The Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder in Adults—An Update for 2012: Practice Parameters with an Evidence-Based Systematic Review and Meta-Analyses
Abstract
A systematic literature review and meta-analyses (where appropriate) were performed to update the previous AASM practice parameters on the treatments, both dopaminergic and other, of RLS and PLMD. A considerable amount of literature has been published since these previous reviews were performed, necessitating an update of the corresponding practice parameters. Therapies with a STANDARD level of recommendation include pramipexole and ropinirole. Therapies with a GUIDELINE level of recommendation include levodopa with dopa decarboxylase inhibitor, opioids, gabapentin enacarbil, and cabergoline (which has additional caveats for use). Therapies with an OPTION level of recommendation include carbamazepine, gabapentin, pregabalin, clonidine, and for patients with low ferritin levels, iron supplementation. The committee recommends a STANDARD AGAINST the use of pergolide because of the risks of heart valve damage. Therapies for RLS secondary to ESRD, neuropathy, and superficial venous insufficiency are discussed. Lastly, therapies for PLMD are reviewed. However, it should be mentioned that because PLMD therapy typically mimics RLS therapy, the primary focus of this review is therapy for idiopathic RLS.
(Reprinted with permission from SLEEP 2012; 35(8):1039–1062)
1.0 Introduction
The purpose of this review is to survey and provide an evidence-based update of the literature and corresponding practice parameters in the area of the treatment of restless legs syndrome (RLS) and periodic limb movement disorder (PLMD). Two previous reviews have been published by the American Academy of Sleep Medicine (AASM): the first was in 1999 and called “The Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder,”2 and the most recent was published in 2004 called “An Update on the Dopaminergic Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder.”3 Two practice parameters have also been published: “Practice Parameters for the Dopaminergic Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder”4 and “Practice Parameters for the Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder.”5
2.0 Background
2.1 Diagnosis
Most studies published after 2003 reference either the ICSD-26 or the International RLS Study Group (IRLS)7 diagnostic criteria. The four cardinal diagnostic features of RLS include (1) an urge to move the limbs that is usually associated with paresthesias or dysesthesias, (2) symptoms that start or become worse with rest, (3) at least partial relief of symptoms with physical activity, and (4) worsening of symptoms in the evening or at night. RLS frequently also has a primary motor symptom that is characterized by the occurrence of periodic leg movements in sleep (PLMS). PLMS occur in approximately 80% to 90% of patients who have RLS and support the diagnosis of RLS. These criteria are based on the published report by Allen et al.7 (IRLS) from a workshop held at the National Institutes of Health and are endorsed by the ICSD-2.
PLMD is characterized by periodic episodes of repetitive limb movements during sleep, which most often occur in the lower extremities, including the toes, ankles, knees, and hips, and occasionally in the upper extremities. These movements may be associated with an arousal, and if so, sleep disruption can cause excessive daytime sleepiness. PLMD is thought to be rare as PLMS are typically associated with RLS, REM sleep behavior disorder (RBD), or narcolepsy and represent a distinct diagnosis from PLMD.6 It should be noted that while an extensive amount of literature on the treatment of RLS has emerged since the prior practice parameter update, the data on therapy for PLMD has essentially remained unchanged. Due to the scarcity of PLMD therapy data and the fact that the occurrence of only PLMD is uncommon, the current practice parameter primarily focuses on the therapies for RLS, while recommendation levels are not given for pharmacological therapies for PLMD.
2.2 Treatment Efficacy Measures
Due to the multifaceted nature of RLS, many different treatment efficacy measures have been used to assess RLS severity, sleep quality, and quality of life, both subjectively and objectively. There is some consensus in recent studies to focus on the IRLS rating scale8 (IRLS) and the Clinical Global Impression (CGI) scale.9 Both of these are subjective rating scales. The IRLS was validated in 2003.8 It consists of a 10-question assessment of RLS in a format of 0 to 4, 0 being “never” or “none,” and 4 being “very severe” or “very often.” The severity of RLS is rated as: 1-10 mild; 11-20 moderate; 21-30 severe; and 31-40 very severe. The CGI has 3 sections: (1) Severity of illness; (2) Global improvement (CGII) or change (CGIC), and (3) Efficacy index. Most, if not all, studies document the proportion of patients with an investigator-rated score of “much improved” (2) or “very much improved” (1) on the CGI-I (or –C) scale (defined as a “response” on this 7-point overall global improvement scale, a non-disease specific outcome measure in which 1 = very much improved and 7 = very much worse).
Other subjective measures include the RLS-6, which was used typically prior to 2003, the Patient Global Impression (PGI), the Sleep Questionnaire Form A, Quality of Life (QoL) for RLS, the Augmentation Severity Rating Scale (ASRS), Visual Analog Scales (VAS), and the Medical Outcomes Study sleep scale (MOS). A variety of other scales have been used occasionally such as the Self-Rating Zung Depression Scale (SDS) and Anxiety Scale (SAS), the SF-36 (MOS short form health survey), the work productivity and activity impairment (WPAI) survey, the Pittsburgh Sleep Quality Index (PSQI), the Hospital Anxiety and Depression Scale (HADS), subjective sleep and awakening quality scale (SSA), and the Epworth Sleepiness Scale (ESS).
The only objective measurements included are sleep-related parameters by polysomnography (PSG) or actigraphy. The most salient include Periodic Limb Movements in Sleep (PLMS), PLM index (PLMI), PLMs arousal index (PLMS-AI), and sleep efficiency.
3.0 Methods
3.1 Literature Search
The literature search was performed using a combination of MeSH terms and keywords. The MeSH terms were Restless Legs Syndrome and Nocturnal Myoclonus Syndrome. The keywords were: restless legs syndrome, periodic limb movement disorder, PLMD, sleep-related movement disorder(s), leg motor activity, myoclonic hyperkinesias, nocturnal myoclonus syndrome, RLS, periodic leg movement(s), periodic limb movement(s), sleep leg movement(s), and PLM. All therapies were searched with a start date of 11-1-1997 (6 months prior to previous search). Results on dopaminergic treatments between 11-1-97 to 11-1-2001 already covered in the 2004 update were excluded. The Cochrane Highly Sensitive Search Strategy10 for identifying randomized trials in MEDLINE was applied to the search. The search was performed first on August 12, 2010, and updated again on June 29, 2011, to capture the latest literature. The limits of the search were: humans, English, all adults (no pediatrics), randomized controlled trials (RCTs), and no editorials, letters, comments, or case reports. Studies on treatments for RLS with fewer than 10 subjects completing the study and for treatments of PLMD with fewer than 5 subjects completing the study were rejected. Also, studies with less than 1 week of treatment time were rejected. A total of 378 hits were obtained and supplemented by pearling. The final number of articles included for all treatments with either benefit/efficacy or harm data is 126.
3.2 PICO Questions
PICO (Population, Intervention, Comparison, Outcome) questions were developed for the review, and are summarized in Table 1.
| Population | Intervention | Comparison | Outcome |
|---|---|---|---|
| Adults diagnosed with RLS using the ICSD-2 or the International RLS Study Group (IRLS) diagnostic criteria | Pramipexole | Control group, those with untreated RLS, or those with RLS using an alternate treatment | Subjective measures: |
| Ropinirole | 1. IRLS rating scale | ||
| Levodopa | 2. Clinical Global Impression (CGI) Scale | ||
| Pergolide | 3. RLS-6 | ||
| Cabergoline | 4. Patient Global Impression (PGI) | ||
| Opioids | 5. Sleep Questionnaire Form A | ||
| Gabapentin Enacarbil | 6. Quality of Life (QoL) for RLS | ||
| Gabapentin | 7. Augmentation Severity Rating Scale (ASRS) | ||
| Pregabalin | 8. Visual Analog Scales (VAS) | ||
| Carbamazepine | 9. Medical Outcomes Study Sleep Scale (MOS) | ||
| Clonidine | 10. Self-Rating Zung Depression Scale (SDS) | ||
| Iron supplementation | 11. Anxiety Scale (SAS) | ||
| Rotigotine | 12. SF-36 | ||
| Lisuride | 13. Work productivity and activity impairment (WPAI) | ||
| Amantadine | 14. Pittsburgh Sleep Quality Index (PSQI) | ||
| Talipexole | 15. Hospital Anxiety and Depression Scale (HADS) | ||
| Peribedil | 16. Subjective sleep and awakening quality scale (SSA) | ||
| Alpha-dihydroergocryptine | 17. Epworth Sleepiness Scale (ESS) | ||
| Clonazepam | Objective measures: | ||
| Valproic acid | 1. Sleep-related parameters by polysomnography | ||
| Valerian | a. PLMS | ||
| Antidepressants | b. PLMI | ||
| c. PLMS-AI | |||
| d. Sleep efficiency | |||
| e. TST | |||
| f. % TIB without leg movements | |||
| g. PLMWI | |||
| 2. Sleep-related parameters by actigraphy | |||
| a. Leg movements | |||
| b. Sleep efficiency |
3.3 Meta-Analysis
To compare the range of treatment options available for RLS and PLMD, one outcome measure was chosen for which the majority of studies presented data: the International Restless Legs Syndrome Rating Scale (IRLS). Data on other outcomes measures besides IRLS are summarized and presented in a descriptive manner for further information for the reader. Thus for medications that were studied prior to the development of the IRLS, meta-analysis was not performed. All meta-analyses were performed using MIX software.11,12 All analyses are presented using the random effects model.
The result of each meta-analysis is shown in a figure with several components. Each study of the meta-analysis is identified along the left-hand column (study ID), and adjacent to it is the year of the study, treatment (exposed, “e”) results, and control (“c”) results. The results are expressed as “n/M/SD” corresponding to “number/mean/standard deviation.” A graphical representation of the data is shown in the center of the figure. The vertical red line indicates the average response of all studies. The zero line represents no effect. The width of the red diamond at the bottom of the plot represents the standard deviation of the meta-analysis. If the red diamond does not touch the zero line, the meta-analysis results indicate that the treatment is different from zero (i.e., it has an effect). The magnitude of the effect across all studies is given by the value of the association measure along with the 95% confidence intervals.
Tables of the data used in the meta-analyses are presented in the online Appendix.
