Parasomnias: A Review for Psychiatrists
Abstract
The category of sleep disorders known as parasomnias includes behavioral disturbances occurring during sleep or states of mixed sleep and wakefulness. They can be minimal and confined to vocalizations or minor movements or of a magnitude that can lead to serious injury, disruption of relationships, and diagnostic ambiguity. Many can be mistakenly thought to represent manifestations of psychiatric disorders. Careful evaluation and therapy can prevent inappropriate psychiatric diagnosis, avoid ineffective treatment, and ameliorate the sleep disorders. Herein, many parasomnias will be brought to the attention of practicing psychiatrists who can learn to recognize enough of the most important clinical features to ensure appropriate consultation with a sleep medicine specialist.
Introduction
Parasomnias are undesirable physical, experiential, or behavioral phenomena that occur exclusively during sleep or are exacerbated by the sleeping state. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM−5) distinguishes non-rapid eye movement (NREM) sleep arousal disorders (sleepwalking, sleep terror types), nightmare disorder (formerly known as dream anxiety disorder), and rapid eye movement (REM) sleep behavior disorder (1). The International Classification of Sleep Disorders, Second Edition (ICSD−2) distinguishes them as disorders of arousal or partial arousal from non-REM sleep, disorders usually associated with REM sleep, and other unusual parasomnias ranging from sleep-related groaning to eating and sexual behavior (3). Clinical significance is in proportion to disruption of sleep and risk of injury to patient as well as bed partner.
The basic premise that underlies parasomnias is that REM sleep, NREM sleep, and wakefulness are not always mutually exclusive states and that behavioral and electrophysiological components of each may dissociate from the parent state and recombine in aberrant form. When components of NREM sleep and wakefulness occur in conjunction, there may be variable juxtapositions or oscillations of consciousness, memory, motor, and/or autonomic activation. When skeletal muscle atonia of REM sleep is deficient, complex dream enactment behavior can be liberated during spells of REM sleep behavior disorder (4). Such admixtures of sleep phenomena and elements of wakefulness can easily be confused with primary psychiatric disorders such as when bizarre motor and/or emotional behavior is mistakenly interpreted as symptomatic of waking psychosis.
Non-REM Sleep Parasomnias: Disorders of Arousal
Sleepwalking and Sleep Terrors
Definition
NREM parasomnias are phenomena that emerge during arousal without complete awakening from sleep. Not included in DSM−5 is the momentary arousal with confusion, disorientation, and minimal movement without ambulation or autonomic activation known as confusional arousal. Sleepwalking (SW) represents motor activation during an abnormal arousal, usually from deep NREM sleep. Current diagnostic criteria in the DSM−5 include:
(A) repeated episodes of arising from bed during sleep and walking about with blank, staring face, unresponsiveness to others, and awakening only with difficulty.
There is typically (B) no or little recall of dream imagery, and
(C) none for the behavioral episodes. Events are associated with
(D) significant distress or impairment in social, occupational, or other areas of functioning,
(E) no influence of an exogenous substance, nor
(F), any coexisting psychiatric or medical disorders to explain the spells (1).
Behaviors can vary from sitting up in the bed (confusional arousal) to full ambulation, and can include complicated behaviors such as walking, running, driving, and eating. These behaviors can occasionally result in sleep-related injury. When mental activity is recalled, it is described as dream-like visual imagery, both less detailed and less bizarre than traditional REM dream reports. Typically, these behaviors occur over minutes and more rarely over an hour or more during the early part of the sleep cycle, and emerging from the “deeper” stage N3 of NREM sleep, they may derive from any NREM stage. Note that sleep stage scoring was revised in 2007 and stages formerly designated as 3 and 4 of NREM sleep have been combined into the single stage now known as N3. Other stages of NREM sleep are now designated as N1 and N2 (2).
This disorder of arousal, like those mentioned below, occurs when there is incomplete transition from NREM sleep to wakefulness. Phenomena that deepen sleep and enhance sleep inertia promote NREM parasomnias by impairing otherwise normal arousal mechanisms. These include sedative medications as well as increased homeostatic sleep drive such as those following sleep deprivation. Also, disorders causing repeated cortical arousals can lead to NREM parasomnias emerging during sleep fragmentation. These can include exogenous stimuli such as noises, or endogenous factors such as obstructive sleep apnea (OSA), periodic limb movements of sleep (PLMS), gastroesophageal reflux disorder, other physical illness, or full bladder, without any specific psychological meaning (3–8).
