Bias Tool evaluates selection, performance, detection, attrition, reporting and other biases and is the current gold standard for assessing bias in randomized controlled trials. Each criterion are categorized as high risk of bias, unclear risk of bias, or low risk of bias and consider whether the risk of bias is sufficient enough to have a notable impact on the results or conclusions of the trial. A treatment fidelity criterion, which evaluated whether or not the intervention was delivered as designed or that potential intervention deviations were assessed, was added as an ‘‘other bias.’’ | ||||
| • | The Quality Assessment Tool used in this study is modeled after the ‘‘Aid to the Evaluation of Therapeutic Studies’’ developed by Reisch et al18 and was modified as recommended by Deeks et al.17,19 It grades study quality on important constructs such as blinding, randomization, adequate reporting, attrition, sample size determination, and control group usage. A quantitative score is calculated that is adjusted for study design by removing questions about randomization, comparisons between groups, and blinding for nonrandomized controlled trial and uncontrolled trials. The result is an adjusted score on a scale of 0 to 100, 100 being a higher quality study. | ||||
Data Synthesis and Evidence Grading
A meta-analysis for this study was not possible because of substantial variation in participant type, interventions, implementation, and outcomes across studies. Therefore, we sought to provide a general understanding of the available evidence for each modality. First, each study was rated as a positive, mixed, negative or neutral study (positive = most posttraumatic stress disorder outcomes are positive; mixed = only 1 to 2 posttraumatic stress disorder subscales are positive; negative = no posttraumatic stress disorder outcomes are positive; neutral = no difference between intervention and active control). Studies were then grouped by modalities. The level of evidence was then graded for each modality according to the Natural Standard evidence-based grading rationale.20 Letter grades of A, B, C, D, and F reflect the level of scientific evidence in support of a given therapy for posttraumatic stress disorder (A = strong scientific evidence; B = good scientific evidence; C = unclear or conflicting scientific evidence; D = fair negative scientific evidence; F = strong negative scientific evidence; L = lack of evidence). The criteria to designate each grade are described in more detail in Table 1. Grades reflect the level of available scientific data for or against the use of each therapy for a specific medical condition. For example, to receive an A level of evidence a modality had to have statistically significant evidence of benefit from >2 properly conducted randomized controlled trials, OR evidence from one properly conducted randomized controlled trial AND one properly conducted meta-analysis, OR evidence from multiple randomized controlled trials with a clear majority of the properly conducted trials showing statistically significant evidence of benefit AND with supporting evidence in basic science, animal studies, or theory. Natural Standard was founded by health care providers and researchers to provide high-quality, evidence-based information about complementary and alternative medicine therapies.
| Level of Evidence Grade | Criteria |
|---|---|
| A (Strong scientific evidence) | Statistically significant evidence of benefit from >2 properly randomized controlled trials, OR evidence from 1 properly conducted randomized controlled trial AND 1 properly conducted meta-analysis, OR evidence from multiple randomized controlled trials with a clear majority of the properly conducted trials showing statistically significant evidence of benefit AND with supporting evidence in basic science, animal studies, or theory. |
| B (Good scientific evidence) | Statistically significant evidence of benefit from 1 to 2 properly randomized trials, OR evidence of benefit from >1 properly conducted meta-analysis OR evidence of benefit from >1 cohort/case-control/nonrandomized trials AND with supporting evidence in basic science, animal studies, or theory. |
| C (Unclear or conflicting scientific evidence) | Evidence of benefit from >1 small randomized controlled trial(s) without adequate size, power, statistical significance, or quality of design by objective criteria, OR conflicting evidence from multiple RCTs without a clear majority of the properly conducted trials showing evidence of benefit or ineffectiveness, OR evidence of benefit from >1 cohort/case-control/nonrandomized trials AND without supporting evidence in basic science, animal studies, or theory, OR evidence of efficacy only from basic science, animal studies, or theory. |
| D (Fair negative scientific evidence) | Statistically significant negative evidence (ie, lack of evidence of benefit) from cohort/case-control/nonrandomized trials, AND evidence in basic science, animal studies, or theory suggesting a lack of benefit. |
| F (Strong negative scientific evidence) | Statistically significant negative evidence (ie, lack of evidence of benefit) from >1 properly randomized adequately powered trial(s) of high-quality design by objective criteria. |
| Lack of evidence | Unable to evaluate efficacy because of lack of adequate available human data. |
Table 1. Natural Standard Evidence-Based Validated Grading Rationale.
Results
Search Results
A total of 1596 studies were identified (Figure 1). After removing duplicates, 1337 titles and abstracts were screened for inclusion criteria. Ninety full-text articles were assessed for eligibility, and of these, 33 were included in the final review (Table 2).
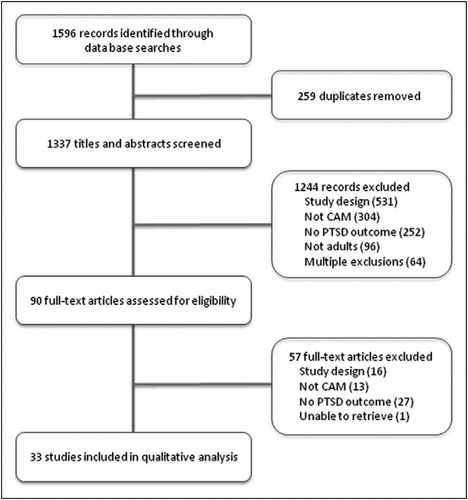
Figure 1. Study flow diagram.