3.4 Quality of Evidence
The assessment of evidence quality was performed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) process. The GRADE system differs from other grading systems as each study is not only evaluated for study design and risk of bias, but, additionally, an estimate of effect (see footnote following article) is generated for each outcome. Multiple aspects of quality are assessed including study limitations, imprecision, inconsistency of results, indirectness of evidence, and likeliness of publication bias. The quality of evidence from observational studies can be adjusted by the presence of large magnitudes of effect, evidence of dose-response associations, and all plausible confounders that increase the confidence in the estimated effects.13 Quality refers to the confidence that the estimates of the effects are correct, and the quality rating is applied to a body of evidence and not to individual studies.1
Briefly, risk of bias includes aspects of study design (randomized control trials [RCTs] versus non-randomized controlled trials or before-after trials)14 and conduct such as blinding, allocation concealment, large loss to follow-up, or selective outcome reporting.15 Imprecision refers to wide confidence intervals around the estimate of effect when there are relatively few patients and few events. Indirectness occurs when the question being addressed is different than the available evidence regarding population, intervention, comparator, or outcome. There is inconsistency when there is unexplained heterogeneity of the results. Reporting bias can occur if there is selective reporting of studies or outcomes, which may occur if the published evidence is limited to a small number of trials funded by a for-profit organization.15
As a first step, all individual studies were assessed by 2 task force members for study design, and limitations to validity (bias) for each outcome of interest.16,17 Randomized control trials (RCTs) were considered a higher level of evidence than observational, nonrandomized, or before-after interventional studies (Table 2). Subsequently, the body of evidence for each outcome was assessed and graded, taking into account the results of the meta-analysis (if applicable) and other factors as described above. The final assessment, as defined in Box 1, was determined for each treatment and outcome measure.
| Study design | Initial quality of a body of evidence | Lower if | Higher if | Quality of a body of evidence |
|---|---|---|---|---|
| Radomized trials | High → | Risk of bias | Large effect | High (four plus:⊕⊕⊕⊕) |
| −1 Serious | +1 Large | |||
| −2 Very serious | +2 Very large | |||
| Inconsistency | Dose response | Moderate (three plus:⊕⊕⊕○) | ||
| −1 Serious | +1 Evidence of a gradient | |||
| −2 Very serious | All plausible residual confounding | |||
| Observational studies | Low → | Indirectness | +1 Would reduce a demonstrated effect | Low (two plus:⊕⊕○○) |
| −1 Serious | +1 Would suggest a spurious effect if no effect was observed | |||
| −2 Very serious | ||||
| Imprecision | Very Low (one plus:⊕○○○) | |||
| −1 Serious | ||||
| −2 Very serious | ||||
| Publication bias | ||||
| −1 Likely | ||||
| −2 Very likely |
Box 1—Final assessments of level of bodies of evidence1
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
The results are reported as evidence profiles in each section that include the number of studies, study design, limitations, inconsistency, indirectness, imprecision, and other considerations that went into the quality of evidence for each outcome of interest. Also reported are the number of patients that were studied, the overall effect that was calculated in the meta-analysis (reported as the mean difference [MD]), and a qualitative assessment of the relative importance of the outcome.
One reviewer extracted the data and graded the studies and another verified this compiled information. The systematic review of the evidence was additionally reviewed by an outside expert who was an author on both previous review papers (2004 and 1999). The AASM Standards of Practice Committee (SPC) then reviewed the assessments of bodies of evidence as well.
3.5 Strength of Recommendations
The SPC developed these practice parameters based on the strength of evidence for efficacy of each therapy counterbalanced by an assessment of the relative benefits of each treatment versus the potential risks as delineated in Table 3. The Board of Directors of the AASM subsequently approved these practice parameters. All members of the AASM SPC and Board of Directors completed detailed conflict-of-interest statements and were found to have no conflicts of interest with regard to this subject. The recommendations were also critically reviewed by an outside expert, and the concerns that were raised were addressed by the SPC prior to approval by the Board.
| Overall quality of evidence | |||||
|---|---|---|---|---|---|
| High | Moderate | Low | Very Low | ||
| Assessment of benefit/harm/burden | Benefits clearly outweigh harm/burden | Standard | Standard | Guideline | Option |
| Benefits closely balanced with harm/burden OR uncertainty in the estimates of benefit/harm/burden | Guideline | Guideline | Option | Option | |
| Harm/burden clearly outweighs benefits | Standard | Standard | Standard | Standard | |
These practice parameters define principles of practice that should meet the needs of most patients in most situations. These guidelines should not, however, be considered inclusive of all proper methods of care or exclusive of other methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding propriety of any specific care must be made by the physician, in light of the individual circumstances presented by the patient, available diagnostic tools, accessible treatment options, and resources.
The AASM expects these guidelines to have an impact on professional behavior, patient outcomes, and, possibly, health care costs. These practice parameters reflect the state of knowledge at the time of publication and will be reviewed, updated, and revised as new information becomes available. Definitions of levels of recommendations used by the AASM appear in Table 3. Particularly noteworthy on this table is that when harm/burden clearly outweighs benefit, a STANDARD level of recommendation against the proposed therapy is given regardless of the overall quality of evidence. Sections titled “Values and Trade-offs” appear under each individual practice parameter. The Values and Trade-offs discussion elucidates the rationale leading to each recommendation. These sections are an integral part of the GRADE system and offer transparency to the process.18
4.0 Recommendations for Therapies for RLS
The salient detailed data from the studies was extracted and can be found in evidence tables, available at http://www.aasmnet.org/practiceguidelines.aspx. Table 4 shows a summary of the recommendation statements organized by strength of recommendation, including the body of evidence level, the assessment of the harm/benefit balance and the FDA status of the intervention.
| Practice Parameter | Strength of Recommendation | Body of Evidence Level | Harm/burden Assessment | FDA status |
|---|---|---|---|---|
| Standards for use in RLS | ||||
| Clinicians should treat patients with RLS with pramipexole. | (STANDARD) | High | Benefits clearly outweigh harms | Approved for indication |
| Clinicians should treat patients with RLS with ropinirole. | (STANDARD) | High | Benefits clearly outweigh harms | Approved for indication |
| Standards against use in RLS | ||||
| Clinicians should not treat RLS patients with pergolide because of the risks of heart valve damage. | (STANDARD) | High | Harms clearly outweigh benefits | Discontinued |
| Guidelines for use in RLS | ||||
| Clinicians can treat RLS patients with levodopa with dopa decarboxylase inhibitor. | (GUIDELINE) | High | Benefits closely balanced with harms. This is particularly true for those with intermittent RLS who use this medication sporadically. | Approved, but off-label use |
| Clinicians can treat RLS patients with opioids. | (GUIDELINE) | Low | Benefits clearly outweigh harms | Approved, but off-label use |
| Clinicians can treat patients with RLS with gabapentin enacarbil. | (GUIDELINE) | High | Uncertainty in balance between benefits and harms | Approved for indication |
| Given the potential of side effects, including heart valve damage, clinicians can treat RLS patients with cabergoline only if other recommended agents have been tried first and failed, and close clinical follow-up is provided. | (GUIDELINE) | High | Benefits closely balanced with harms | Approved, but off-label use |
| Options for use in RLS | ||||
| Clinicians may treat RLS patients with gabapentin. | (OPTION) | Low | Unclear benefit/harm balance | Approved, but off-label use |
| Clinicians may treat patients with RLS with pregabalin. | (OPTION) | Low | Benefits closely balanced with harms | Approved, but off-label use |
| Clinicians may treat RLS patients with carbamazepine. | (OPTION) | Low | Benefits closely balanced with harms | Approved, but off-label use |
| Clinicians may treat RLS patients with clonidine. | (OPTION) | Low | Unclear benefit/harm balance | Approved, but off-label use |
| Clinicians may use supplemental iron to treat RLS patients with low ferritin levels. | (OPTION) | Very Low | Unclear benefit/harm balance | Approved, but off-label use |
| PLMD | ||||
| There is insufficient evidence at present to evaluate the use of pharmacological therapy in patients diagnosed with PLMD alone. | (NO RECOMMENDATION) | Insufficient | N/A | N/A |
4.1 Introduction to Therapies for RLS
There are 2 types of therapies for RLS: pharmacotherapy and non-pharmacotherapy. The use of pharmacotherapy has been more widespread. Newer non-pharmacotherapies, such as cognitive behavioral therapy or exercise therapy, are still being investigated.
One interesting recent study has highlighted the importance of the placebo effect in RLS studies. Fulda and Wetter19 performed a meta-analysis on the treatment of RLS and estimated the magnitude of this effect at 40%. This reinforces the need for placebo-controlled studies to determine the true effect of any treatment.
4.2 Pharmacotherapy
4.2.1 Dopaminergic medications
Overall, dopaminergic agents are the most extensively investigated and used therapies for the treatment of RLS. Since the prior practice parameter update, the literature has advanced considerably with regards to both the number and quality of studies for dopaminergic treatment of RLS. While these agents confer many benefits, there are some adverse effects that should be recognized. Similar to patients with Parkinson's disease, RLS patients treated with dopamine agonists may develop dopamine dysregulation syndrome.20-25 These patients may exhibit an addictive pattern of dopamine replacement therapy use and/or behavioral disturbances including punding and impulse control disorders such as pathologic gambling, compulsive shopping, compulsive eating, and hypersexuality. One report20 indicated a prevalence of 7% for pathologic gambling and 23% for compulsive eating in RLS subjects treated with a dopaminergic medication. Case reports indicate that discontinuation of the dopamine agonist results in resolution or improvement of the impulse control disorder,26-28 although these patients may be particularly susceptible to dopamine agonist withdrawal syndrome.29 The levodopa review encompassed an aggregate of medications with varying dopa decarboxylase inhibitor types (DDCI).
4.2.1.1 Non-ergot derived dopamine agonist: pramipexole
The dopamine agonist pramipexole is effective in the treatment of moderate-to severe RLS. (Level of evidence: High) This recommendation was a guideline in the previous practice parameter. An additional 8 short-term studies30-37 (3 to 12 weeks) of treatment of patients with moderate-to-severe idiopathic RLS and 2 studies38,39 on long-term efficacy up to 1 year have been published. All studies showed improved RLS symptom severity according to IRLS30-37 as well as other measures of RLS including: MOS sleep disturbance and sleep adequacy30; CGI-I30-32,34,37; PGI-I30,33,34,37; RLS QoL30,31,33; PLM index33-35; PSQI37; and VAS.31 In patients with RLS-related mood disturbance, Montagna et al.36 also reported an improvement in mood impairment (Beck Depression Inventory II). In patients with RLS and mood impairment, Hornyak et al.40 reported a statistically significant decrease in RLS-related limb pain as assessed by VAS. Lastly, Inoue et al.37 reported that older age and mild RLS severity were significantly associated with early response to low-dose pramipexole therapy in their study of Japanese patients.
A meta-analysis was performed on all RCT studies with placebo control, and the results are shown in Figure 1. The results show an average improvement of 6.7 points (95% CI 4.9 to 8.5) in the IRLS scale with pramipexole use over placebo. The trials with larger patient populations trend toward an approximate improvement of 5 points. Figure 2 summarizes the evidence profile.
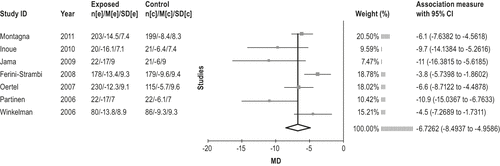

The long-term studies (open label, 26 to 52 weeks in length) report a 17-point improvement in IRLS scores over baseline with pramipexole use. See the online supplement at http://www.aasmnet.org/practiceguidelines.aspx for the detailed data.
Some other studies have been published that were low level evidence (Saletu et al.,41 Stiasny-Kolster and Oertel,42 Silber et al.43), and all reported improvements in RLS symptoms with pramipexole use. The study by Trenkwalder et al.44 on the effects of pramipexole withdrawal after 6 months of use showed that patients switched to placebo experienced worsening symptoms over those who continued to receive pramipexole.
Pramipexole is well tolerated.30–34,36,45 Inoue et al.,38 Montagna et al.,36 and Partinen et al.39 also reported that adverse events (AEs) were mild to moderate in intensity and typical for non-ergot dopamine agonists. These included nausea and somnolence, which typically decreased in frequency over time, and nasopharyngitis. Winkelman and Johnston46 and Silber et al.43 reported that augmentation occurred in one-third of the patients on extended pramipexole use, but was manageable by earlier dosing in the day, small dose increases,46 or increased doses earlier in the day.43 Silver et al.47 reported that (1) over 10 years the average annual rate of augmentation leading to discontinuation of pramipexole was 7% in the 164 patients studied; (2) the percentage continuing medication over 5 years was 58%; and (3) the daily dose of pramipexole at the time of discontinuation for augmentation, as opposed to all other reasons, was 1.28 ± 1.0 vs. 0.66 ± 0.5 mg. Trenkwalder et al.44 reported no augmentation after 9 months of use in 150 patients, and Inoue et al.38 reported no augmentation in 140 patients after 1 year of use.