Sleep terrors (ST) are spells of abrupt “terror” arousals from sleep usually initiated with a scream. There is intense fear along with profound autonomic arousal including mydriasis, tachycardia, tachypnea, and diaphoresis. The remaining diagnostic criteria (B–F) are identical to those listed above for SW (1). While not distressing to the amnestic individual, ST are very troubling to parents and other witnesses. As with SW, the individual is relatively unresponsive to attempts by others to provide comfort and consolation. Often, there can be accompanying motor behavior, including sitting up in bed and possible progression to SW. SW and ST, often occurring in combination, represent a continuum of events with exclusively motor or autonomic activation as polar examples (3, 9, 10).
Various predisposing and priming factors frequently lead to SW either in isolation or in combination. Many individuals report that the frequency and severity of SW increases with stressful life experiences (11, 12). Cases have been associated with migraine and thyrotoxicosis, fever, prior sleep deprivation, and central nervous system depressant drugs including alcohol. SW and ST both appear to demonstrate familial patterns. In one study, 80% of sleepwalkers and 96% of patients with ST could identify a family member who suffered from similar phenomena (13). Central nervous system acting polypharmacy is often the setting for prolonged dangerous behavior such as sleep driving (14, 15). Other examples include patients with OSA who are prescribed sedative-hypnotic medication to assist with continuous positive airway pressure (CPAP) compliance. A similar situation occurs often in patients with restless legs syndrome (RLS) misdiagnosed as having insomnia and subsequently treated with a benzodiazepine receptor agonist (BZRA) drug. As patients with RLS have a strong drive to ambulate, it is not unexpected that agents that suppress memory and executive function would lead to amnestic sleepwalking behaviors. When SW is associated with sedative hypnotics, it is of particular importance to reconsider the diagnosis for which the medication was originally prescribed. In these cases, patients may not have insomnia (for which the sedating agent was prescribed) but rather another disorder of sleep initiation such as RLS or a delayed circadian rhythm (3, 9, 10, 15–20).
Epidemiology and Biopsychosocial Underpinnings
Commonly beginning in childhood, SW peaks between 11 and 12 years of age. SW and ST generally subside in later childhood and adolescence but may continue into, and rarely arise during adulthood. The prevalence of disorders of arousal has been estimated at 1%−6.5% for ST and 5%−30% for SW in children and adolescents (21–23). It has been estimated that 2%−5% of adults may experience SW (24–26). A large systematic telephonic survey of individuals aged 15 years and older in the United Kingdom documents ST in 2.2% (2.6% for ages 15–24 and 1.0% for ages >65), SW episodes in 2.0% (4.9% for ages 15–24 and 0.5% for ages >65), and confusional arousals in 4.2% (8.9% for ages 15–24 and 1.4% for ages >65). In the same population, 2.0% of all respondents reported some violent behavior during sleep. Pure ST occurs more frequently in young children, arising in approximately 3% of children and less than 1% of adults (24, 27, 28).
Historically, SW has been thought to be associated with psychopathology in adults (29–33). A study of 54 adults with SW/ST from a study of 100 patients with sleep-related injury based upon PSG, psychiatric interview, MMPI, Beck Depression Inventory (BDI), and SCL-90 (34), includes current DSM–III axis I diagnoses in only 19/54 (35.2%) cases. Of these, 14/19 (37.6%) were mood disorders and 4/19 (21.1%) alcohol and/or substance use disorders. These did not appear to be temporally associated with the onset of parasomnias. MMPI data available for 36 of the 54 adults (66.7%) suggested possible personality disorders in only 12 (33.3%) (5). A retrospective review of 11 cases of ST cites 7/11 patients reporting an influence of stress on their disturbance, though none had a diagnosis of panic disorder and the course of the sleep disorders did not overlap significantly with any lifetime mood or substance use disorders. The authors conclude that ST are “not simply symptoms of a psychiatric disorder” (10, 12, 35). Recent epidemiological evidence suggests that there is some association between SW, major depression, and obsessive-compulsive disorders, though with no established causal relationship (36).