| Study/Design | n | % Dropout or Missing Data | Gender Female: Male | Mean Age, Years ± SD (Range) | Intervention Versus Comparator | PTSD Dx Required | Participant Characteristics | Duration of Intervention | PTSD Outcomes | Results | Overall Study Outcome** | Reisch Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy Medicine | ||||||||||||
| Boggio, 2010/RCT | 30 | 13 | 21:9 | 44.5 ± 4.4 | rTMS 20 Hz to right or left dIPFC vs Sham rTMS | Yes, SCID criteria | Mixed trauma types | 5 sessions (1600 pulses per session)/wk for 2 wk | PCL, TOPS | Right and left rTMS sig dec PTSD outcomes vs sham (PCL P< .001; TOPS P< .001). | Positivea | 92 |
| Cohen, 2004/RCT | 29 | 17 | 7:17* | 41.7 ± 11.4 (22-68) | rTMS 10 Hz or rTMS 1 Hz to right dIPFC vs Sham rTMS | Yes, SCID criteria | Mixed trauma types | Five 20-min sessions/wk for 2 wk | PCL, TOPS, CAPS-Hebrew version | For PCL and TOPS: 10 Hz rTMS sig better than sham and 1 Hz (P < .002). No diff I Hz rTMS vs sham. | Positivea | 85 |
| Grisaru, 1998/Pre-post | 10 | 0 | 3:7 | 47 (21-53) | rTMS 0.3 Hz | Yes, DSM-IV criteria | Mixed trauma types | Single session of 30 pulses at I-min intervals | IES | No sig change in total IES or IES-1; Sig dec IES-A at 7-day followup (P = .033); no sig diff 28-day follow-up. | Mixedc | 71 |
| Osuch, 2009/Crossover | 9 | 0 | 8:1 | 41.4 + 12.3 (24-56) | Imaginal exposure therapy plus rTMS 1 Hz to right dIPFC v. Imaginal exposure therapy plus sham rTMS | Yes, no criteria specified | Mixed trauma types | Three to five 30-min sessions/wk for a total of 20 sessions | CAPS, IES | No sig diff between exposure + active rTMS vs exposure + sham rTMS except for moderate dec CAPS hyperarousal subscale (P = .08). | Mixeda | 70 |
| Watts, 2012/RCT | 20 | 0 | 2:18 | Tx: 54 ± 12.3; Ctrl: 57.8 ±11.8 | rTMS 1 Hz to right dIPFC vs Sham rTMS | Yes, SCID criteria | Veterans with mostly combat trauma | Five 20-min sessions/wk for 2 wk | CAPS, PCL | rTMS sig reduced CAPS (P = .009) and PCL (P = .0002) vs sham rTMS. | Positivea | 80 |
| Chinese Medicine | ||||||||||||
| Hollifield, 2007/RCT | 84 | 27 | 57:27 | Acu: 42.3 ± 12.1; CBT: 40.9 ± 13.4; Ctrl: 43.4 ± 13.5 | Acupuncture vs (1) CBT; (2) 12-wk waitlist control | Yes, SCID criteria | Mixed trauma types | Acupuncture: two I-h sessions/wk for 12 wk; CBT: one 2-h session/wk for 12 wk | PSS-SR | Both acupuncture and CBT dec PTSD outcomes vs waitlist controls (P < .01). No diff between acup and CBT as both groups improved (P = .29). | Neutrala; Positiveb | 88 |
| Zhang, 2011 RCT | 91 | 1 | 55:36 | 35.0 ± 19.3 (4-89) | Acupoint stimulation (50 Hz) + CBT vs CBT | Yes, WHO criteria | Acute PTSD from China’s 2008 Zhejiang Province earthquake | Acupoint and CBT: 30-min session every other day over 1 wk | IES-R Chinese version | Acupoint + CBT more effective reducing IES-R than CBT alone (P < .01). | Positivea | 77 |
| Mind-body: Biofeedback | ||||||||||||
| Lande, 2010/CT | 49 | 20 | 6:33* | Mean/range not reported | HRV Biofeedback vs TAU | No | Active duty combat soldiers with self-report PTSD | Two 20-min sessions/wk for 3 wk | PCL-M | Both biofeedback and control groups experienced sig dec in PTSD symptoms overtime, no sig diff between groups. | Negativeb | 62 |
| Muller, 2009/Pre-post | 13 | 15 | 8:3* | 35.7 ± 6.1 | Pain-focused cognitive behavioral biofeedback | Yes, MINI criteria | Refugees with PTSD, chronic pain, and experience of torture or war | One 90-min session/wk for 10 wk | Posttraumatic Diagnostic Scale | No sig changes in PTSD symptoms over time. | Negativec | 76 |
| Tan, 2011/RCT | 20 | 5 | 0:20 | 36.0 ± 13.1 (24-62) | HRV Biofeedback vs TAU | Yes, no criteria specified | Veterans with combat-related PTSD | One 30-min session/wk for 8 wk | CAPS, PCL | Biofeedback sig dec CAPS (P< .001) and PCL (P= .035) pre-post; Only CAPS-AN better in biofeedback group vs TAU; no other between-group diff but moderate effect sizes for change in overall sx (Cohen’s d= 0.52 and 0.70 for CAPS and PCL, respectively). | Mixedb | 80 |
| Zucker, 2009/RCT | 50 | 24 | 17:21* | (18-60) | RSA Biofeedback vs progressive muscle relaxation recording | No | Substance use disorder and elevated PTSD sx; mixed trauma types | Personal instruction for portable biofeedback device or PMR recording: 20 min/day for 4 wk | PCL, PTS-T | Both groups decreased PTSD scores over time (both groups P < .01). Biofeedback did not improve PCL scores (P = .32) or PTS-T scores (P = 0.73) over control. | Neutrala | 81 |
| Mind-body: Thought field therapies | ||||||||||||
| Folkes, 2002/Pre-post | 61 | 49 | Not reported | 27.7 (5-48) | TFT | No | Adult and child refugees from 5 language groups | One 60- to 90-min session | PCL (adult or child version) | 50% dropout rate, analysis completed on 31 individuals with complete data sets. PCL-C scores dropped 40% from pre- to postintervention (P = .05). | Positivec | 62 |
| Karatzias, 201 l/RCT | 46 | 43 | 26:20 | EFT: 39.7 ± 10.9; EMDR: 41.5 ± 10.8 | EFT vs EMDR | Yes, DSM-IV criteria | Mixed trauma types | Up to eight I-h sessions. EMDR group received 3.7 + 2.3 h, EFT group received 3.8 ± 2.6 h. | CAPS, PCL | 43.5% dropped out from the EMDR group, and 39.1% dropped out from the EFT group. Both EFT and EMDR improved all outcomes (P < .001). Effect size Cohen’s d = 0.80 for both modalities. | Neutrala | 85 |
| Mind-body: Hypnosis | ||||||||||||
| Abramowitz, 2008/RCT | 32 | 0 | 0:32 | 31.7 (21-40) | Hypnosis vs Zolpidem 10 mg | Yes, DSM-IV criteria | Chronic combat-related PTSD with insomnia | Hypnosis: Two 1.5-h sessions/wkfor 2 wk; Zolpidem: 10 mg nightly for 2 wk | IES-R, PDS | Hypnosis group had sig reductions in PDS (P < .034) and IES scores (P < .0005) compared with Zolpidem over the course of the study. | Positivea | 77 |
| Abramowitz, 2010/Pre-post | 37 | 3 | 0:37 | 41.2 ± 12.2 (24-64) | Hypnosis paired with olfactory-based exposure | Yes, semistructured interview with DSM-IV criteria | Combat trauma | One 90-min session/wk for 6 wk | IES-R | Hypnosis technique decreased stress reaction after 6 wk (P < .0001). | Positivec | 81 |
| Bryant, 2006/RCT | 87 | 46 | 53:34 | (17-60) | Hypnosis + CBT vs (1) CBT; (2) Supportive counseling | No | Acute stress disorder from motor vehicle accident or sexual assault | CBT and CBT plus hypnosis: Both groups five 90-min sessions | CAPS, IES | No diff in IES scores among groups. CAPS scores for CBT and CBT hypnosis groups were 43% lower than counseling group at 3-year follow-up (P = .05). | Neutrala | 77 |
| Mind-body: Meditation | ||||||||||||
| Bormann, 2005/Pre-post | 101 | 39 | 6:56* | 61.8 ± 13.2 (33-84) | Mantram meditation | No | Veterans with combat-related trauma | One 90-min instructional session/wk for 5 wk plus home practice | PCL | PTSD scores (only available for n = 30) decreased 13.7% from pre- to postintervention (P = .02). | Positivec | 90 |