4.2.1.1a: Clinicians should treat patients with RLS with pramipexole. (STANDARD)
Values and Trade-Offs: Pramipexole is upgraded to standard from the previous practice parameter based on multiple studies showing efficacy in RLS. Pramipexole is typically well tolerated and side effects are self-limited with cessation of pramipexole therapy.
4.2.1.2 Non-ergot derived dopamine agonist: ropinirole
The dopamine agonist ropinirole is effective in the treatment of moderate-to-very severe RLS (Level of evidence: High). This recommendation was an option in the previous practice parameter. Since then an additional 5 RCTs 48-52 have been published. The studies were well conducted and reported consistent results, with just one48 outlier as shown in Figure 3. This study included only 22 patients. Four other studies reported results that could not be used in the meta-analysis: Allen et al.53 gave only the adjusted treatment difference without any standard deviations or details; Kushida et al.54 provided imprecise data with very large standard deviations; Montplaisir et al.55 reported results compared to 24 weeks treated instead of baseline; and Garcia-Borreguero et al.56 reported a non-randomized treatment trial without placebo control. All data can be found in the online supplement at http://www.aasmnet.org/practiceguidelines.aspx.

The data from the RCT studies were combined into a meta-analysis. The average improvement in IRLS score over placebo was 4 points (95% CI: 2 to 6) as shown in Figure 3. The body of evidence level is judged to be high. The evidence profile is summarized in Figure 4.
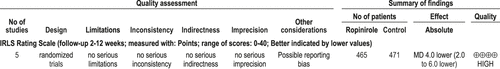
The patients had moderate-to-severe idiopathic RLS. Eight studies showed significant improvement with ropinirole versus placebo on IRLS48-52,54-56 and other measures (RLS symptom diary,48 PLMS,52,53 PLM with arousal,53 PLM while awake,53 ability to initiate sleep,53 sleep adequacy [MOS],49,50,53,56 PLMI [actigraphy],49 CGII responders,49,50,54,56 PGI,54 MOS sleep disturbance,50,55,56 MOS somnolence,50,55,56 MOS sleep quantity,50,55,56 RLS QoL,50,55,56 anxiety [HADS],49 WPAI,56 SF-36,56 and patient relapse55).
Ropinirole is also effective in the treatment of severe-to-very severe RLS. This conclusion is based on an analysis of pooled data57 from four 12-week clinical trials49-51,53 of 223 patients with IRLS scores ≥ 24 compared to those receiving placebo (n = 240). The mean treatment difference was > 3 points in these patients. An increasing treatment effect with ropinirole and not placebo was reported with increasing RLS severity. Additional improvements in global symptoms, sleep, and quality of life were also reported.
Two studies did not show greater efficacy than placebo. Although IRLS was significantly better than baseline in the 4 weeks of open testing by Bliwise et al.,52 no difference compared to placebo was noted after an additional 2 weeks of randomized testing. Allen also reported a nonsignificant effect of ropinirole on IRLS after 12 weeks.53 Unfortunately, the data were not reported in a format that allowed inclusion in the meta-analysis.
Ropinirole was found to be effective48-51,53,55,56 and generally well tolerated.48-51,55,56 The most common side effects were nausea,48,50,52-54,56 headache,50,52-54,56 dizziness,48,53 somnolence,52,54 and vomiting.54 Adverse events led to discontinuation in 8.7% of patients in one study.56 The incidence of augmentation was reported to be between 0%50,51 and 2.3%.56 The mean daily dose ranged from 1.551 to 4.648 mg/d taken 1-3 hours before bedtime50,51,53 or in divided doses.54 A significant placebo effect was reported in one study.54
4.2.1.2a: Clinicians should treat patients with RLS with ropinirole. (STANDARD)
Values and Trade-Offs: This recommendation is upgraded to standard from the previous practice parameter based on multiple studies with RCT data showing efficacy in RLS therapy. Ropinirole is typically well tolerated and side effects are self limited with cessation of ropinirole therapy.
4.2.1.3 Levodopa
Levodopa is effective in the treatment of RLS, but carries the risk of augmentation (Level of evidence: High). This conclusion and evidence level is based primarily on the data from the previous review paper.3 Since the last review in 2004, new formulations of levodopa have been studied (combinations of sustained and regular release L-dopa58,59 or Stalevo,60 which contains L-dopa, carbidopa, and entacapone [LCE]) and there has been progress in understanding augmentation.61 The newer studies were limited by short duration RCT but followed by an open clinical trial (Saletu et al.58), outcome measures other than IRLS reported (Polo et al.60), nonrandomized and open label (Hogl et al.61 and Trenkwalder et al.59).
Both Trenkwalder et al.59 and Saletu et al.58 found improvements in RLS symptoms with the combination of sustained release (sr) and regular release (rr) L-dopa, although Trenkwalder et al. found that roughly 66% of the subjects terminated therapy before the end of a year due to probable augmentation. The dose at 1-year in the Trenkwalder study (mean rr-L-dopa 203 ± 101 mg with 185 ± 93 mg sr-L-dopa) was higher than the 4-week dose in the Saletu study (mean rr-L-dopa 100 ± 38.5 mg with 112 ± 33.2 mg sr-L-dopa). Trenkwalder reported improved quality of sleep, reduced sleep latency, increased total sleep time, reduced severity of RLS at time of falling asleep and during the night, but increased severity of RLS during the day. Global improvement was found in 56% of patients, 30% were unchanged, and 9% were slightly worse. Saletu reported a significant reduction of PLM/h TST from 20.0 ± 14.7 to 4.5 ± 4.9 (P < 0.01), as well as reduction of all other objective RLS/PLM variables. However, treatment did not improve sleep efficiency or subjective sleep quality with respect to placebo. Other scales (IRLS, PSQI, SSA, VAS) also improved significantly. Trenkwalder et al.59 recommended that other treatments be sought if more than 400 mg L-dopa is required to treat RLS patients.
In a randomized controlled crossover trial of 2 days for each treatment, Polo et al.60 studied Stalevo, a new formulation of L-dopa that potentially provides longer symptom control throughout the night by incorporating entacapone. The mean PLMI and TIB were significantly reduced compared with placebo. Compared with levodopa/carbidopa 100/25 mg, levodopa/carbidopa/entacapone 100/25/200 mg and levodopa/carbidopa/entacapone 150/37.5/200 mg reduced PLMs during the second half (P = 0.06 and P < 0.001, respectively) or the last 3 h of the night (P < 0.05 and P < 0.01, respectively). Single doses of LCE with up to 150 mg L-dopa were effective and also well tolerated without typical side effects such as nausea. Of note, Stalevo is on an FDA watch list with concerns about possible increased risk of both prostate cancer and cardiovascular disease.
Hogl et al.61 reported during a 6-month multi-center, open-label trial with flexible dosing of levodopa that augmentation with L-dopa occurred in 60% of the patients and caused 12% to discontinue treatment by 6 months. The median time to occurrence of augmentation was 71 days. Compared to those without augmentation, patients with augmentation were significantly more likely to be on higher doses of levodopa (≥ 300 mg, 83 vs. 54%, P = 0.03) and to show less improvement of symptom severity.
4.2.1.3a: Clinicians can treat RLS patients with levodopa with dopa decarboxylase inhibitor. (GUIDELINE)
Values and Trade-Offs: This recommendation is changed from the previous practice parameter, where it was given a STANDARD level of recommendation for use. Levodopa has longstanding clinical use in RLS with concomitant concerns for daytime RLS augmentation and early morning rebound of RLS symptoms. The use of levodopa may be most advantageous for those patients with intermittent RLS symptoms that do not require daily therapy. For those that require daily therapy for RLS, the newer dopaminergic agents may be a better choice. Therapy should be tailored to the individual patient's specific circumstances and needs. Vigilance for secondary impulsive behavior as an adverse reaction is needed.
4.2.1.4 Ergot-derived dopamine agonists: pergolide and cabergoline
The dopamine agonist pergolide is effective in the treatment of RLS but has been withdrawn in the U.S. because of the risk of cardiac valvulopathy. (Level of evidence: High) Although determined as effective based on the previous review,3 pergolide has been voluntarily withdrawn by the manufacturer in the United States because of risk of heart valve damage. Only 1 study by Trenkwalder et al.62 has been published since the last review was written. Pergolide was found to significantly reduce PLMS-AI, PLMI, RLS severity (IRLS), CGI response, and PGI response versus placebo; however, sleep efficiency did not improve. The mean dose for the double-blinded patients was 0.52 ± 0.22 mg/d and for the open-label patients was 0.72 ± 0.42 mg/d at 12 months. With regard to side effects; nausea and headache were more frequent with pergolide than with placebo. The authors conclude that low-dose pergolide was well tolerated and maintained its efficacy in the long term.
The dopamine agonist cabergoline is effective in the treatment of moderate-to-severe RLS. (Level of evidence: High) The dopamine agonist cabergoline is more effective in the treatment of RLS than levodopa, but is not as well tolerated. (Level of evidence: Moderate) The recommendation was an option in favor of cabergoline use in the previous practice parameter because of 1 low-level study. A significant amount of evidence63-66 has been published since the previous review. These studies investigated the effects of cabergoline in patients with moderate-to-severe idiopathic RLS and one in severe-to-very severe RLS patients.66 The average effective dose was approximately 2 mg, at least 3 h before bedtime.64 All studies reported significant improvement in IRLS.63-66 The meta-analysis of the before-after treatment data show an average decrease in IRLS of 17.5 points (95% CI: 14 to 21 point improvement; see Figure 5), and the 2 RCTs show a decrease in IRLS of 1663 and 1264 with an average decrease of 14 (95% CI: 9 to 18 point improvement] over the control group (Figure 6). Other secondary measures including PLMS-AI, PLM-I, PLMS-I, sleep efficiency, sleep time, sleep quality,63,62 QoL, RLS-6 (day and night),63,64,66 CGI severity,63,62 sleep diaries,64 and nocturnal activity (actigraphy) also improved.62,65Figure 7 summarizes the evidence profile.
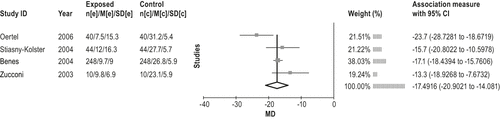
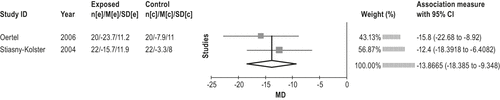

An additional RCT67 compared the effect of cabergoline versus levodopa. Direct comparison67 showed cabergoline to be superior to L-dopa with respect to efficacy (by IRLS, time to discontinuation of therapy or augmentation, RLS-6, QoL, SF-A, CGI, and ASRS). In terms of IRLS, cabergoline showed an improvement over L-dopa of 6.6 (95% CI 8.6 to 4.7) points, and, versus baseline, of 15.6 ± 10.8. However, L-dopa was found to be better tolerated: 95% of patients on L-dopa vs. 85% on cabergoline were determined to have no or mild side effects.