Of particular interest to psychiatrists are cases of parasomnias related to the use of psychotropic drugs, in particular sedative hypnotics. One group of investigators noted a high frequency of SW and other amnestic complex behaviors among psychiatric patients taking BZRA medication (15, 37, 38). These findings are consistent with other reports of abnormal nocturnal behavior induced by these drugs, especially zolpidem (15, 18, 38–46). These parasomnia behaviors can be prolonged and include amnestic nocturnal eating, sexual activity, and even sleep-driving. Not unexpectedly, these events have increased in parallel with the rise in the use of these sedative-hypnotic agents (15). Note that it is the short duration of action hypnotics that seem to be associated with this phenomenon, whereas the longer duration benzodiazepines tend to suppress them. It is likely that these drugs dull the transition between sleep and wakefulness, permitting behavior that is unconstrained by executive function in susceptible individuals. Other drugs associated with SW and ST include the use of neuroleptics such as olanzapine (47, 48) and quetiapine (49), antidepressants including paroxetine (50, 51), reboxetine (52), and bupropion (53), mood stabilizers and antiepileptics including lithium (54–56), topiramate (57), valproic acid combined with zolpidem (39), stimulants, and antihistamines, often in various combinations (6, 7, 18, 58–63). Two other medications associated with SW/ST are metoprolol (64) and fluoroquinolone (65).
Nathaniel Kleitman, the “father” of American sleep research, wrote, “all the characteristics of somnambulism underline the difference between wakefulness and consciousness” (66). This idea is reinforced by a report of single photon emission computed tomography (SPECT) imaging during a polygraphically documented SW episode, demonstrating increased cerebral blood flow (CBF) in the anterior cerebellum (vermis) and posterior cingulate cortex when compared with quiet slow wave sleep. There were also large areas of frontal and parietal cortical decrements of CBF when compared with normal awake subjects. As anticipated by Kleitman, SW appears to represent a concurrence of increased motor activation and decreased executive function during incomplete, disordered arousals from sleep (67).
On PSG, there are few diagnostic markers of disorders of arousal in the absence of a spell. Bursts of slow EEG waveforms known as hypersynchronous delta activity are possible indicators of a drive to enhance depth of sleep but are not specific to these disorders (68). Actual episodes of sleepwalking or sleep terrors are not observed very often during PSG. When they occur, they appear as abrupt arousals from non-REM sleep, typically but not exclusively from stages 3/4. With sleep terrors, there may be impressive tachycardia and tachypnea. Muscle activity often obscures the underlying EEG, which can demonstrate diffuse rhythmic delta activity, diffuse delta and theta activity intermixed with alpha and beta activity, and/or prominent alpha and beta activity. Hence, the EEG during episodes of disorders of arousal can show either the complete persistence of sleep, the admixture of sleep and wakefulness, or complete wakefulness in spite of the behavioral manifestations of a mixed state (3, 9, 10, 69, 70).
Case example
A 25-year-old man has a history of childhood sleep terrors, reporting numerous episodes between the ages of 6 and 10. He would awaken his parents by screaming loudly but be unresponsive to their attention and inconsolable about 2 hours after he had initially fallen asleep. He would return to sleep after a few minutes and subsequently be totally amnestic for the event. He was otherwise healthy with no daytime psychiatric symptoms. After age 19, he would find himself awakening every few months outside of his bed in his home and unaware of how he had gotten there. He began experiencing such events 2–3 times weekly after starting a new job. He was admitted to the emergency room with a large laceration of his right forearm after punching a bedroom window. He recalled only vague dream content with frightening dark animal figures. He felt no pain, but was awakened by his wife to discover copious bleeding. A PSG study was eventually performed and demonstrated an abrupt arousal from stage N3 sleep with loud moaning, flailing of upper extremities, and lower extremity kicking that all subsided after 30 seconds with resumed NREM sleep (Figure 1). The use of clonazepam 0.5 mg h.s. for 2 months was associated with the elimination of these spells and the patient also learned a self-hypnotic relaxation-mental imagery exercise that he continues to practice nightly. Remission has persisted for 1 year.
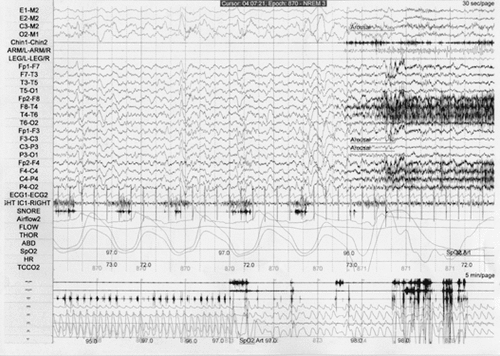
a During this 30-second epoch of polysomnography, there was an abrupt arousal from non-REM stage N3 sleep with moaning, arm flailing movement. Patient was amnestic for this episode.
Treatment and outcomes of Sleepwalking/Sleep Terrors
Sleepwalking and sleep terrors are often benign and self-limiting, especially in children, and may require no treatment beyond reassurance and attention to sleep hygiene. Attention should be paid to safety features of the sleep environment such as placement of dangerous obstacles, accessible windows and stairways, and other dangers. If falls from bed are possible, consider placing the mattress on the floor.