| Bormann, 2008/RCT | 33 | 14 | 0:33 | 56 ± 6.6 (40-76) | Mantram meditation vs Waitlist control | Yes, no criteria specified | Veterans with combat-related PTSD | One 90-min session/wk for 6 wk | PCL, CAPS | Intervention improved CAPS score (effect size −0.33) and PCL score (effect size −0.72), no P values provided. | Positiveb | 85 |
| Brooks, 1985/CT | 25 | 28 | 0:18* | 33.3 | Transcendental meditation vs psychotherapy | No | Vietnam veterans with chronic PTSD | Meditation: One 60-min session/wk for 12 wk; Therapy: One 60-min session/wk for 12 wk | Nonstandard PTSD Scale (no reference provided in article) | Meditation showed positive effect compared with psychotherapy for PTSD and related subscales of emotional numbness, anxiety, depression, alcohol use, insomnia, and family problems (all P < .05). | Positivea | 54 |
| Harris, 2011/RCT | 54 | 6 | 6:48 | 45.5 ± 13.5 | Spiritual prayer and/or meditation vs waitlist control | No | Veterans with trauma exposure, mixed trauma types | One 2-h session/wk for 8 wk | PCL | Spiritual prayer/mediation group dec PCL vs waitlist control (P < .02) | Positiveb | 73 |
| Kearney, 2012/Pre-post | 92 | 20 | 22:70 | 51.0 ± 10.6 | Mindfulness-Based Stress Reduction | No | Veterans, 74% screened positive for PTSD at baseline | One 2.5-h session/wk for 8 wk | PCL | MBSR decreased PCL total and all subscores (P < .001). | Positivec | 86 |
| Kimbrough, 2010/Prepost | 27 | 22 | 24:3 | 45 (23-68) | Mindfulness-Based Stress Reduction | No | Adults with history of childhood sexual abuse | One 3-h session/wk for 8 wk, followed by 3 refresher courses | PCL | MBSR decreased PCL total and all subscores at 8 and 24 wk postenrollment (P < .0001). | Positivec | 95 |
| Price, 2005/RCT | 25 | 4 | 25:0 | 41 (median) (26-56) | Mindful Awareness in Body-Oriented Therapy vs massage | No | Adult women currently in therapy for childhood sexual abuse | Two 60-min sessions/wk for 4 wk | Crime-related PTSD Scale | Both body-oriented therapy and regular massage improved PTSD symptoms, no sig diff between the groups (P > .05). | Neutrala | 81 |
| Price, 2006/RCT | 8 | 0 | 8:0 | (28-52) | Mindful Awareness in Body-Oriented Therapy vs waitlist control | No | Adult women currently in therapy for childhood sexual abuse | One 60-min session/wk for 8 wk | Crime-related PTSD Scale | Body-oriented therapy group had sig pre-post improvement in PTSD scale (P < .01), control group did not experience sig improvements. | Positiveb | 72 |
| Rosenthal, 2011/Pre-post | 6 | 17 | 0:6 | (25-40) | Transcendental meditation | PTSD as judged by investigator | OEF/OIF vets with combat-related PTSD | 3 to 5 h of instruction followed by home practice: 20 min twice a day for 12 wk | CAPS, PCL-M | Participants showed sig improved CAPS (P = .02) and PCL-M (P = .02) scores | Positivec | 86 |
| Mind-body: Relaxation | ||||||||||||
| Colosetti, 2000 Cross-over | 5 | 0% | 5:0 | 38.8 (25-50) | Relaxation (control condition) vs EMDR | Yes, CAPS criteria | Incarcerated women with history of abuse in an intimate relationship | One session relaxation training/wk for 3 to 6 wks followed by one session of EMDR/wk for 3 wks | IES | Neither relaxation training or EMDR exhibited sig changes in PTSD outcomes, no statistics provided due to small sample size. | Negativea | 65 |
| Echeburua, 1997/CT | 20 | 0 | 20:0 | 20 ± 7.1 | PMR (control condition) vs gradual self-exposure with cognitive restructuring | Yes, ADIS-R DSM-III criteria | Women with history of sexual abuse | 1 x/wk for 6 wk; home practice 2x/day | Scale of Severity of Posttraumatic Stress Disorder Symptoms | Cognitive restructuring lead to the reversal of DSM-III PTSD diagnosis in 100% of participants while relaxation was only 40% by 12 months. PTSD scale score was 4x lower in the cognitive restructuring group. | Negativea | 60 |
| Mitani, 2006/Pre-post | 22 | 0 | 0:22 | 42.2 ± 9.7 | Relaxation | No | Japanese fire fighters in a select fire station | One 60-min instructional session followed by home practice: 2-3x/wk for 2 mo | IES-R (Japanese version) | Total IES-R scores dec 60% from pre- to post in PTSD stress-related group (P = .04). Intrusion subscale dec sig in the PTSD stress-related group (P = .038); hyperarousal and avoidance did not change sig. No sig changes in the non-PTSD stress-related group IES scores noted (P = .76-1.0). | Mixedc | 68 |
| Taylor, 2003/RCT | 60 | 25 | 45:15 | 37 ± 10 | Relaxation (control condition) vs (1) exposure therapy; (2) EMDR | Yes, DSM-IV criteria | Mixed trauma types | Eight 90-min individualized session of relaxation, exposure, or EMDR therapy | CAPS, PTSD Symptom Severity Scale, PTSD dx | Exposure superiorto relaxation in reducing number who met PTSD dx (P < .02); no sig diff between EMDR and exposure or EMDR and relaxation for this outcome. CAPS and Symptom Severity Scale dec sig in all groups with no difference between groups. | Neutrala | 85 |
| Mind-body: Guided imagery | ||||||||||||
| Jain, 2012/RCT | 123 | 17 | 11:112 | Tx: 27.1 (20-42); Ctrl: 27.9 (20-48) | Healing touch plus guided imagery vs TAU | No | Returning combat-exposed active duty military with sig PTSD sx | Two 60-min sessions/wk for 3 wk | PCL-M | Healing touch/guided imagery group had sig dec in PCL score compared with controls (P < .0005). | Positiveb | 92 |
| Mind-body: Yogic breath work | ||||||||||||
| Descilo, 2010/CT | 183 | 3 | 160:23 | Tx 1: 30.8; Tx 2: 35.1; Ctrl: 34.7 | Yogic breath work-vs (1) Yoga breath work with exposure therapy; (2) 6-wk waitlist control | No | 2004 Southeast Asian tsunami survivors living in refugee camps who scored > 50 on the PCL | Breath work: one 2-h session/day x 4 days. Exposure therapy: as above + 3-5 exposure sessions | PCL | Both treatment groups showed improvement in PCL scores over waitlist control (P < .0001), no diff between active treatments. | Neutrala; Positiveb | 83 |
| Nutraceutical | ||||||||||||
| Kaplan, 1996/Crossover | 17 | 24 | 5:8* | 39.7 (25-56) | Inositol powder 12 g/d vs placebo (glucose powder) 12 g/d | Yes, DSM-III-R criteria | Trauma type not reported | Inositol or placebo daily for 4 wk, 2 wk washout between crossover | lES-Hebrew version | No sig diff between inositol and placebo for total IES scores or avoidance and intrusion subscales, no P values reported. | Negativeb | 73 |
| Shams, 2007/RCT | 40 | 0 | 34:6 | Tx: 38.2 ± 11.2; Ctrl: 38.5 ± 13.7 | Gingko biloba 200 mg vs placebo | Yes, DSM-IV | Earthquake survivors | 12 wk | Watson’s PTSD Scale | Sig improvement in gingko group over control (P < .01). | Positivea | 73 |
Table 2. Characteristics of Included Studies.