Cabergoline is primarily indicated in treatment of prolactinoma with associated risk of visual field loss. Cabergoline carries a comparatively much stronger risk-to-benefit ratio in prolactinoma therapy than that seen in RLS therapy. Cabergoline risks include valvular heart disease.68 The data seem to agree that there is valve risk, but the defined risk in each study varies by incidence and degree of valve injury.68-75 Other side effects were mostly mild and transient and included nausea, dizziness, and headache.66 If unacceptable gastrointestinal side effects were experienced, domperidone could be prescribed.63 Some possible or probable mild augmentation was reported.64,66
4.2.1.4a: Clinicians should not treat RLS patients with pergolide because of the risks of heart valve damage. (STANDARD)
Values and Trade-Offs: Pergolide risks include heart valve damage and retroperitoneal fibrosis making any future use of pergolide in RLS strongly contraindicated.
4.2.1.4b: Given the potential of side effects, including heart valve damage, clinicians can treat RLS patients with cabergoline only if other recommended agents have been tried first and failed, and close clinical follow-up is provided. (GUIDELINE)
Values and Trade-Offs: The risks of cabergoline are sufficient to recommend cabergoline not be used in routine clinical practice for RLS particularly since there are multiple alternative RLS dopaminergic therapies with a better side effect profile. Because the risk is unclear, it is prudent to remain cautious with respect to recommending cabergoline.
4.2.2 Opioid medications
Opioids are effective in the treatment of RLS, especially for patients with RLS that is not relieved by other treatments. (Level of evidence: Low) In addition to 2 small RCTs that studied oxycodone and propoxyphene discussed in the 1999 review,76,77 3 new studies (1 open-label and 2 retrospective reviews) were found on the effects of opioids on RLS.78-80 Lauerma and Markkula78 reported that 10 of 12 patients found tramadol to be more effective than drugs they had tried in the past, 1 experienced some relief, and 1 had no relief. Some patients alternated tramadol with levodopa or clonazepam while other patients took “drug holidays” or used it intermittently to minimize concerns of abuse. There is one report of augmentation with long-term tramadol treatment.81 In a retrospective review of 113 patients on long-term (up to 5 years) opioid therapy (most commonly tilidine, dihydrocodeine, codeine, propoxyphene, or methadone), Walters et al.79 reported that opioids seem to have long-term effectiveness in the treatment of RLS and PLMS, but patients on long-term opioid therapy should be clinically or polysomnographically monitored periodically for the development of sleep apnea, as 3 of 7 subjects developed worsening sleep apnea. Lastly, Ondo80 reported on the effect of methadone (5-40 mg/day) in 29 patients who had failed dopaminergics. Sixty-three percent of the patients remained on methadone for 23 ± 12 months, all of whom reported at least a 75% reduction in symptoms and no augmentation. Silver et al.47 reported no augmentation leading to the end of treatment with methadone in a 10-year retrospective review of 76 patients on methadone. The median daily dose after 8-10 years on methadone treatment was no more than 10 mg greater than at 6 months, indicating minimal change in narcotic requirement over time.
4.2.2a: Clinicians can treat RLS patients with opioids. (GUIDELINE)
Values and Trade-Offs: Opioid data shows clinical effectiveness in treating RLS with a low level of evidence. As mentioned above, side effects can include an undefined potential for abuse in predisposed patients and a possible risk for the development or worsening of sleep apnea. Therefore, patients should be clinically monitored for the development of symptoms. In general, however, this medication is very well tolerated and has a lower risk of augmentation than is seen in the dopaminergic medications.
4.2.3 Anticonvulsant medications
4.2.3.1 Gabapentin enacarbil
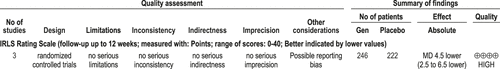
Gabapentin enacarbil is effective in the treatment of moderate-to-severe RLS. (Level of evidence: High) Four studies provided data on the change in IRLS score with gabapentin enacarbil treatment over placebo.82-85 All were well-conducted studies with no limitations. Three studies82,84,85 provided data comparing the change in IRLS vs. baseline of 1200 mg/d of gabapentin enacarbil vs. the same change with placebo. A meta-analysis was performed on these data. Two studies83,85 were 12 weeks in duration, and 184 was only 2 weeks long. The meta-analysis (Figure 8) showed an improvement in IRLS of −4.5 over placebo (95% CI −6.5, −2.5). Other doses have also been studied (60084,85and 180086 mg/d). All data are presented in the Appendix. The evidence profile is shown in Figure 9.
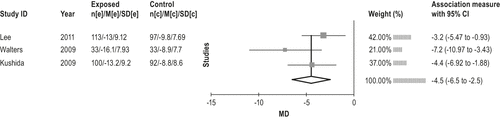
The studies also reported improvements in other outcomes, including CGI-I,82,84,85 sleep architecture, mood,84 and sleep disturbance (MOS, PSQ, or WASO).82,84,85 A recent multicenter, randomized, double-blind, placebo-controlled, 2-period cross-over study87 reported the effect of 1200 mg/d gabapentin enacarbil on polysomnographically measured wake time during sleep and periodic limb movements with arousal per hour of sleep on 136 subjects after 4 and 10 weeks of treatment. There was a statistically significant decrease in both outcomes (adjusted mean treatment difference of −26 minutes for wake time during sleep and −3.1 periodic limb movements with arousal/h).
The short-term studies83,84 reported an increase in adverse events over placebo of approximately 40%, whereas the longer-term study82 reported an increase of only 8%. The most common adverse events were somnolence and dizziness, which were mild-to-moderate in intensity, and generally remitted. A 52-week open label trial88 reported AEs in 80.1% of subjects, 10.3% of which led to withdrawal from the study. Most (67.7%) were mild-to-moderate in intensity, while 3.5% were serious. An additional double-blind, placebo-controlled, 9-month study89 reported RLS relapse comparing maintenance on gabapentin enacarbil to withdrawal and introduction of placebo in gabapentin enacarbil responders. Patients on gabapentin enacarbil had fewer relapses and longer time to relapse.
An additional consideration discussed by Ellenbogan et al.88 is the following: although their study was not prospectively designed to assess augmentation, there were no reported or suspected cases of augmentation based on a retrospective analysis of AEs. Also, there was no evidence of reemergence/rebound of symptoms and no reports of compulsive behavior or impulse control disorder.
4.2.3.1a: Clinicians can treat patients with RLS with gabapentin enacarbil. (GUIDELINE)
Values and Trade-Offs: This is a new recommendation from the prior practice parameter. Sufficient evidence has emerged since the last practice parameter to support gabapentin enacarbil as a guideline level for treatment in RLS therapy. Gabapentin enacarbil therapy is generally well tolerated with self-limited side effects. High level evidence is encouraging. However, this medication is relatively new, thereby warranting a conservative recommendation level of guideline at this time.
4.2.3.2 Gabapentin
Gabapentin is effective in the treatment of mild-to-moderate RLS. (Level of evidence: Low) Two small studies (1690 and 2491 patients) were identified on gabapentin that indicated improvement in RLS symptoms. Although the patient description was not explicitly denned, from the data it is judged that the patients in both studies were primarily in the mild-to-moderate category. In a randomized open clinical trial, Happe et al.90 compared gabapentin to ropinirole and found gabapentin to be as effective as ropinirole (IRLS, PLMS, and PLMS index significantly improved in both groups; ESS, QoL, and SAS were not significantly changed in both groups; PLMS-AI, PSQI, and SDS were significantly better in the gabapentin but not ropinirole groups). In a 12-week randomized cross-over trial (6 weeks for each treatment), Garcia-Borreguero et al.91 reported an improvement in IRLS for gabapentin to 9.5 ± 6.1 versus placebo to 17.9 ± 1.3 from a baseline of 20 for both groups. The evidence profile is shown in Figure 10. Sleep studies showed a significantly reduced PLMS index (11.3 ± 15.5 vs. 20.8 ± 15.5; P = 0.05) and improved sleep architecture. Patients whose symptoms included pain benefited most from gabapentin. It should be noted that gabapentin has the following potential side effects: sedation, dizziness, vision changes, and suicidal behavior and ideation.

4.2.3.2a: Clinicians may treat RLS patients with gabapentin. (OPTION)
Values and Trade-Offs: Low level evidence supports use of gabapentin for RLS therapy. Pain relief with gabapentin supports consideration of gabapentin in patients with both RLS and pain. There are some concerning potential side effects which makes the balance of benefits versus harms uncertain.
4.2.3.3 Pregabalin
Pregabalin is effective in the treatment of moderate-to-severe RLS. (Level of evidence: Low) Two studies have recently been published on the use of pregabalin to treat moderate-to-severe RLS. Allen et al.92 reported the results of a dose-finding investigation of 50-450 mg/day over 6 weeks. There were 22-24 patients in each of 6 arms of the study. Calculations indicated that 123.9 mg/day would provide 90% efficacy in symptom reduction. Garcia-Borreguero et al.93 reported the results of a 12-week RCT of 30 patients randomized to pregabalin and 28 to placebo. Twenty-four pregabalin and 19 placebo patients completed the trial. The baseline adjusted mean difference in IRLS was 4.9 (95% CI 0.7 to 9.1) with a mean dose of 337 mg/d. CGI-I showed significant improvements, as did measures of sleep quality and architecture including PLMS and PLMS-AI. Eighty-three percent of patients on pregabalin experienced AEs compared with 32% on placebo. The most common AEs were unsteadiness (39% higher with pregabalin over placebo) and daytime sleepiness (29% higher with pregabalin over placebo). More information is needed regarding long-term use, including augmentation occurrence. Also, the effect of pregabalin on the working population is needed.93 The optimal dose has not yet been identified.92,93Figure 11 shows the evidence profile.
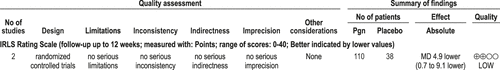
4.2.3.3a: Clinicians may treat patients with RLS with pregabalin (OPTION)
Values and Trade-Offs: Preliminary data shows therapeutic efficacy in pregabalin therapy for RLS. However, long-term follow up and published experience in pregabalin therapy for RLS is lacking. Thus, other better-studied RLS therapies should be considered before prescribing pregabalin.
4.2.3.4 Carbamazepine
Carbamazepine is effective in the treatment of RLS (Level of evidence: Low). This assessment is based on the data presented in 1999, which are considered low according to the methods of this update. Of the 3 studies, there was one large (n = 181 patients) but short-term (5 weeks) double-blind RCT94 with placebo control that showed carbamazepine to be significantly more effective than placebo using a visual analogue scale. One study95 did not meet current inclusion criteria because the number of patients was too small (n = 6), and the other study was a clinical series.96 No new evidence was found on the use of carbamazepine since the last review (1999).
4.2.3.4a: Clinicians may treat RLS patients with carbamazepine. (OPTION)
Values and Trade-offs: This has been downgraded from GUIDELINE in the prior practice parameter to OPTION in this practice parameter. Although carbamazepine efficacy in RLS was shown in prior studies, these data are dated with no new additional supportive work. There are other RLS therapies with comparatively more supportive evidence, risk-to-benefit ratios, and clinical experience than carbamazepine. The benefits of carbamazepine therapy are closely balanced with potential adverse side effects which include sedation, liver abnormalities and, rarely, the potential suicidal ideation and behavior, and Stevens-Johnson syndrome.