Reversing the comorbid conditions that predispose, prime, and precipitate parasomnias often dramatically diminishes nocturnal behaviors. Discontinuing offending agents will typical resolve the parasomnia, particularly if another underlying condition is identified and treated (15, 17, 71). Identifying and reversing sleep-disordered breathing often results in a resolution of NREM parasomnias. In one study, 60 SW patients were studied with PSG, treated accordingly, and followed for 1 year. A high number (N=53) were diagnosed as having sleep-disordered breathing. The majority of patients had only a mild burden of disease, often not reaching the criteria for OSA, but instead reaching the criteria for upper airway resistance syndrome (UARS); they did not demonstrate daytime sleepiness. However, the results were striking. Only three patients dropped out of the study while of the remaining 50, all reported resolution of SW after treatment (42 reported CPAP, eight reported upper airway surgery). These dramatic results suggest that treatment of even mild, asymptomatic sleep-disordered breathing may result in the resolution of SW (72).
In cases with a low risk of imminent injury, nonpharmacological therapy with clinical hypnosis may be preferred. This can be offered initially in combination with medication, which can be gradually withdrawn. Patients are instructed in the induction of a relaxed, meditative state, with visual imagery of quiet, restful sleep associated with comfort, safety, and reinforcement of a possible but minimally probable need for physical mobilization. With self-hypnosis utilized before retiring to bed, benefit has been reported in 20/27 (74%) patients who reported significant or very significant improvement (73). In another study with similar techniques, 3/6 (50%) SW patients were greatly improved or spell-free after 18 months and likewise for 2/3 (67%) patients after a 5-year follow-up (74). Progressive muscle relaxation training has also been utilized (75). In children, the use of anticipatory awakenings (76), clinical hypnosis (77, 78), and a combination of acupuncture with medicinal herbs have been documented as helpful (79).
When NREM parasomnias persist despite the resolution of inducing and exacerbating factors, pharmacological intervention is considered for disorders associated with the risk of injury or disturbance of the home environment. Evidence for all therapies for these disorders is limited to case reports, and rare controlled clinical trials with limited sample sizes. Complicating things further are some contradictory findings in the literature (80). The earliest pharmacological agents offered to individuals at serious risk of injury were diazepam (81) and imipramine (82). Other agents considered to be anecdotally effective include other benzodiazepines, carbamazepine, doxepin, trazodone (61), and melatonin (83). All pharmacotherapy for parasomnias is currently utilized as “off label,” without formal FDA indications.
Intermediate and long-acting agents in the benzodiazepine class of sedative hypnotics (BZD) have become the most commonly reported pharmacological treatments for NREM parasomnias. Clonazepam has been reported to be effective in doses of 0.25–2.0 mg taken about 30–120 minutes before sleep. When given to 28/54 (51.9%) patients with SW/ST, it produced substantial benefit in over 80% (5). In 1996, a series of 170 patients with mixed sleep disorders (69 with SW/ST) were treated with benzodiazepines, primarily clonazepam (N=136), and followed for clinical response (84). The vast majority of all patients (86%) reported good control after an average follow-up of 3.5 years. We reported that clonazepam efficacy was sustained with low risk of dosage escalation. A separate clinical case series reported on six SW patients who were initiated on clonazepam. SW was suppressed in five of six patients (85). The drug may work through the suppression of cortical arousals, but this is clearly paradoxical when considering the induction of amnestic nocturnal behaviors by the BZRA drugs (15).
Conversely, a more recent report claims that clonazepam failed to demonstrate sustained efficacy in five SW patients. This investigation carefully excluded even subtle sleep-disordered breathing. After 1 year, all patients treated with clonazepam dropped out of the study and reported a persistence of SW (72).
Distinct from BZD and BZRA drugs, a number of antidepressants have been reported to treat NREM parasomnias, most commonly ST. One report described two patients with a history of combined ST and SW, both of whom failed diazepam therapy but responded well to imipramine (a tricyclic antidepressant) (82). The selective serotonin reuptake inhibitor (SSRI) paroxetine appears to be particularly effective in the treatment of ST. In one report, six patients had a significant reduction if not outright elimination of ST events. We suggested that SSRIs might be uniquely effective for ST through the effects of serotonin on terror centers in the midbrain periaqueductal gray matter (86). In contrast to these successful ST cases, a more recent series of SW patients describes eight patients who were treated with various serotonergic agents and/or benzodiazepine. After a 1-year follow-up, all eight patients described a persistence of SW (72). Further, there have been reports of paroxetine and sertraline allegedly inducing SW (50, 51).