Description of Included Studies
All articles were published between 1985 and 2012. Eighteen of the studies were conducted within the past 5 years (2008-2012). There were 17 randomized controlled trials, 4 nonrandomized controlled trials, 9 pre-post designs, and 3 crossover interventions. The mean sample size was 40 ± 38 (range 5-183). In total, 1329 participants were included. Of the controlled trials, 13 used active control groups (either another intervention or placebo intervention), 6 used nonactive controls (waitlist or treatment as usual), and 2 used active and nonactive controls. Eighteen studies confirmed posttraumatic stress disorder diagnosis of participants and 15 did not. Posttraumatic stress disorder diagnosis was confirmed using clinician assessed diagnostic criteria and/or through structured clinical interviews (DSM-III [2]; DSM-IV [6], Clinician-Administered Posttraumatic Stress Disorder Scale [1], Mini International Neuropsychiatric Interview [1], World Health Organization criteria [1]; Structured Clinical Interview DSM-IV [4]) for most but not all studies. Three studies did not note criteria for confirming posttraumatic stress disorder diagnosis. Participants experienced diverse traumatic events: combat exposure (10 studies), natural disaster (3), sexual assault (3), abuse (2), war (2), firefighting (1), mixed traumatic events (11), and 1 study did not report the trauma type. Seven studies had all male participants and four studies had all female participants. The remaining studies had an average of 45% ± 38% females (range 9% to 89%). One study did not report gender data. Most studies used mind-body therapies, including biofeedback (4 studies), hypnosis (3), meditation (9), relaxation (4), Emotional Freedom and Thought Field therapies (2), visualization (1), and yogic breath work (1). Other modalities represented were repetitive transcranial magnetic stimulation (5), acupuncture (2), and natural products—inositol and ginkgo biloba (2). Posttraumatic stress disorder outcomes varied and included one or more of the following: the Posttraumatic Stress Disorder Checklist (civilian and military versions), Clinician-Administered Posttraumatic Stress Disorder Scale, Impact of Events Scale, Crime-Related Posttraumatic Stress Disorder Scale, Posttraumatic Diagnostic Scale, Severity of Symptoms Scale for Posttraumatic Stress Disorder (Spanish adaptation of Posttraumatic Stress Disorder Symptom Scale), Treatment Outcome Posttraumatic Stress Disorder Scale, and the Clinical Global Impressions.
Methodological Quality of Included Studies
Methodological quality for all studies as determined by the Quality Assessment Tool is presented in Table 2. The mean score was mean 78 ± 9 (median 80, range 54-95). Several criteria were met by the majority of studies (at least 31 out of 33): the purpose of the study was stated, outcomes were validated and adequately described, the intervention was reasonable and appropriate to answer questions posed by researchers, intervention protocols were adequately described, participant demographic information was reported, and descriptive measures were identified for all important variables. Five studies reported a power calculation to determine adequate sample size. Blinding of participants or outcome assessors was discussed in 11 of the 24 controlled and cross-over design studies. Thirteen studies reported adverse events.
Table 3 summarizes the risk of bias for the 17 randomized controlled trials included in this review. Several of the studies failed to provide enough detail for adequate assessment; methods of random sequence generation and allocation concealment were particularly poorly reported. The repetitive transcranial magnetic stimulation studies and the nutraceutical studies used a sham or placebo control group that allowed for participant blinding. One study21 assessed treatment credibility as it was perceived by the participants (ie, expectation). Nine of the studies explicitly stated that outcomes assessors were blinded to treatment group. In general, randomized controlled trial sample sizes were small (7 of the 17 randomized controlled trials had n < 33). Three studies performed intention-to-treat analyses.22-24
| Study | Intervention | Random Sequence Generation | Allocation Concealment | Blinding of Participants/Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Fidelity of Intervention Assessed |
|---|---|---|---|---|---|---|---|---|
| Energy modalities | ||||||||
| Boggio, 2010 | rTMS | + | ? | + | + | + | + | N/A |
| Cohen, 2004 | rTMS | ? | ? | + | + | ? | + | N/A |
| Watts, 2012 | rTMS | ? | ? | + | + | + | + | N/A |
| Chinese medicine | ||||||||
| Hollifield, 2007 | Acupuncture | + | + | – | + | + | + | + |
| Zhang, 2011 | Acupoint stimulation | ? | ? | – | – | + | + | – |
| Nutraceutical | ||||||||
| Shams, 2007 | Ginkgo biloba | ? | ? | + | ? | + | + | ? |
| Mind-body modalities | ||||||||
| Tan, 2011 | Biofeedback | ? | ? | – | ? | + | + | + |
| Zucker, 2009 | Biofeedback | + | ? | – | ? | + | + | ? |
| Karatzias, 2011 | EFT | + | ? | – | + | + | + | ? |
| Abramowitz, 2008 | Hypnosis | ? | ? | – | ? | + | + | ? |
| Bryant, 2006 | Hypnosis | + | – | – | + | – | + | + |
| Bormann, 2008 | Meditation | + | ? | – | + | + | + | + |
| Harris, 2011 | Meditation | + | ? | – | ? | + | + | + |
| Price, 2005 | Meditation | ? | ? | – | ? | + | + | + |
| Price, 2006 | Meditation | ? | ? | – | ? | + | + | + |
| Taylor, 2003 | Relaxation | ? | ? | + | + | – | + | + |
| Jain, 2012 | Guided imagery | + | + | – | + | ? | + | ? |
Table 3. Summary of Risk of Bias for Randomized Controlled CAM Trials for PTSD.