4.2.4 Medications acting on the adrenergic systems
Clonidine is effective in the treatment of RLS (Level of evidence: Low). No new evidence was found on the use of clonidine since the last complete review2 where there were 2 small studies of 1197 and 2098 patients. The studies were also short-term (3 days98 to 2-3 weeks97). Both were double-blind and placebo controlled, but randomization was unclear in one.98 Because of these limitations and other considerations, the data using the current methodology is considered low. The results of Wagner et al.,97 as reported by Hening et al.,2 are that “clonidine resulted in significant improvement compared to baseline in subjective measures and sleep latency, though PLMI was not significantly decreased. There was no correlation between plasma clonidine concentration and control of symptoms. Side effects were frequent (8 of 10 patients); however, no patients left the study due to them.” Side effects were generally considered mild, and included dry mouth, decreased cognition, lightheadedness, sleepiness post dose, constipation, decreased libido, and headache. Ausserwinkler and Schmidt98 reported that “clonidine significantly improved RLS symptoms, with 8/10 patients having complete relief of symptoms.”
4.2.4a: Clinicians may treat patients with RLS with clonidine (OPTION)
Values and Trade-Offs: Clonidine has minimal supporting data in treating RLS and carries a considerable risk for side effects. Clonidine might be considered in treating hypertension and RLS concomitantly. The risk of side effects (such as hypotension in normotensive patients) associated with clonidine in the treatment of RLS makes the benefit-to-harm ratio unclear.
4.2.5 Iron supplementation
Iron supplementation has not been shown to be effective in the treatment of RLS, except perhaps in patients with iron deficiency or refractory RLS. (Level of evidence: Very low)
There were 6 studies in total on 3 forms of iron treatment: oral iron sulfate, IV iron sucrose, and IV iron dextran. Overall, the data are conflicting, but show some improvement in select cases, typically those with low serum ferritin levels. Davis et al.99 and Wang et al.100 studied oral iron sulfate. Davis et al. reported no significant effect on quality after 12 weeks. Wang et al. also examined the use of oral iron sulfate. In an RCT with 18 patients with low serum ferritin levels, the investigators showed a statistically significant improvement in IRLS with 2 doses of 325 mg/d. Earley et al.101 and Grote et al.102 assessed 1000 mg iron sucrose administered in 2 doses of 500 mg or 5 doses of 200 mg. Earley et al. stopped the trial early (after 2 weeks) because of no effect demonstrated on the global rating scale and PLMS. Grote et al. reported on patients with variable degrees of iron deficiency at several lengths of follow up including 2 months and 12 months. There was no statistically different change in IRLS observed at either endpoint. The dropouts for lack of treatment effect were higher in the placebo group (61% vs. 17%). The use of IV iron dextran for treatment of RLS has also been examined. Earley et al.103 and Ondo104 investigated IV iron dextran. The study by Earley et al. consisted of 11 patients and was open-label. Results were mixed. Ondo reported on 25 subjects in a retrospective review of severe refractory RLS. It was demonstrated that iron dextran can dramatically improve refractory RLS, but results were inconsistent and not predicted by patient demographics. Anaphylactic symptoms are a risk. In 2009, the FDA issued a warning that analphylactic-type reactions, including fatalities, have followed the parenteral administration of iron dextran injection. The boxed warning recommends administering a test dose prior to the first therapeutic dose and observing reactions. However, it should be noted that parenteral infusion risk with low molecular weight iron dextran is lower (1 per 200,000)105 than that with high molecular weight iron dextran. Additionally, parenteral iron therapy with iron sucrose, iron gluconate or ferumoxytol carries no anaphylactic risk.106
4.2.5a: Clinicians may use supplemental iron to treat RLS patients with low ferritin levels. (OPTION)
Values and Trade-Offs: RLS therapy with iron may be effective in patients with RLS associated with low ferritin levels. Parenteral high molecular weight iron dextran therapy carries the potential for anaphylactic reaction. The parenteral infusion risk with low molecular weight iron dextran is substantially lower. Moreover, parenteral iron therapy with iron sucrose, iron gluconate, or ferumoxytol carries no anaphylactic risk. However, whenever possible, oral iron replacement is recommended. Oral supplemental iron carries fewer side effects—primarily constipation and rare cases of iron overload.
4.3 Therapies For Which No Recommendations Are Made
The following section contains information on those pharmacological and nonpharmacological RLS therapies for which a recommendation level could not be given secondary to either insufficient evidence to support any recommendation or because the therapy is no longer available in the U.S.
4.3.1 Non-ergot-derived dopamine agonists: rotigotine
Rotigotine as a transdermal patch is effective in the treatment of moderate-to-severe RLS, but was withdrawn from the U.S. in 2008 (Level of evidence: High). This is a new treatment since the last review, and the evidence base is 5 studies107-111 for the meta-analysis. No limitations were noted with the studies, and the results were consistent. All data are presented in the online supplement at http://www.aasmnet.org/practiceguidelines.aspx. Rotigotine was found to improve RLS symptom severity according to IRLS by 7.0 (95% CI: 5.6 to 8.4)107-111; the results are shown in Figure 12. Other outcomes measures including PSG-measured PLMI and PLM arousal index,111 RLS severity (RLS-6),108,109 CGI-I,107-110 and QoL107,109 also improved significantly. Figure 13 shows the evidence profile.
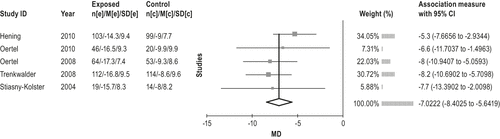

Two long-term continuation studies were reported, Oertel et al.112 (220 patients for 1 year) and Hogl et al.113 (190 patients for 2 years). The IRLS total score improved by −17.4 ± 9.9 points between baseline and end of year 1 (P < 0.001) and by −15.4 ± 10.3 for the 2-year study. The other measures of symptom severity, sleep satisfaction, and QoL supported the efficacy of rotigotine.
Doses ranged from 1107 to 4.5 mg,108 with increasing effectiveness up to approximately 3 mg/d. Oertel et al.112 reported the mean daily dose after 1 year was 2.8 ± 1.2 mg/24 h with 4 mg/24 h (40.6%) being the most frequently applied dose. Braun et al.114 concluded from their pharmacokinetic interaction data that rotigotine dose adjustment would not be needed if domperidone was added to the treatment regimen. In 2008, this drug was withdrawn from the U.S. market because of concerns about inconsistent absorption from the patch.
The transdermal patch was safe and generally well tolerated by the majority of patients. Oertel et al.112 reported after 1 year of study that the tolerability was described as “good” or “very good” by 80.3% of all patients. Side effects were mostly mild to moderate, including application site reactions (40%112−43%109), nausea (9.5%112), and fatigue (6.4%112). Hogl et al.113 reported at a median dose of 4 mg/d that 87% of patients experienced at least 1 adverse event, the majority of which were mild or moderate, but 22% of these were severe. Additionally, the most frequent adverse event in year 2 was any application site disorder (16.4%), followed by 4.5% with back pain and 4.1% with nasopharyngitis. Transdermal rotigotine was withdrawn from the market because of drug crystallization that resulted in suboptimal absorption.
4.3.2 Other dopaminergic medications: lisuride and amantadine
There is insufficient evidence at this time to support the use of lisuride in the treatment of RLS, and it is not FDA-approved. Two small studies by Benes et al.,115,116 1 a non-randomized treatment trial116 and the other a randomized controlled trial,115 reported on the effect of lisuride on patients with severe and/or advanced RLS. The non-randomized treatment trial116 of 20 patients reported that lisuride given orally as a monotherapy (0.3 mg) as well as in conjunction with L-dopa (150 mg) significantly improved CGI-I and PLM index. The randomized controlled trial115 of 10 patients reported that lisuride transdermal patches significantly improved RLS-6, CGI-I, PLM index (actigraphy), and IRLS (−23.5 with lisuride and −10.6 with placebo). Side effects were typical for dopaminergic drugs. With the exception of nausea and dizziness in one patient, none of the adverse events were rated as severe.
No new studies were found on amantadine since the previous practice parameter, which reported that in 1 clinical series (Evidente et al.117) of 21 patients, half the patients benefited acutely by amantadine as an add-on medication, with long-term benefit in a minority. In 2004, the strength of this recommendation was OPTION level. However, currently no recommendation has been given for amantadine as several superior options are available; there was limited existing evidence, and no new evidence for the use of amantadine in RLS.
4.3.3 Other dopamine agonists
There is insufficient evidence at this time to support the use of talipexole, peribedil, and alpha-dihydroergocryptine in the treatment of RLS. In the previous practice parameter (2004), these agents were given an OPTION level of recommendation based on very low level evidence (1 small case series for each drug), one of which (Inoue, talipexole) would not have been accepted in this paper because there were only 5 patients, 2 of whom had uremia.
4.3.4 Benzodiazepines (clonazepam)
There is insufficient information on the effect of benzodiazepines on the treatment of RLS. In addition to 3 studies118–120 discussed in the last review, 2118,119 of which were RCTs with small numbers of patients (n = 6) and showed contradictory results, 1 non-randomized treatment trial on 10 patients with RLS by Saletu et al.121 reported that 1 mg clonazepam improved objective sleep efficiency and subjective sleep quality but did not reduce the PLM index. The authors concluded that clonazepam had an acute therapeutic effect on insomnia, which is a different mode of action than dopamine agonists. An additional paper122 suggested that clonazepam was not as effective as pramipexole in the treatment of RLS.
Although clonazepam received an OPTION level of recommendation in 1999 as described in the evidence review, benzodiazepines lack clinical data necessary to assess efficacy in treating RLS. The committee strongly recommends that alternate and better studied RLS medications be considered in RLS therapy. The “no recommendation” status applies to the use of benzodiazepines as a first line agent. For example, clonazepam could still be considered as an adjunctive medication in treatment of RLS.
4.3.5 Valproic acid
There is insufficient evidence at present to evaluate the use of valproic acid for RLS. A single, small RCT by Eisensehr et al.123 reported no major difference between the efficacy of valproic acid (VPA) and levodopa on 20 patients with moderate-to-severe idiopathic RLS. Follow-up 6 to 18 months after the study end revealed that VPA was still effective in 75% (9 of 12 patients), whereas only 29% (2 of 7 patients) were still satisfied with levodopa (P = 0.048). The authors conclude that slow-release VPA provides an alternative or adjunctive treatment for patients unable to tolerate dopaminergics or those suffering from augmentation, and not as a first-line treatment for RLS. In 2009, the FDA issued a warning that there is an increased risk of neural tube defects and other major birth defects, such as craniofacial defects and cardiovascular malformations, in babies exposed to valproate sodium and related products (valproic acid and divalproex sodium) during pregnancy.
4.3.6 Valerian
There is insufficient evidence at present to evaluate the use of valerian for RLS. In one RCT by Cuellar and Ratcliffe on 48 patients,124 it was reported that although PSQI, ESS, and IRLS all decreased, no significant differences were found between placebo and 800 mg/d valerian. In patients with ESS > 10, valerian significantly improved symptoms of RLS and decreased daytime sleepiness. Higher doses should be considered in future studies.
4.3.7 Avoidance of antidepressants
The evidence on the issue of whether or not antidepressant use can cause or exacerbate RLS symptoms is conflicting. Three studies were identified that reported there is an association between antidepressants and the occurrence of RLS. Baughman et al.125 interviewed 1693 veterans and reported on the relationship between antidepressants and gender. Men were found to have an increased risk of developing RLS with antidepressant use, RR = 1.77 (95% CI 1.26, 2.48), whereas for women, there was no increased risk—RR = 0.79 (0.43, 1.47). For men, the highest odds ratios were found for citalopram, paroxetine, and amitryptiline. One antidepressant, fluoxetine, was found to show an increased odds ratio for women (RR = 2.47 [1.33, 4.56]). Kim et al.126 performed a retrospective chart review of 181 charts and found that 8% of patients who were treated with mirtazapine developed RLS symptoms, typically within a few days after introduction of the drug. A higher odds ratio was found with the concomitant use of tramadol and dopamine-blocking agents. Lastly, Rottach et al.127 studied second-generation antidepressants in a prospective observational study of 271 participants. Nine percent of patients developed RLS as a side effect with the use of these second generation antidepressants with the exception of reboxetine. Twenty-eight percent of mirtazapine users reported RLS. In another investigation, 243 subjects with affective and anxiety disorders were studied systematically for the emergence of symptoms of RLS after antidepressant use. In contrast to the previously discussed studies, in this study antidepressants were not found to be a major risk factor for RLS.128 Furthermore, Brown et al.129 reported the results of a retrospective chart review of 200 consecutive patients presenting with insomnia. There were no statistically significant associations between RLS and antidepressant use or any specific class of antidepressant.