Other Non-REM Parasomnias
Sleepwalking with Sleep-Related Eating
An interesting variant of SW is sleepwalking with sleep-related eating, usually designated as sleep-related eating disorder (SRED). SRED is characterized by episodes of eating during behavioral arousal without clearly established wakefulness. Typically, foods high in carbohydrates and fats are ingested, and binging is common. Food choices are often not those ordinarily consumed during daytime hours. Food preparation and consumption can result in safety concerns with adverse health consequences as inedible and toxic substances have been eaten. Studies have demonstrated that hunger is noticeably absent during feeding episodes. Rather, patients often describe restless eating, meaning an urge to eat in order to fall back asleep. It is important for psychiatrists to query patients receiving monoamine oxidase inhibitor antidepressants and their families regarding nocturnal eating or SW/ST because of the risk of possible hypertensive crisis (87, 88).
As in SW, sedating agents in the setting of RLS frequently trigger SRED. The most common offending medications include the benzodiazepam receptor agonists, in particular, zolpidem. In addition, sporadic cases have been reported with tricyclic antidepressants, anticholinergics, lithium, triazolam, olanzapine, and risperidone (66, 71, 88–91). SRED demonstrates some overlap with the night eating syndrome (NES is not designated in DSM−5), which is distinguished by evening hyperphagia along with fully wakeful nocturnal eating (88, 92).
Various forms of psychotherapy, hypnotherapy, and behavior therapy have proven ineffective for SRED. The first step in treating SRED is to identify and treat comorbid sleep disorders, in particular RLS. Of note, effective therapies for SRED are often also anti-RLS treatments including dopaminergics (pramipexole, carbidopa/levodopa), codeine, and clonazepam (87, 93). In a series of 44 RLS patients unexposed to prior use of dopaminergics, the frequency of SRED decreased proportionally with the motor symptoms of RLS during dopaminergic therapy (16). Other reported therapies include combinations of a dopaminergic with bupropion and trazodone (93–95). Monotherapy with the anticonvulsant topiramate has been efficacious in two open-label series. It suppresses nocturnal eating in roughly two-thirds of patients with either the sleepwalking or the idiopathic variant of SRED (96, 97).
Sleepwalking with Sleep-Related Sexual Behavior
Sleep-related abnormal sexual behaviors are generally regarded as variants of confused arousals and SW. There is an established and growing literature on this phenomenon that has also been designated “sleepsex,” “sexsomnia,” and “atypical sexual behaviors in sleep.” Behaviors during sleep can vary from coital movements to fondling and attempted or completed intercourse with a bed partner. A recent review has provided the first classification of all reported abnormal sexual behaviors associated with sleep disorders, including the sexual parasomnias. The majority of published cases involve young-adult males with rich histories of NREM sleep parasomnias, such as sleepwalking and sleep terrors. Most reported cases resolve once the predisposing, priming, and precipitating factors have been addressed. Pharmacotherapy (e.g., clonazepam at h.s.) is typically reserved for recalcitrant cases (98–100).
Parasomnias Usually Associated with REM-Sleep
Nightmare Disorder
Formerly termed dream anxiety attacks, nightmare disorder is now recognized as a REM sleep phenomenon, distinct from NREM ST. As defined in DSM−V, this is classified as an actual disorder if it includes (A) repetitive, extended, extremely dysphoric, and well recalled dreams usually involving threats to survival, security, or physical integrity, usually occurring during the second half of the sleep period. (B) Typically, the individual becomes rapidly alert and oriented. (C) This disturbance causes significant distress or impairment of functioning, (D) is not attributable to a substance, and (E), it is not adequately explained by coexisting psychiatric or medical disorders (1). Emotional manifestations of fear, anger, and sadness may predominate. Nightmares occur in 5%−8% of adults, more commonly women, and individuals with “type A” personality characteristics (101–103). Nightmares can be induced by a number of drugs including some antidepressants and neuroleptics, amantadine, lisuride, clonidine, methyldopa, reserpine, ciprofloxacin, donepezil, and amiodarone. Dopaminergic antiparkinsonian drugs and lipophilic beta adrenergic blockers are particularly notable. Conversely, withdrawal from REM suppressing agents such as tricyclic antidepressants, monoamine oxidase inhibitors, clonidine, alcohol, and amphetamines may cause nightmares as a result of REM sleep rebound. It is not uncommon for nightmares to occur following traumatic experiences, and occasional psychotic episodes may be heralded by their occurrence (3, 104).