Quality of the Body of Evidence for Each Modality
The body of evidence for each modality for posttraumatic stress disorder was reviewed. Letter grades were derived for each modality using the Natural Standard evidence-based grading rationale. Table 1 describes the criterion for each grade. The individual study outcomes that determined the grades are listed in Table 2.
Repetitive Transcranial Magnetic Stimulation.
Five trials (3 randomized controlled trials, 1 pre-post, and 1 crossover) were included. The randomized controlled trials were of generally high quality (Quality Assessment Tool scores ranging from 71 to 88) and all had positive results. Both the pre-post and crossover trials had lower quality ratings and showed mixed result. GRADE A.
Acupuncture.
Two randomized controlled trials, both of which demonstrated significantly improved symptoms over control conditions were included. Hollifield et al23 found that acupuncture was as effective as cognitive behavioral therapy when compared with a waitlist control, and Zhang et al25 found that acupoint stimulation combined with cognitive behavioral therapy was more effective than cognitive behavioral therapy alone. Because these are slightly different modalities, further research is warranted. GRADE B.
Biofeedback.
Four trials (1 randomized controlled trial, 1 nonrandomized controlled trial, and 2 pre-post studies) were included. The controlled trials had high Quality Assessment Tool scores, with either mixed results or no difference from the control group. The other studies had small sample sizes and methodological concerns. GRADE C.
Emotional Freedom Technique/Thought Field Therapy.
The body of evidence for these mind-body techniques includes 1 randomized controlled trial showing no difference between Emotional Freedom Technique and Eye Movement Desensitization and Reprocessing, and 1 pre-post trial with positive results. While the non-inferiority of Emotional Freedom Technique to Eye Movement Desensitization and Reprocessing is an intriguing finding, both trials had significant dropout rates (43% and 49%, respectively), bringing into question the validity of results. GRADE C.
Hypnotherapy.
Three studies (2 randomized controlled trials and 1 pre-post) were included. Both randomized controlled trials had active control groups. In one study, the hypnotherapy group did just as well as the cognitive behavioral therapy group at a 3-year follow-up.22 In the other study, the hypnotherapy group had significantly greater improved outcomes than Zolpidem.26 The Zolpidem study was targeted at improving insomnia for people with posttraumatic stress disorder rather than posttraumatic stress disorder symptoms directly and Zopidem as a hypnotic pharmaceutical would not be expected to improve posttraumatic stress disorder symptoms. The grade remains the same if this study is excluded. GRADE B.
Meditation.
Nine studies were included, 5 randomized controlled trials and 4 pre-post studies. Meditation represents the largest number of complementary and alternative medicine studies for any modality included in this review. All the prepost studies showed positive outcomes and high Quality Assessment Tool scores (all >86). While the randomized controlled trials were of variable quality and sample size, the majority favored meditation over waitlist controls. Several different types of meditation were assessed and heterogeneity complicated the grading of this modality. GRADE B.
Relaxation.
Four studies (2 randomized controlled trials, 1 prepost, and 1 crossover) were included, 3 of which had significant methodological issues and showed mixed or negative results. GRADE C.
Visualization.
One large, high-quality randomized controlled trial was included. This study combined healing touch with guided imagery and demonstrated significant improvements in posttraumatic stress disorder symptoms compared with treatment as usual. GRADE B.
Yoga Breath Work.
One large nonrandomized controlled trial was included demonstrating that yoga breath work alone and in combination with exposure therapy is better than wait list for acute trauma survivors. GRADE C.
Natural Products.
Two randomized controlled trials were included. One small randomized controlled trial showed no effect of inositol compared with placebo. Another small randomized controlled trial showed a positive effect of ginkgo biloba on posttraumatic stress disorder outcomes compared with placebo. GRADE C.
Grades were reassessed for 2 subgroups: (1) studies where posttraumatic stress disorder was required and (2) randomized controlled trials. These subgroup analyses are important because they reflect more stringent inclusion criteria and lend credibility to the application of these modalities under more specific circumstances. When evaluating only those studies where a posttraumatic stress disorder diagnosis was required, all grades remained the same except for 3 modalities. Meditation was reduced from a B to a C because only 1 out of the 9 meditation studies required a posttraumatic stress disorder diagnosis. Guided imagery and yoga breath work were downgraded to ‘‘lack of evidence’’ because neither of these studies required a posttraumatic stress disorder diagnosis. When evaluating only the randomized controlled trials, all grades remained the same except, again, yoga breath work was downgraded to ‘‘lack of evidence’’ because it did not include a randomized controlled trial.
Discussion
The objectives of this review were to systematically characterize and evaluate complementary and alternative medicine studies for posttraumatic stress disorder. We believe that at this relatively young stage of complementary and alternative medicine research it is important to evaluate all the available evidence for a particular modality, and inclusion criteria were deliberately kept broad to capture as many studies as possible. We found 33 complementary and alternative medicine studies that used 10 different modalities to assess posttraumatic stress disorder outcomes. Scientific evidence of benefit for posttraumatic stress was ‘‘strong’’ for repetitive transcranial magnetic stimulation, and ‘‘good’’ for acupuncture, hypnotherapy, meditation, and visualization. Evidence was ‘‘unclear or conflicting’’ for biofeedback, relaxation, Emotional Freedom and Thought Field therapies, yoga breath work, and natural products.
Implications for Research
Studies included in this review were of variable quality. Important aspects of rigorous research design were often not conducted or not reported. In order to improve the quality of the complementary and alternative medicine research field and accurately determine efficacy of complementary and alternative medicine modalities, investigators are encouraged to consider the following when designing studies: (1) choose an appropriate control (ie, active, nonactive, or both) depending on the research question27; (2) assess for expectancy and placebo effects because they play a pivotal role in mind-body studies28,29; (3) blind research staff and participants if possible; (4) randomize participants; (5) clearly define the population being studied; (6) determine an appropriate sample size; and (7) follow standard rigorous clinical design and reporting guidelines.30,31 This will help improve the quality of complementary and alternative medicine studies and thus the quality of evidence.