4.3.8 Non-pharmacological therapy
There is insufficient evidence at present to evaluate the use of non-pharmacological therapy for RLS, including accommodative strategies, sleep hygiene, behavioral and stimulation therapies, compression devices, exercise, and nutritional considerations.
No studies were found on accommodative strategies, sleep hygiene, or nutritional considerations since the last review. Regarding cognitive behavioral therapy, one non-randomized, non-blinded treatment trial (Hornyak et al.130) reported that IRLS, QoL-RLS, and mental health status (SCL-90-R) scores improved significantly through 3 months with 8 weekly 90-min sessions in group therapy consisting of mindfulness-based exercises, stress-reduction strategies, diary-based analysis, and medical education.
Lettieri and Eliasson131 (RCT) and Eliasson and Lettieri132 (non-randomized, non-blinded treatment trial) reported that wearing compression devices a minimum of 1 h per day for 1 to 3 months was significantly superior to sham treatment on IRLS, JHRLSS, RLS-QoL, ESS, and the Fatigue Visual Analog Scale; furthermore, one-third of patients experienced complete resolution of symptoms. The authors suggested that these devices may be potential adjunctive or alternative therapies for RLS patients.
One small (11 therapy and 12 controls) unblinded RCT by Aukerman et al.133 reported that 12 weeks of exercise therapy (aerobic and lower-body resistance training for 3 days/week) significantly decreased RLS symptoms (IRLS rating scale and an ordinal RLS scale) versus the control group. The exercise program was shown to be an effective treatment to improve the symptoms of RLS.
4.3.9 Secondary RLS and special patient groups
There is insufficient evidence on the effectiveness of any one therapy or the balance of benefits to harm in the treatment of secondary RLS, children, pregnant women, or other special patient groups for a recommendation to be made.
End Stage Renal Disease (ESRD)—Six studies discussed the treatment of patients with ESRD and/or those on hemodialysis who also had RLS. Various treatments were used in the studies. The first was an RCT by Thorp et al.,134 who reported that 200-300 mg gabapentin after each hemodialysis session on 13 patients significantly improved RLS symptoms according to an author-developed questionnaire based on the IRLS rating scale. A small (14 patients) unblinded RCT by Micozkadioglu et al.135 compared the effects of 200 mg/d gabapentin versus 125 mg/d L-dopa on hemodialysis patients. They reported that gabapentin was significantly superior to L-dopa on RLS symptom severity relief, improvement of general health, body pain, social functions, and sleep parameters according to the IRLS rating scale, SF-36, and PSQI. Sloand et al.136 reported in an RCT on 25 patients that 4 weeks of 1000 mg IV iron dextran during dialysis resulted in significant, but transient, reduction in symptoms of RLS in patients with ESRD according to an author-developed questionnaire. Pellecchia et al.137 reported in an unblinded RCT on the effects of 6 weeks each of ropinirole (mean dosage 1.45 mg/d) versus sustained-release levodopa (mean dosage 190 mg/d) in 10 patients on chronic hemodialysis with RLS. Ropinirole resulted in a significantly higher improvement (73.5% vs. 33.5%) in IRLS scores, sleep time, and PGI. No adverse events were reported during ropinirole treatment. Mirada et al.138 reported in a nonrandomized treatment trial on the effects of 0.125-0.75 mg of pramipexole on 10 patients with RLS that was severe enough to interfere with their dialysis treatment such that they required disconnection. At a mean follow-up time of 8 months, the IRLS and PLMI were significantly reduced, whereas differences in sleep latency, total hours of sleep, number of awakenings, and sleep efficiency were not statistically significant. Lastly, 2 small studies (14139 and 18140 patients) showed promising results of the effect of exercise on IRLS139 and PLM during hemodialysis140 in hemodialysis patients.
Other information from the 1999 review paper2 includes: “Dialysis itself does not appear to alter the RLS or PLMD secondary to end-stage renal disease, but the dialysate temperature may influence symptoms.141Erythropoietin supplementation may reduce symptoms, and symptoms often largely resolve with kidney transplantation as reported in a case report and abstract.142,143 Two clinical trials meeting study criteria included patients with end-stage renal disease and found efficacy for levodopa.144,145 One did not.146 One trial98 with clonidine revealed efficacy in this group of patients. Some case reports suggest efficacy for benzodiazepines (clonazepam).147 Nephrologists, noting that carbidopa is a pyridoxine (B6) inhibitor, suggest providing an additional daily B6 supplement of 10 mg.148 In considering other potential medications for dialysis patients, the elimination patterns of the medications and their active metabolites need to be considered (e.g., gabapentin is dialyzable, whereas meperidine, propoxyphene, valproic acid and carbamazepine are not148).”
Neuropathy—In a nonrandomized treatment trial, Sommer et al.149 reported on the effect of pregabalin on 16 patients with secondary RLS, most with neuropathy and neuropathic pain and 3 with idiopathic RLS. The final mean daily dose was 305 ± 185 mg. All patients self-rated a satisfactory or good alleviation of RLS symptoms and maintained pregabalin, 5 with additional medication, for a mean duration of 217 ± 183 days.
Superficial venous insufficiency (SVI)—A study by Hayes et al.150 reported that RLS symptoms were alleviated in 18 treatment subjects but not in 15 controls who all had concurrent moderate-to-very severe RLS (IRLS rating scale ≥ 15) and duplex-proven SVI. The treatment consisted of endovenous laser ablation of refluxing superficial axial veins and ultrasound-guided sclerotherapy of the associated varicose veins with post-operative ACE wrap for 48 h followed by compression stockings for 2 weeks. The mean IRLS score decreased significantly by 21.4 points from 26.9 to 5.5 for treatment subjects, whereas control scores did not decrease. Fifty-three percent of patients had a 6-week follow-up score ≤ 5, and 31% had a follow-up score of 0, indicating a complete relief of RLS symptoms. The 1999 review included the results of an unblinded study151 on 113 selected patients with documented SVI and complaints of RLS. After 1-10 treatments of intravenous sclerotherapy with sodium tetradecyl sulfate, 98% of patients reported notable improvement in RLS symptoms, although 28% of these relapsed by the 2-year follow up.
5.0 Therapies for PLMD
Periodic limb movements of sleep (PLMS) are frequently seen as an incidental finding during sleep studies. In some cases in which there are frequent PLMS and a subjective perception of poor sleep in the absence of RLS or sleep-related breathing disorder, PLMD can be diagnosed.6 Although there are no studies of dopaminergic treatment of PLMD, many of the studies of dopaminergic medication effects on RLS looked at PLMS and periodic limb movements during wakefulness (PLMW). Some studies demonstrated statistically significant falls in PLM indices with Stalevo,60 pramipexole,35,41,42,45 ropinorole,49,52,53 and rotigotine.111 In addition, gabapentin90,91 and pregabalin93 were also shown to decrease PLM indices in subjects with RLS. Thus, although there were no studies on the efficacy of these medications in a population with PLMD, they have been noted to decrease PLM indices in subjects with RLS and might be effective in treating the sleep dysfunction of PLMD. The following sections review medications tried in subjects with PLMD.
5.1 Clonazepam
In a nonrandomized treatment trial, Saletu et al.121 discussed the acute effects of 1 mg clonazepam (1 night each of trial drug and placebo) on idiopathic PLMD. Clonazepam significantly improved objective sleep efficiency and subjective sleep quality, PLM during time in bed, PLM during REM, and PLM during wake-time, but did not reduce the PLM index. The authors concluded that clonazepam had an acute therapeutic effect on insomnia rather than limb movements.
5.2 Melatonin
In a 6-week open clinical trial on 9 patients, Kunz et al.152 reported that 3 mg of melatonin taken 30 min prior to bedtime significantly improved the movement parameters associated with PLMD (4 severe [PLM index > 50], 3 moderate [PLM index 26-50], and 2 mild [PLM index 5 thru 25]). Melatonin improved Zerssen well-being (a self-rating mood scale) in 7 of the 9 patients; significantly reduced PLMs, PLM index, PLMs with arousals and PLM-arousal index; and significantly reduced movement rate and minutes with movements during time in bed as measured by actigraphy.
5.3 Valproate
In a nonrandomized treatment trial, Ehrenberg et al.153 reported on the effects of low-dose valproate (125-600 mg at bedtime) on 6 patients with PLMD for a mean of 6 months of treatment. All patients experienced statistically significant improvement in subjective daytime alertness and objective sleep parameters including sleep efficiency (76% to 88%), stage 1 sleep (26% to 13%), stage 3 and 4 sleep (19% to 30%). REM sleep was unchanged, and non-significant reductions in the number of PLMs per hour of sleep and in the percentage of arousals associated with PLMs were observed.
5.4 Selegiline
In the 2004 practice parameters, 1 study (Grewal et al.,154 a case series on 31 patients) was discussed where selegiline was used successfully to treat PLMD. No new studies were found on selegiline.
5.0a: There is Insufficient Evidence at Present to Comment on the use of Pharmacological Therapy in Patients Diagnosed With PLMD Alone. (NO RECOMMENDATION)
Values and Trade Offs: There is insufficient evidence to comment on pharmacologic therapies in isolated PLMD. Existing data in RLS therapy does, in some cases, support some medical interventions in both RLS and PLMD. Clinical judgment must be used in any pharmacologic intervention in PLMD.
6.0 Conclusions and Future Directions
Since the prior practice parameter, a considerable amount of literature has been published on the effects of dopaminergic medications for RLS. However, there were a significant number of therapies, both pharmacological and nonpharmacological, that received “no recommendation” due to the dearth of information regarding their use in the setting of RLS. Furthermore, there is a paucity of data comparing medications in head-to-head trials to determine their relative effectiveness and adverse event profiles. For this reason, and the fact that therapy should always be tailored to the individual, a dopaminergic “drug of choice” cannot be recommended. It is worth noting that the late development of augmentation (even after one year of continuous therapy on dopaminergic agents) remains a significant concern, and patients need to be monitored throughout therapy for this particular side effect.
Additionally, Godau et al.155 have noted that the RLS treatment successes that have been demonstrated in pharmacological trials have not been consistently replicated in the clinical setting. The authors suggest that this could be related to the fact that approximately two-thirds of the patients with idiopathic RLS evaluated in clinical practice are excluded from pharmacological trials secondary to the presence of neuropsychiatric comorbidities. These comorbidities include anxiety, depression, chronic pain, and various somatoform disorders. A possible way to circumvent this limitation is to include cognitive behavioral therapies or psychotherapy as part of the treatment regimens. Investigations including patients with both RLS and neuropsychiatric comorbidities would be more clinically germane.
Finally, randomized controlled trials evaluating treatment options for patients with secondary RLS and PLMD are lacking. Multiple medications that can be considered for idiopathic RLS do not have sufficient evidence in the setting of secondary RLS or PLMD to warrant a recommendation level. These practice parameters highlight the need for further investigations assessing treatments for secondary RLS and PLMD.