Reported treatments include a cognitive strategy known as imagery rehearsal, which treats the disorder as a sleep disturbance rather than a manifestation of specific psychopathology. Patients are taught to restructure the dream scenario into a more acceptable experience by rewriting the script as an exercise during wakefulness (105). Instruction in lucid dreaming has also been cited in a case report (106). Pharmacotherapy, based largely on experience with posttraumatic stress disorder, includes a reported benefit with cyproheptadine and prazosin (107–109).
REM Sleep Behavior Disorder
REM sleep behavior disorder (RBD) is now included in the DSM−5 classification of sleep disorders, characterized by
(A) repeated episodes of arousal during sleep associated with vocalization and/or complex motor behaviors that
(B) arise during REM sleep, usually more than 90 minutes after sleep onset and more frequent during the later part of the sleep period when REM periods tend to be longer. These episodes are therefore uncommon during daytime naps.
(C) When awakening from the episodes, the individual is completely awake, alert, and not confused or disoriented.
(D) The overnight sleep study shows REM sleep without atonia, which could be absent but the diagnosis is sustained if there is a history suggestive of RBD and an established diagnosis of a synucleinopathy such as Parkinson’s disease or multiple system atrophy.
(E) The behaviors cause significant distress or impairment and can be associated with injury to the patient or bed partner.
(F) The disturbance is not attributable to physiological effects of a substance or another medical condition, and
(G), it is not explained by a coexisting psychiatric or medical disorder (1).
RBD typically involves a prolonged, chronic course and is identified most frequently in elderly males. There tends to be a lengthy prodrome in about 25% of patients with increased action-packed dream content along with somniloquy and limb-jerking. As the disorder becomes established, there is a tendency for abrupt, often violent movement concordant with dream content. Dream reports tend to be much more vivid than those reported when dream mentation is recalled during SW/ST. This is related to the intensity of REM mentation and to the low arousal threshold of REM sleep. Patients typically dream of themselves as defenders, rarely as aggressors. Violent dream enactment can result in injury to the patient and/or bed partner whose presence is often incorporated into the dream content. RBD spells are likely to occur during the latter part of the night when REM sleep tends to be more prolonged and intense, in contrast to SW/ST, which may occur earlier in the course of the night when stage N3 is more likely.
PSG is diagnostic of the disorder with fluctuating levels of skeletal motor (rarely autonomic) activation during REM sleep. Normally, alpha motor neurons are hyperpolarized during REM sleep with resulting atonia of voluntary muscles. This is disrupted in RBD, and manifested by varying degrees of muscle tone on electromyogram (EMG) monitoring of submental (chin), and limb muscles. Increased muscular twitching is also seen, even when complex behavior is not recorded but documented by history. There is often an increase in the percentage of sleep time spent in stage REM (>25%), a reduction of the REM sleep latency <75 minutes, and an increase in the actual number of rapid eye movements. There tends to be an increase of stage N3 beyond that expected for age. A technique for quantitative scoring of motor activation during REM sleep has been described in the literature (110–112).
Epidemiology and Biopsychosocial Underpinnings
During the decade following the initial description of RBD, approximately half of newly diagnosed cases appeared to be associated with central nervous system disorders, ranging among degenerative processes, narcolepsy, vascular disorders, cerebral astrocytoma, multiple sclerosis, and Guillain-Barré syndrome. With time, the gradual development of Parkinson’s disease was reported in 38% of 29 previously idiopathic cases, suggesting a pathophysiological relationship (113). After another 7 years, the same cohort revealed an increased prevalence of delayed emergence of parkinsonism and/or dementia of 65% (114). Recent data now indicate the eventual development of neurodegenerative disorders in 81% of cases (115). Indeed, RBD is now recognized as “cryptogenic” rather than idiopathic, and appears to be an early component of neurodegenerative disease. More specifically, these are disorders marked by deposits of alpha synuclein such as in Parkinson’s disease, Lewy body disease, and multisystem atrophy (116, 117). Pathophysiology of REM sleep motor control seems to be localized in the subcoeruleus region of the pons, which is involved in the generation of REM sleep components including atonia (118). In cases of various synucleinopathies, RBD prevalence appears to be 19%−77%. Conversely, in tauopathies such as Alzheimer’s disease, corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia, RBD is rare and if present usually follows the onset of the neurological condition (119). In RBD, neuropsychological testing has revealed dysfunctional visuospatial constructional ability and altered visuospatial learning consistent with an impending Lewy body type of dementia. Anosmia can be an early feature suggesting the possibility of synucleinopathy (120).