Overall Completeness and Applicability of Evidence
Studies in this review recruited from a variety of settings (eg, Veteran’s Administration facilities, outpatient clinics, prison) and countries (North America, Asia, Iran, and Israel). Participants were from the general population, combat veterans, firefighters, and sexual abuse survivors. Approximately one third of the studies were specific for combat-related trauma and another one third enrolled participants with a diverse mix of trauma exposure. Studies varied widely with respect to participant gender. Some studies enrolled only men, other studies only women, and several had a diverse mix of male and female participants. All-male studies most frequently targeted combat-related trauma whereas all-female studies more often addressed sexual abuse, thus reflecting gender differences associated with these trauma exposures. While the grades did not distinguish by trauma type or gender, the results lend preliminary support to the acceptability of complementary and alternative medicine for people with a variety of trauma exposures and genders. Additional research and synthesis of evidence is needed to address the efficacy of each modality by trauma exposure and gender.
Implications for Clinical Practice
There is positive evidence of effectiveness for repetitive transcranial magnetic stimulation, acupuncture, hypnotherapy, meditation, and visualization for the treatment of posttraumatic stress disorder symptoms. Repetitive transcranial magnetic stimulation had the strongest scientific evidence followed by acupuncture, hypnotherapy, meditation, and visualization. Practitioners may take this evidence into account when considering these complementary and alternative medicine modalities for treating patients with posttraumatic stress disorder symptoms.
Repetitive transcranial magnetic stimulation is a noninvasive and painless technique that directly stimulates cortical neurons and is approved by the Food and Drug Administration for the treatment of depression.32 Transcranial magnetic stimulation induces significant changes on monoamine neurotransmitters and cortisol, neuroendocrine factors also affected in posttraumatic stress disorder. Future transcranial magnetic stimulation research would clarify dosing relationships to efficacy (ie, frequency used [low or high], area of brain treated, and timing and duration of sessions). Acupuncture is a Chinese medicine energy modality that uses needles inserted into specific points along the body’s energetic meridians. Acupuncture may help posttraumatic stress disorder through its effects on the autonomic nervous system and prefrontal and limbic brain structures, systems that are intrinsically involved in posttraumatic stress disorder pathophysiology.23,33
Meditation also appears to be helpful for posttraumatic stress disorder. While there are various meditation styles, all types incorporate self-observation of mental activity, attention training, and cultivating an attitude that highlights process rather than content.34 Meditation studies show positive benefit for a variety of symptoms related to posttraumatic stress disorder such as depressive symptoms or relapse,35-41 anxiety,40,42-45 suicidal behavior,46 and sleep disturbances.47,48 Meditation may affect posttraumatic stress disorder symptoms through attention training, improving prefrontal cortex activity and autonomic nervous system function, changing thought patterns, increasing emotional acceptance and reducing avoidance, and regulating the hypothalamic-pituitary-adrenal axis.49
Hypnotherapy, another mind-body medicine, is a psychotherapeutic technique based on the hypnotist providing suggestions for changes in sensation, perception, cognition, affect, mood, or behavior.50 Hypnotherapy may allow people with posttraumatic stress disorder to downregulate their autonomic nervous system and thus become more receptive to changes in cognition, mood, or behavior.
Similarly, visualization is designed specifically for the patient’s imagination (mind) to have an effect on a physiological system (body). Visualization is a lived experience that is a dynamic, quasi-real, psychophysiological process.51 Guided imagery is a variation on visualization where another person leads an individual through experiences in the mind to access the physical, emotional and spiritual dimensions that effect physiological change, modulating the individual’s response.52 Both hypnotherapy and visualization/guided imagery could be modified to specifically address the symptoms the person with posttraumatic stress disorder is experiencing.
At this point, the evidence is ‘‘unclear or conflicting’’ for biofeedback, Emotional Freedom and Thought Field Therapies, yoga breath work, relaxation, and natural products. Future studies are warranted to clarify results before practitioners should recommend them specifically for posttraumatic stress disorder symptoms.
Agreement and Disagreements With Other Studies or Reviews
Our work builds on a previous review conducted by Strauss et al16 that found inconclusive evidence for all the complementary and alternative medicine modalities they assessed using very stringent inclusion and exclusion criteria. Similarly, we found inconclusive evidence for some modalities. However, because we included study designs other than randomized controlled trials, participants with posttraumatic stress disorder symptoms (and not just a posttraumatic stress disorder diagnosis), and used a different grading schema, we were able to present a broader view of the state of complementary and alternative medicine research. Our study was also different in that we included repetitive transcranial magnetic stimulation as a complementary and alternative medicine modality, evaluated natural products, and included non-English articles in our search criteria. While our overall search strategy was not as comprehensive, both reviews highlight the importance of improved complementary and alternative medicine clinical trial methods, more rigorous reporting, and the need for more randomized controlled trials in complementary and alternative medicine research.
Limitations
Various limitations must be considered when reviewing these results. Some modalities included in this review may not be considered complementary and alternative medicine modalities (eg, repetitive transcranial magnetic stimulation because of its psychiatry heritage; biofeedback because of its common use in mainstream academic medicine and clinical psychology; and hypnotherapy as a psychosocial treatment). For this study, we used the complementary and alternative medicine definition a priori as explained on the National Center for Complementary and Alternative Medicine Web site11 at the time of designing the study. Those definitions included magnet therapy under the ‘‘Energy Medicine’’ category and hypnotherapy under the ‘‘Mind-Body Medicine’’ category. Repetitive transcranial magnetic stimulation, biofeedback, and hypnotherapy could also be included as complementary because they are not evidence-based standard care treatments for posttraumatic stress disorder, even though they may be considered conventional for other conditions.
Although we included all languages in our search strategy we only found English language articles. We searched only published articles; gray literature resources were not included and hand-searches of relevant bibliographies were not conducted. Publication bias is present when positive trials are more frequently published over negative studies. It is possible that this affected our review as we found 17 positive trials and 5 negative trials (7 neutral and 4 mixed). Another limitation is that we did not take into account outcome measure timing in our grading schema. For example, we were not able to distinguish if each modality had more or less evidence for posttraumatic stress disorder symptoms immediately after the treatment versus at a longer term follow-up. Additional studies with less heterogeneity in methods, participants and outcomes need to be conducted before rigorous meta-analyses can be done. Because of this, the results from this study must be viewed as qualitative trends rather than conclusions.