Footnote
Estimate of effect: The observed relationship between an intervention and an outcome expressed as, for example, a number needed to treat, odds ratio, risk difference, risk ratio, relative risk reduction, standardized mean difference, or weighted mean difference.
1. . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6.Crossref, Google Scholar
2. . The treatment of restless legs syndrome and periodic limb movement disorder. Sleep 1999;22:970–99.Google Scholar
3. . An update on the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep 2004;27:560–83.Crossref, Google Scholar
4. . Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep 2004;27:557–9.Crossref, Google Scholar
5. . Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. Sleep 1999;22:961–8.Crossref, Google Scholar
6.
7. . Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 2003;4:101–19.Crossref, Google Scholar
8.
9.
10. Higgins J, Green S, (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. In: The Cochrane Collaboration.Google Scholar
11. . Development and validation of MIX: Comprehensive free software for meta-analysis of causal research data. BMC Medical Res Methodol 2006 Oct 13;6:50.Crossref, Google Scholar
12. : Comprehensive free software for meta-analysis of causal research data. Version 1.7. http://mix-for-meta-analysis.info. 2008.Google Scholar
13. . GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94.Crossref, Google Scholar
14. . Data collection instrument and procedure for systematic reviews in the Guide to Community Preventive Services. Am J Prev Med 2000;18:44–74.Crossref, Google Scholar
15. . GRADEPro [Computer program]. In: Versions 3.0.1 and 3.2.2 for Windows, 2008.Google Scholar
16. . Grading and quality of evidence and strength of recommendations. BMJ 2004;328:1490–94.Crossref, Google Scholar
17. . An official ATS statement: Grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006;174:605–14.Crossref, Google Scholar
18. . On the Goodness of Recommendations: The Changing Face of Practice Parameters. Sleep 2010;33:1273–76.Crossref, Google Scholar
19. . Where dopamine meets opioids: a meta-analysis of the placebo effect in restless legs syndrome treatment studies. Brain 2008;131:902–17.Crossref, Google Scholar
20. . Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case-control study. Sleep 2010;33:81–7.Google Scholar
21. . Predictors of impulsivity and reward-seeking behavior with dopamine agonists. Parkinsonism Relat Disord 2008; 14:28–32.Crossref, Google Scholar
22. . Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol 2007;30:249–55.Crossref, Google Scholar
23. . Dopamine dysregulation syndrome: an overview of its epidemiology, mechanisms and management. CNS Drugs 2009;23:157–70.Crossref, Google Scholar
24. . Dopamine dysregulation syndrome in a patient with restless legs syndrome. Sleep Med 2009; 10:494–6.Crossref, Google Scholar
25. . Drug hoarding: a case of atypical dopamine dysregulation syndrome in a RLS patient. Mov Disord 2009;24:627–8.Crossref, Google Scholar
26. . Patho logic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology 2007;68:301–3.Crossref, Google Scholar
27. . Pathological gambling associated with dopaminergic agonist use in restless legs syndrome. Parkinsonism Relat Disord 2007;13:535–6.Crossref, Google Scholar
28. . Dopamine agonist-induced pathological gambling in restless legs syndrome due to multiple sclerosis. Mov Disord 2007;22:590–1.Crossref, Google Scholar
29. . Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol 2010;67:58–63.Crossref, Google Scholar
30. . Effect of pramipexole on RLS symptoms and sleep: A randomized, double-blind, placebo-controlled trial. Sleep Med 2008;9:874–81.Crossref, Google Scholar
31. . Efficacy and safety of pramipexole in restless legs syndrome. Neurology 2006;67:1034–9.Crossref, Google Scholar
32. . Efficacy of pramipexole in restless legs syndrome: A six-week, multicenter, randomized, double-blind study (Effect-RLS Study). Mov Disord 2007;22:213–9.Crossref, Google Scholar
33. . Efficacy and safety of pramipexole in idiopathic restless legs syndrome: A polysomnographic dose-finding study - the PRELUDE study. Sleep Med 2006;7:407–17.Crossref, Google Scholar
34. . Efficacy and safety of pramipexole in Japanese patients with primary restless legs syndrome: A polysomnographic randomized, double-blind, placebo-controlled study. Sleep Med 2010;11:11–6.Crossref, Google Scholar
35. . A dose-ranging study of pramipexole for the symptomatic treatment of restless legs syndrome: polysomnographic evaluation of periodic leg movements and sleep disturbance. Sleep Med 2009; 10:630–6.Crossref, Google Scholar
36. . Randomized trial of pramipexole for patients with restless legs syndrome (RLS) and RLS-related impairment of mood. Sleep Med 2011;12:34–40.Crossref, Google Scholar
37. . Efficacy, safety and dose-response of pramipexole in Japanese patients with primary restless legs syndrome: Randomized trial. Neuropsychobiology 2011;63:35–42.Crossref, Google Scholar
38. . Long-term open-label study of pramipexole in patients with primary restless legs syndrome. J Neurol Sci 2010;294:62–6.Crossref, Google Scholar
39. . Open-label study of the long-term efficacy and safety of pramipexole in patients with Restless Legs Syndrome (extension of the PRELUDE study). Sleep Med 2008;9:537–41.Crossref, Google Scholar
40. . Evaluation of painful sensory symptoms in restless legs syndrome: experience from two clinical trials. Sleep Med 2011;12:186–9.Crossref, Google Scholar
41. . Acute placebo-controlled sleep laboratory studies and clinical follow-up with pramipexole in restless legs syndrome. Eur Arch Psychiatry Clin Neurosci 2002;252:185–94.Crossref, Google Scholar
42. . Low-dose pramipexole in the management of restless legs syndrome. Neuropsychobiology 2004;50:65–70.Crossref, Google Scholar
43. . Pramipexole in the management of restless legs syndrome: An extended study. Sleep 2003;26:819–21.Crossref, Google Scholar
44. . Controlled withdrawal of pramipexole after 6 months of open-label treatment in patients with restless legs syndrome. Mov Disord 2006;21:1404–10.Crossref, Google Scholar
45. . Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study–the PRELUDE study. Sleep Med 2006;7:407–17.Crossref, Google Scholar
46. . Augmentation and tolerance with long-term pramipexole treatment of restless legs syndrome (RLS). Sleep Med 2004;5:9–14.Crossref, Google Scholar
47. . A 10-year, longitudinal assessment of dopamine agonists and methadone in the treatment of restless legs syndrome. Sleep Med 2011;12:440–4.Crossref, Google Scholar
48. . Ropinirole for restless legs syndrome: A placebo-controlled crossover trial. Neurology 2004;62:1405–7.Crossref, Google Scholar
49. , for the TREAT RLS US (Therapy with Ropinirole Efficacy and Tolerability in RLS US) Study Group. Ropinirole in the treatment of patients with restless legs syndrome: A US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc 2006;81:17–27.Crossref, Google Scholar
50. . Ropinirole in the treatment of restless legs syndrome: Results from the TREAT RLS 1 study, a 12-week, randomised, placebo-controlled study in 10 European countries. J Neurol Neurosurg Psychiatry 2004;75:92–7.Google Scholar
51. . Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: A12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord 2004;19:1414–23.Crossref, Google Scholar
52. . Randomized, double-blind, placebo-controlled, short-term trial of ropinirole in restless legs syndrome. Sleep Med 2005;6:141–7.Crossref, Google Scholar
53. . Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep 2004;27:907–14.Crossref, Google Scholar
54. . Patient- and physician-rated measures demonstrate the effectiveness of Ropinirole in the treatment of restless legs syndrome. Clin Neuropharmacol 2008;31:281–6.Crossref, Google Scholar
55. . Ropinirole is effective in the long-term management of restless legs syndrome: A randomized controlled trial. Mov Disord 2006;21:1627–35.Crossref, Google Scholar
56. . A 52-week open-label study of the long-term safety of ropinirole in patients with restless legs syndrome. Sleep Med 2007;8:742–52.Crossref, Google Scholar
57. . Efficacy and tolerability of ropinirole in patients with restless legs syndrome and a baseline IRLS total score > or = 24 points–data from the ropinirole clinical trial programme. Curr Med Res Opin 2006;22:1867–77.Crossref, Google Scholar
58. . Acute double-blind, placebo-controlled sleep laboratory and clinical follow-up studies with a combination treatment of rr-L-dopa and sr-L-dopa in restless legs syndrome. J Neural Transm 2003; 110:611–26.Crossref, Google Scholar
59. . One-year treatment with standard and sustained-release levodopa: Appropriate long-term treatment of restless legs syndrome? Mov Disord 2003;18:1184–9.Crossref, Google Scholar
60. . Entacapone prolongs the reduction of PLM by levodopa/carbidopa in restless legs syndrome. Clin Neuropharmacol 2007;30:335–44.Crossref, Google Scholar
61. . Progressive development of augmentation during long-term treatment with levodopa in restless legs syndrome: results of a prospective multi-center study. J Neurol 2010 Feb;257:230–7.Crossref, Google Scholar
62. . Efficacy of pergolide in treatment of restless legs syndrome. Neurology 2004;62:1391–7.Crossref, Google Scholar
63. . Efficacy of cabergoline in restless legs syndrome: A placebo-controlled study with polysomnography (CATOR). Neurology 2006;67:1040–6.Crossref, Google Scholar
64. . Effective cabergoline treatment in idiopathic restless legs syndrome. Neurology 2004;63:2272–9.Crossref, Google Scholar
65. . Cabergoline is an effective single-drug treatment for restless legs syndrome: Clinical and actigraphic evaluation. Sleep 2003;26:815–8.Crossref, Google Scholar
66. . Long-term safety and efficacy of cabergoline for the treatment of idiopathic restless legs syndrome: Results from an open-label 6-month clinical trial. Sleep 2004;27:674–82.Crossref, Google Scholar
67. . Cabergoline compared to levodopa in the treatment of patients with severe restless legs syndrome: Results from a multi-center, randomized, active controlled trial. Mov Disord 2007;22:696–703.Crossref, Google Scholar
68. . Valvular heart disease and the use of dopamine agonists for Parkinson's Disease. N Engl J Med 2007;356:39–46.Crossref, Google Scholar
69. . Cardiac and noncardiac fibrotic reactions caused by ergot- and nonergot-derived dopamine agonists. Mov Disord 2009;24:129–33.Crossref, Google Scholar
70. . Valvular heart disease and the use of cabergoline for the treatment of prolactinoma. Clin Endocrinol (Oxf) 2009;70:104–8.Crossref, Google Scholar
71. . Gender effects on cardiac valvular function in hyperprolactinaemic patients receiving cabergoline: a retrospective study. Clin Endocrinol (Oxf) 2010;72:53–8.Crossref, Google Scholar
72. . Valvular heart disease in Parkinson's disease vs. controls: An echocardiographic study. Mov Disord 2006;21:1109–13.Crossref, Google Scholar
73. . Meta-analysis of heart valve abnormalities in Parkinson's disease patients treated with dopamine agonists. Mov Disord 2007;22:1936–42.Crossref, Google Scholar
74. . Assessment of cardiac valve dysfunction in patients receiving cabergoline treatment for hyperprolactinaemia. Clin Endocrinol (Oxf) 2010;73:369–74.Crossref, Google Scholar
75. . Dopamine agonists and cardiac valvulopathy in Parkinson disease: a case-control study. Neurology 2006;67:1225–9.Crossref, Google Scholar
76. . A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep 1993; 16:717–23.Crossref, Google Scholar
77. . Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep 1993; 16:327–32.Crossref, Google Scholar
78. . Treatment of restless legs syndrome with tramadol: an open study. J Clin Psychiatry 1999;60:241–4.Crossref, Google Scholar
79. . Long-term follow-up on restless legs syndrome patients treated with opioids. Mov Disord 2001;16:1105–9.Crossref, Google Scholar
80. . Methadone for refractory restless legs syndrome. Mov Disord 2005;20:345–8.Crossref, Google Scholar
81. . Augmentation of restless legs syndrome with long-term tramadol treatment. Mov Disord 2007;22:424–7.Crossref, Google Scholar
82. . Randomized, double-blind, placebo-controlled study of XP13512/GSK1838262 in patients with RLS. Neurology 2009;72:439–46.Crossref, Google Scholar
83. . A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep 2009;32:159–68.Crossref, Google Scholar
84. . Gabapentin enacarbil in restless legs syndrome: a phase 2b, 2-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol 2009;32:311–20.Crossref, Google Scholar
85. . A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med 2011;7:282–92.Crossref, Google Scholar
86. . A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep 2009;32:159–68.Crossref, Google Scholar
87. . Randomized polysomnography sudy of gabapentin enacarbil in subjects with restless legs syndrome. Mov Disord 2011;Epub ahead of print.Crossref, Google Scholar
88. . A 52-week study of gabapentin enacarbil in restless legs syndrome. Clin Neuropharmacol 2011;34:8–16.Crossref, Google Scholar
89. . Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study. Mayo Clin Proc 2010;85:512–21.Crossref, Google Scholar
90. . Gabapentin versus ropinirole in the treatment of idiopathic restless legs syndrome. Neuropsychobiology 2003;48:82–6.Crossref, Google Scholar
91. . Treatment of restless legs syndrome with gabapentin: A double-blind, cross-over study. Neurology 2002;59:1573–9.Crossref, Google Scholar
92. . A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med 2010; 11:512–9.Crossref, Google Scholar
93. . Treatment of restless legs syndrome with pregabalin: a double-blind, placebo-controlled study. Neurology 2010;74:1897–904.Crossref, Google Scholar
94. . Treatment of the restless legs syndrome with carbamazepine: a double blind study. Br Med J 1984;288:444–6.Crossref, Google Scholar
95. . Carbamazepine in restless legs: A controlled pilot study. Eur J Clin Pharmacol 1983;25:323–4.Crossref, Google Scholar
96. . Nocturnal myoclonus in restless legs syndrome: effect of carbamazepine treatment. Funct Neurol 1989;4:263–71.Google Scholar
97. . Randomized, double-blind, placebo-controlled study of clonidine in restless legs syndrome. Sleep 1996;19:52–8.Google Scholar
98. . [Successful clonidine treatment of restless legs syndrome in chronic kidney insufficiency]. Schweiz Med Wochenschr 1989; 119:184–6.Google Scholar
99. . A randomized, double-blind placebo-controlled trial of iron in restless legs syndrome. Eur Neurol 2000;43:70–5.Crossref, Google Scholar
100. . Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo-controlled study. Sleep Med 2009;10:973–5.Crossref, Google Scholar
101. . A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med 2009;10:206–11.Crossref, Google Scholar
102. . A randomized, double-blind, placebo controlled, multi-center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord 2009;24:1445–52.Crossref, Google Scholar
103. . The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med 2004;5:231–5.Crossref, Google Scholar
104. . Intravenous iron dextran for severe refractory restless legs syndrome. Sleep Med 2010;11:494–6.Crossref, Google Scholar
105. . Low-molecular weight iron dextran and iron sucrose have similar comparative safety profiles in chronic kidney disease. Kidney Int 2008;73:528–30.Crossref, Google Scholar
106. . Update on adverse drug events associated with parenteral iron. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 2006;21:378–82.Crossref, Google Scholar
107. . Efficacy of rotigotine transdermal system in severe restless legs syndrome: A randomized, double-blind, placebo-controlled, six-week dose-finding trial in Europe. Sleep Med 2008;9:228–39.Crossref, Google Scholar
108. . Patch application of the dopamine agonist rotigotine to patients with moderate to advances stages of restless legs syndrome: A double-blind, placebo-controlled pilot study. Mov Disord 2004; 19:1432–510.Crossref, Google Scholar
109. . Efficacy of rotigotine for treatment of moderate-to-severe restless legs syndrome: A randomised, double-blind, placebo-controlled trial. Lancet Neurol 2008;7:595–604.Crossref, Google Scholar
110. . Rotigotine improves restless legs syndrome: a 6-month randomized, double-blind, placebo-controlled trial in the United States. Mov Disord 2010;25:1675–83.Crossref, Google Scholar
111. . Rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome: a randomized, placebo-controlled polysomnographic study. Sleep Med 2010;11:848–56.Crossref, Google Scholar
112. . One year open-label safety and efficacy trial with rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome. Sleep Med 2008;9:865–73.Crossref, Google Scholar
113. . Treatment of moderate to severe restless legs syndrome: 2-year safety and efficacy of rotigotine transdermal patch. BMC Neurol 2010;10:86.Crossref, Google Scholar
114. . Influence of domperidone on pharmacokinetics, safety and tolerability of the dopamine agonist rotigotine. Br J Clin Pharmacol 2009;67:209–15.Crossref, Google Scholar
115. . Transdermal lisuride: short-term efficacy and tolerability study in patients with severe restless legs syndrome. Sleep Med 2006;7:31–5.Crossref, Google Scholar
116. . Lisuride treatment of restless legs syndrome: First studies with monotherapy in de novo patients and in combination with levodopa in advanced disease. J Neural Transm 2006;113:87–92.Crossref, Google Scholar
117. . Amantadine is beneficial in restless legs syndrome. Mov Disord 2000;16:579–81.Crossref, Google Scholar
118. . The treatment of the restless legs syndrome with clonazepam: a prospective controlled study. Can J Neurol Scie 1986;13:245–7.Crossref, Google Scholar
119. . Clonazepam and vibration in restless legs syndrome. Acta Neurol Scand 1984;69:428–30.Crossref, Google Scholar
120. . Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med 1996;100:333–7.Crossref, Google Scholar
121. . Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) Acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol 2001;11:153–61.Crossref, Google Scholar
122. . Proposed dose equivalence between clonazepam and pramipexole in patients with restless legs syndrome. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:522–6.Crossref, Google Scholar
123. . Treatment of idiopathic restless legs syndrome (RLS) with slow-release valproic acid compared with slow-release levodopa/benserazid: A randomized, placebo-controlled, double-blind, cross-over study. J Neurol 2004;251:579–83.Crossref, Google Scholar
124. . Does Valerian improve sleepiness and symptom severity in people with restless legs syndrome? Altern Ther Health Med 2009;15:22–8.Google Scholar
125. . Gender differences in the association between antidepressant use and restless legs syndrome. Mov Disord 2009;24:1054–9.Crossref, Google Scholar
126. . Factors potentiating the risk of mirtazapine-associated restless legs syndrome. Hum Psychopharmacol 2008;23:615–20.Crossref, Google Scholar
127. . Restless legs syndrome as side effect of second generation antidepressants. J Psychiatr Res 2008;43:70–5.Crossref, Google Scholar
128. . Regular intake of non-opioid analgesics is associated with an increased risk of restless legs syndrome in patients maintained on antidepressants. Eur J Med Res 2002;7:368–78.Google Scholar
129. . Antidepressant medication use and restless legs syndrome in patients presenting with insomnia. Sleep Med 2005;6:443–50.Crossref, Google Scholar
130. . Cognitive behavioural group therapy to improve patients' strategies for coping with restless legs syndrome: a proof-of-concept trial. J Neurol Neurosurg Psychiatry 2008;79:823–5.Crossref, Google Scholar
131. . Pneumatic compression devices are an effective therapy for restless legs syndrome. Chest 2009;135:74–80.Crossref, Google Scholar
132. . Sequential compression devices for treatment of restless legs syndrome. Medicine 2007;86:317–23.Crossref, Google Scholar
133. . Exercise and restless legs syndrome: A randomized controlled trial. J Am Board Fam Med 2006;19:487–93.Crossref, Google Scholar
134. . A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. Am J Kidney Dis 2001;38:104–8.Crossref, Google Scholar
135. . Gabapentin versus levodopa for the treatment of restless legs syndrome in hemodialysis patients: An open-label study. Ren Fail 2004;26:393–7.Crossref, Google Scholar
136. . A double-blind, placebo-controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. Am J Kidney Dis 2004;43:663–70.Crossref, Google Scholar
137. . Ropinirole as a treatment of restless legs syndrome in patients on chronic hemodialysis. Clin Neuropharmacol 2004;27:178–81.Crossref, Google Scholar
138. . Pramipexole for the treatment of uremic restless legs in patients undergoing hemodialysis. Neurology 2004;62:831–2.Crossref, Google Scholar
139. . Intradialytic aerobic exercise training ameliorates symptoms of restless legs syndrome and improves functional capacity in patients on hemodialysis - A pilot study. ASAIO Journal 2008;54:185–90.Crossref, Google Scholar
140. . Non-pharmacological management of periodic limb movements during hemodialysis session in patients with uremic restless legs syndrome. ASAIO Journal 2010;56:538–42.Crossref, Google Scholar
141. . Assessment of the symptomtic benefit of cool dialysate. Nephron 1989;1989:166–9.Crossref, Google Scholar
142. . Restless legs syndrome treated successfully by kidney transplantation - A case report. Clin Transplants 1986:138.Google Scholar
143. . Comparison of idiopathic and uremic restless legs syndrome: results of a database of 134 patients (abstract). Mov Disord 1996;11(Suppl):98.Google Scholar
144. . L-DOPA therapy of uremic and idiopathic restless legs syndrome: a double-blind crossover trial. Sleep 1995;18:681–8.Crossref, Google Scholar
145. . L-dopa in uremic patients with the restless legs syndrome. Int J Neurosci 1987;35:233–5.Crossref, Google Scholar
146. . L-DOPA/carbidopa for nocturnal movement disorders in uremia. Sleep 1996; 19:214–18.Google Scholar
147. . Clonazepam: effective treatment for restless legs syndrome in uraemia. Br Med J 1981;283:885–6.Crossref, Google Scholar
148. Bennett WMAronoff GR, eds. Drug prescribing in renal failure. Philadelphia: American College of Physicians, 1994.Google Scholar
149. . Pregabalin in restless legs syndrome with and without neuropathic pain. Acta Neurol Scand 2007;115:347–50.Crossref, Google Scholar
150. . The effect of endovenous laser ablation on restless legs syndrome. Phlebology 2008;23:112–7.Crossref, Google Scholar
151. . The effect of sclerotherapy on restless legs syndrome. Dermatol Surg 1995;21:328–32.Crossref, Google Scholar
152. . Exogenous melatonin in Periodic Limb Movement Disor der: An open clinical trial and a hypothesis. Sleep 2001 ;24:183–7.Google Scholar
153. . Valproate for sleep consolidation in Periodic Limb Movement Disorder. J Clin Psychopharmacol 2000;20:574–8.Crossref, Google Scholar
154. . Treatment of periodic limb movements in sleep with selegiline HCl. Mov Disord 2002;17:398–401.Crossref, Google Scholar
155. . Poor effect of guideline based treatment of restless legs syndrome in clinical practice. J Neurol Neurosurg Psychiatry 2010;81:1390–5.Crossref, Google Scholar