RBD is becoming a more frequent disturbance in younger individuals and in females, because of its relationship with the use of antidepressant drugs that can cause REM sleep without atonia and frank oneiric behavior. It is not yet known if this form of the sleep disorder carries a similar risk of neurodegenerative disorders (121). There is a recent report of 100 patients with “idiopathic” RBD associated with antidepressant use. Features of prodromal neurodegeneration such as olfactory and color vision deficits, mild cognitive impairment, and other markers were present more than in matched control subjects. Though having lower risk of neurodegenerative disorders than typical “idiopathic” RBD, it is possible that drug-related RBD may still have some association with underlying neurodegeneration (122). This creates ambiguity because the diagnostic criteria above technically disallow a diagnosis of RBD if attributable to drug effect.
Case Example
A 70-year-old man was admitted to the emergency room for treatment of right metatarsal fractures sustained when he kicked the wall adjacent to his bed at 4 a.m. when dreaming vividly of fighting with a large, brown, aggressive dog that was chasing him. This sort of dream-enactment behavior with varying vivid imagery had been occurring a few times weekly over the previous 6 months with a longer history of action-packed dreaming without injurious behavior. His wife continues to share their bed in spite of numerous bruises to her legs from his kicking. When studied in the sleep laboratory, there was variable continuation of electromyographic submental muscle tone during stage R, with intermittent vigorous upper and lower extremity movements and unintelligible vocalization (Figure 2). These spells were eliminated with clonazepam 1.0 mg and melatonin 6 mg at h.s. and have not recurred during 5 years of follow-up. He has recently described anosmia and has developed a parkinsonian tremor of both hands.
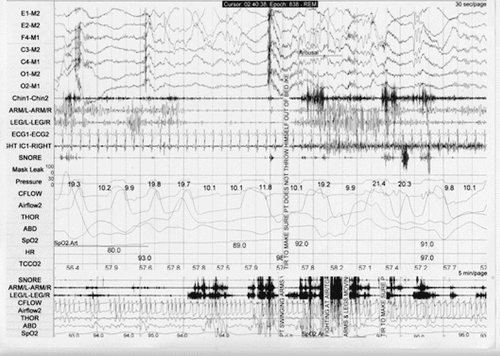
a During this 30-second epoch of polysomnography, there was increased muscle tone in the chin electromyogram as well as upper and lower extremity movement during stage REM. The technologist entered the room to ensure that the patient would not injure himself if we were to “throw himself out of bed.”
Treatment and Outcomes of REM Behavior Disorder
Thus far, there are no randomized clinical trial data or any FDA indication for pharmacotherapy of RBD. Most original reports suggested that clonazepam was the first line of therapy for RBD. The highly activated dream and behavioral manifestations of the disorder generally respond to modest doses of 0.5–2.0 mg taken about 30 minutes before retiring. The mechanism of action is apparently suppression of phasic motor activity and behavioral release rather than restoration of normal REM atonia. While the majority of patients respond to clonazepam at first, long-term follow up studies are mixed. The results range from sustained benefit without dose escalation to others with a high incidence of dose escalation and treatment failure (89, 123–125). In one series, 58% of patients on clonazepam reported clinically significant adverse effects with 50% either stopping the agent or reducing the dose (125). Clonazepam is particularly problematic in the setting of advanced neurodegenerative disease where its prolonged duration of action may result in morning sedation as well as cognitive and gait impairment (126, 127).
Melatonin is now considered to be a first-line therapy of RBD, with the mechanism of action apparently involving substantial restoration of REM atonia (128–130). Importantly, a recent direct comparison study noted that melatonin (median 6 mg, maximum 25 mg) was equal to clonazepam (median dose 0.5 mg, maximum 3 mg) in treatment efficacy and superior in side effect profile. Patients on melatonin reported fewer adverse effects, particularly with respect to falls and injuries as compared with clonazepam (131). Pramipexole may be effective in mild cases of RBD particularly those associated with frequent periodic limb movements (PLMs) (132, 133). There is anecdotal support for other medications being beneficial in patients who do not respond to clonazepam. These include carbidopa/levodopa, donepezil, quetiapine, clozapine, clonidine, l-tryptophan, carbamazepine, rivastigmine (134), and gabapentin.