Conclusions
Several complementary and alternative medicine modalities may be helpful for improving posttraumatic stress disorder symptoms. Repetitive transcranial magnetic stimulation has the strongest evidence for benefit followed by acupuncture, hypnotherapy, meditation, and visualization. There is insufficient evidence to recommend biofeedback, Emotional Freedom and Thought Field Therapies, relaxation, yoga breath work, and natural products at this time. Future research should include larger, properly randomized, controlled trials with appropriately selected control groups and rigorous methodology.
. (2008). Hypnotherapy in the treatment of chronic combat-related PTSD patients suffering from insomnia: A randomized, zolpidem-controlled clinical trial. International Journal of Clinical & Experimental Hypnosis, 56(3), 270–280.Crossref, Google Scholar
. (2010). A new hypnotic technique for treating combat-related posttraumatic stress disorder: A prospective open study. International Journal of Clinical & Experimental Hypnosis, 58(3), 316–328.Crossref, Google Scholar
. (2010). Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. Journal of Clinical Psychiatry, 71(8), 992–999.Crossref, Google Scholar
. (2005). Efficacy of frequent mantram repetition on stress, quality of life, and spiritual well-being in veterans: a pilot study. Journal of Holistic Nursing, 23(4), 395-414.Crossref, Google Scholar
. (2008). A spiritually based group intervention for combat veterans with posttraumatic stress disorder: Feasibility study. Journal of Holistic Nursing, 26(2), 109–116.Crossref, Google Scholar
. (1985). Transcendental Meditation in the treatment of post-Vietnam adjustment. Journal of Counseling & Development, 64(3), 212–215.Crossref, Google Scholar
. (2006). Hypnotherapy and cognitive behaviour therapy of acute stress disorder: a 3-year follow-up. Behaviour Research and Therapy, 44(9), 1331–1335.Crossref, Google Scholar
. (2004). Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: A double-blind, placebo-controlled study. American Journal of Psychiatry, 161(3), 515–524.Crossref, Google Scholar
. (2000). The relative effectiveness of EMDR versus relaxation training with battered women prisoners. Behavior Modification, 24(5), 719–739.Crossref, Google Scholar
. (2010). Effects of a yoga breath intervention alone and in combination with an exposure therapy for post-traumatic stress disorder and depression in survivors of the 2004 South-East Asia tsunami. Acta Psychiatrica Scandinavica, 121(4), 289–300.Crossref, Google Scholar
. (1997). Psychological treatment of chronic posttraumatic stress disorder in victims of sexual aggression. Behavior Modification, 21(4), 433–456.Crossref, Google Scholar
. (2002). Thought field therapy and trauma recovery. International Journal of Emergency Mental Health, 4(2), 99–103.Google Scholar
. (1998). Effect of transcranial magnetic stimulation in posttraumatic stress disorder: A preliminary study. Biological Psychiatry, 44(1), 52–55.Crossref, Google Scholar
. (2011). The effectiveness of a trauma-focused spiritually integrated intervention for veterans exposed to trauma. Journal of Clinical Psychology, 67(4), 425–438.Crossref, Google Scholar
. (2007). Acupuncture for posttraumatic stress disorder: a randomized controlled pilot trial. Journal of Nervous & Mental Disease, 195(6), 504–513.Crossref, Google Scholar
. (2012). Healing touch with guided imagery for PTSD in returning active duty military: A randomized controlled trial. Military Medicine, 177(9), 1015–1021.Crossref, Google Scholar
. (1996). Inositol treatment of post-traumatic stress disorder. Anxiety, 2(1), 51–52.Crossref, Google Scholar
. (2011). A controlled comparison of the effectiveness and efficiency of two psychological therapies for posttraumatic stress disorder: eye movement desensitization and reprocessing vs. emotional freedom techniques. Journal of Nervous & Mental Disease, 199(6), 372–378.Crossref, Google Scholar
. (2012). Association of participation in a mindfulness program with measures of PTSD, depression and quality of life in a veteran sample. Journal of Clinical Psychology, 68(1), 101–116.Crossref, Google Scholar
. (2010). Mindfulness intervention for child abuse survivors. Journal of Clinical Psychology, 66(1), 17–33.Google Scholar
. (2010). Efficacy of biofeedback for post-traumatic stress disorder. Complementary Therapies in Medicine, 18(6), 256–259.Crossref, Google Scholar
. (2006). Effect of autogenic training on cardiac autonomic nervous activity in high-risk fire service workers for posttraumatic stress disorder. Journal of Psychosomatic Research, 60(5), 439–444.Crossref, Google Scholar
. (2009). Biofeedback for pain management in traumatised refugees. Cognitive Behaviour Therapy, 38(3), 184–190.Crossref, Google Scholar
. (2009). Repetitive TMS combined with exposure therapy for PTSD: A preliminary study. Journal of Anxiety Disorders, 23(1), 54–59.Crossref, Google Scholar
. (2005). Body-oriented therapy in recovery from child sexual abuse: an efficacy study. Alternative Therapies in Health & Medicine, 11(5), 46–57.Google Scholar
. (2006). Body-oriented therapy in sexual abuse recovery: A pilot-test comparison. Journal of Bodywork and Movement Therapies, 10, 58–64.Crossref, Google Scholar
. (2011). Effects of transcendental meditation in veterans of Operation Enduring Freedom and Operation Iraqi Freedom with posttraumatic stress disorder: a pilot study. Military Medicine, 176(6), 626–630.Crossref, Google Scholar
. (2011). Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): A pilot study. Applied Psychophysiology & Biofeed-back, 36(1), 27–35.Crossref, Google Scholar
. (2003). Comparative efficacy, speed, and adverse effects of three PTSD treatments: exposure therapy, EMDR, and relaxation training. Journal of Consulting & Clinical Psychology, 71(2), 330–338.Crossref, Google Scholar
. (2012). A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimulation, 5(1), 38–43.Crossref, Google Scholar
. (2011). Clinical study on treatment of the earthquake-caused post-traumatic stress disorder by cognitive-behavior therapy and acupoint stimulation. Journal of Traditional Chinese Medicine, 31(1), 60–63.Crossref, Google Scholar
. (2009). The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: a pilot study. Applied Psychophysiology & Biofeedback, 34(2), 135–143.Crossref, Google Scholar
1 . Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005; 62:617–627.Crossref, Google Scholar
2 . The cost of treating anxiety: the medical and demographic correlates that impact total medical costs. Depress Anxiety. 2005; 21:178–184.Crossref, Google Scholar
3 . Psychoneuroimmunology and psychosomatic medicine: back to the future. Psychosom Med. 2002; 64:15–28.Crossref, Google Scholar
4 . Trauma: prevalence, impairment, service use, and cost. J Clin Psychiatry. 1997; 58(suppl 9):5–11.Google Scholar
5 . Posttraumatic stress disorder: diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology. 2001; 43:150–162.Crossref, Google Scholar
6
7 . Treatment of PTSD: An Assessment of the Evidence. Washington, DC: National Academies Press; 2007.Google Scholar
8 . Imagery rescripting and reprocessing therapy after failed prolonged exposure for post-traumatic stress disorder following industrial injury. J Behav Ther Exp Psychiatry. 2007; 38:317–328.Crossref, Google Scholar
9 . Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences. New York, NY: Oxford University Press; 2007.Google Scholar
10 . Conceptualizing treatment nonadherence in patients with bipolar disorder and PTSD. CNS Spectr. 2011; 16:11–20.Crossref, Google Scholar
11
12 . Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Rep. 2008; (12):1–23.Google Scholar
13 . Use of complementary and alternative medicine in a large sample of anxiety patients. Psychosomatics. 2012; 53:266–272.Crossref, Google Scholar
14 . Complementary and alternative medicine in VA specialized PTSD treatment programs. Psychiatr Serv. 2012; 63:1134–1136.Crossref, Google Scholar
15 . Meditative therapies for reducing anxiety: a systematic review and meta-analysis of randomized controlled trials. Depress Anxiety. 2012; 29:545–562.Crossref, Google Scholar
16 . Efficacy of Complementary and Alternative Medicine Therapies for Posttraumatic Stress Disorder. Washington, DC: Department of Veterans Affairs; 2011.Google Scholar
17 . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed.). 2011; 343:d5928.Crossref, Google Scholar
18 . Aid to the evaluation of therapeutic studies. Pediatrics. 1989; 84:815–827.Google Scholar
19 . Evaluating non-randomised intervention studies. Health Technol Assess. 2003; 7(27):iii-x, 1-173.Crossref, Google Scholar
20
21 . Comparative efficacy, speed, and adverse effects of three PTSD treatments: exposure therapy, EMDR, and relaxation training. J Consult Clin Psychol. 2003; 71:330–338.Crossref, Google Scholar
22 . Hypnotherapy and cognitive behaviour therapy of acute stress disorder: a 3-year follow-up. Behav Res Ther. 2006; 44:1331–1335.Crossref, Google Scholar
23 . Acupuncture for posttraumatic stress disorder: a randomized controlled pilot trial. J Nerv Ment Dis. 2007; 195:504–513.Crossref, Google Scholar
24 . A controlled comparison of the effectiveness and efficiency of two psychological therapies for posttraumatic stress disorder: eye movement desensitization and reprocessing vs. emotional freedom techniques. J Nerv Ment Dis. 2011; 199:372–378.Crossref, Google Scholar
25 . Clinical study on treatment of the earthquake-caused post-traumatic stress disorder by cognitive-behavior therapy and acupoint stimulation. J Tradit Chin Med. 2011; 31:60–63.Crossref, Google Scholar
26 . Hypnotherapy in the treatment of chronic combat-related PTSD patients suffering from insomnia: a randomized, zolpidem-controlled clinical trial. Int J Clin Exp Hypnosis. 2008; 56:270–280.Crossref, Google Scholar
27 . Methodological challenges in meditation research. Adv Mind Body Med. 2005; 21:4–11.Google Scholar
28 . Randomized, controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern Ther Health Med. 2006; 12:40–47.Google Scholar
29 . The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review. Health Technol Assess. 1999; 3:1–96.Crossref, Google Scholar
30 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010; 7(3):e1000251.Crossref, Google Scholar
31 . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008; 148:295–309.Crossref, Google Scholar
32 , Van den Eynde F, JeffDaskalakis Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013; 38:543–551.Crossref, Google Scholar
33 . Acupuncture for posttraumatic stress disorder: conceptual, clinical, and biological data support further research. CNS Neurosci Ther. 2011; 17:769–779.Crossref, Google Scholar
34 . Meditation practices for health: state of the research. Evid Rep Technol Assess (Full Rep). 2007; (155):1–263.Google Scholar
35 . Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004; 57:35–43.Crossref, Google Scholar
36 . Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004; 72:31–40.Crossref, Google Scholar
37 . A qualitative study of mindfulness-based cognitive therapy for depression. Br J Med Psychol. 2001; 74(pt 2): 197–212.Crossref, Google Scholar
38 . Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000; 68:615–623.Crossref, Google Scholar
39 . Treatment-resistant depressed patients show a good response to mindfulness-based cognitive therapy. Behav Res Ther. 2007; 45:617–625.Crossref, Google Scholar
40 . An exploratory mixed methods study of the acceptability and effectiveness of Mindfulness-Based Cognitive Therapy for patients with active depression and anxiety in primary care. BMC Psychiatry. 2006; 6:14.Crossref, Google Scholar
41 . Mindfulness meditation alleviates depressive symptoms in women with fibromyalgia: results of a randomized clinical trial. Arthritis Rheum. 2007; 57: 77–85.Crossref, Google Scholar
42 . Mindfulness meditation, anxiety reduction, and heart disease: a pilot study. Fam Community Health. 2003; 26:25–33.Crossref, Google Scholar
43 . Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiatry. 1995; 17:192–200.Crossref, Google Scholar
44 . Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992; 149:936–943.Crossref, Google Scholar
45 . Emotional-vulnerability and mindfulness: a preliminary test of associations among negative affectivity, anxiety sensitivity, and mindfulness skills. Cogn Behav Ther. 2007; 36:91–101.Crossref, Google Scholar
46 . Mindfulness-based cognitive therapy for prevention of recurrence of suicidal behavior. J Clin Psychol. 2006; 62:201–210.Crossref, Google Scholar
47 . The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbance in women with breast cancer: an exploratory study. J Psychosom Res. 2003; 54:85–91.Crossref, Google Scholar
48 . Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int J Behav Med. 2005; 12:278–285.Crossref, Google Scholar
49 . Mindfulness meditation for posttraumatic stress disorder. In: Ie A, Ngnoumen CT, Langer E, eds. The Wiley Blackwell Handbook of Mindfulness. 1st ed. Chichester, England: Wiley; 2014:776–793.Crossref, Google Scholar
50 . Hypnosis for cancer care: over 200 years young. CA Cancer J Clin. 2013; 63:31–44.Crossref, Google Scholar
51 . Effects of guided imagery on outcomes of pain, functional status, and self-efficacy in persons diagnosed with fibromyalgia. J Altern Complement Med. 2006; 12:23–30.Crossref, Google Scholar
52 . Imagery in Healing Shamanism and Modern Medicine. Boston, MA: Shambhala; 1985.Google Scholar