Recurrent Isolated Sleep Paralysis
Sleep paralysis is essentially the atonia of REM sleep that has become dissociated and occurring at times other than the typical periods of REM sleep during the night. It can either intrude into light NREM sleep at sleep onset or persist into awakening at the offset of sleep. In either case, its occurrence during sleep-wake transitions is often experienced as discomforting or frightening. It is classically found in association with the quintessential dissociation of REM sleep components in narcolepsy, but it is not specific for this disorder. It is similar to cataplexy, which occurs exclusively with narcolepsy during full wakefulness and typically in response to emotional arousal such as laughter. Isolated sleep paralysis may occur with a lifetime prevalence of 2.3%−40%, depending on the country and study population. Unless the history includes hypersomnia or cataplexy, there is no need for PSG. Treatment is usually unnecessary unless there is significant sleep disruption of subjective distress. In that case, REM-suppressing agents such as fluoxetine or imipramine may be indicated (138).
Other Parasomnias of Interest to Psychiatrists
Sleep-Related Dissociative Disorders (not Included in DSM−5)
Dissociative disorders may also occur during the sleep period, typically in individuals suffering daytime syndromes such as dissociative identity disorder (DID), dissociative amnesia, and unspecified dissociative disorder. In one report, 6/21 (27.5%) patients with daytime dissociative disorders were noted to demonstrate nocturnal dissociative episodes, and two of them showed a clear transition to an alter personality (139). In the original published report, Schenck et al. include only one case of exclusively sleep-related DID without daytime dissociation in a 19-year-old male. Other patients with diurnal dissociative disorders demonstrated nocturnal behaviors resembling SW/ST that can be prolonged, often with amnesia. If observed during PSG, these complex, lengthy, and repetitive behaviors are clearly seen to follow the development of EEG wakefulness in spite of no obvious behavioral awakening (140). Therapy is typically that provided for the wakeful dissociative disorder.
Sleep-Related Groaning (catathrenia, Not Included in DSM−5)
Catathrenia (nocturnal groaning) is typically a longstanding, nightly disorder characterized by expiratory groaning during sleep, especially during the second half of the night in adults of any age and usually without any other sleep disturbance. PSG shows recurrent bradypneic episodes occurring more often during REM sleep and often appearing in clusters, in which a deep inspiration is followed by prolonged expiration accompanied by a monotonous vocalization closely resembling groaning. The prevalence is unknown, but appears to be quite rare. No predisposing factors have been identified and few of the reported patients have a history of any other parasomnia. The main complication is disrupted sleep for the bed partner or others in the same household. Therapy can be challenging; however, some cases have responded to CPAP (149, 150).
Exploding Head Syndrome (not Included in DSM−5)
Exploding head syndrome is characterized by the sudden sensation of a loud noise or sense of a violent, though painless “explosion” in the head occurring as the affected person is falling asleep or waking during the night. It is not a headache disorder. The prevalence is unknown. The median age of onset is 58 years, but onsets at all ages have been reported. Most patients cannot identify precipitating factors. The course is benign with no reported neurological sequelae. Symptoms often appear to remit spontaneously. Exploding head symptoms often coincide with the sleep onset motor phenomena of hypnic myoclonia and may represent a sensory variant of those wake-sleep transition phenomena (143, 144).
Sleep-Related Hallucinations (not Included in DSM−5)
The most common hallucinations emerging in relation to sleep involve hypnagogic (occurring in the twilight of sleep onset) and hypnopompic (occurring on awakening in the morning) hallucinations. These experiences are often components of the narcolepsy syndrome that includes sleep paralysis, hypersomnia, cataplexy, and disturbed nocturnal sleep. Like sleep paralysis, sleep-related hallucinations can occur in isolation among otherwise normal individuals. There may be auditory, tactile, or kinetic features, and spells are often associated with sleep paralysis (145). In contrast, the visual hallucinations of sleep-related complex partial epileptic seizures are usually brief, stereotyped, and fragmentary. Sleep-related migraine with aura at times can be associated with complex visual hallucinations, but a headache should follow. Complex nocturnal visual hallucinations can be associated with the use of beta-adrenergic receptor blocking medications, dementia with Lewy bodies, visual loss (Charles Bonnet hallucinations), and other brain pathology (peduncular hallucinosis) (145).
Additional Varieties
Sleep-Related Epilepsy
Approximately 10% of epileptic disorders will present with exclusively or predominantly sleep-related seizures. There is frequently no history of classical generalized tonic–clonic convulsions and conventional EEG studies may be inconclusive. All night PSG studies utilizing a full montage of EEG leads and continuous audiovisual recording with technologist observation and written commentary are essential. In spite of careful recording, ictal events may be unaccompanied by EEG dysrhythmias, but are frequently responsive to anticonvulsant therapy. Diagnostic confusion is compounded by atypical, unconventional seizures that can present as recurrent dreams, nightmares, sleepwalking, sleep terrors, or psychiatric illness. In particular, orbitofrontal seizures may cause bizarre behavior, peculiar clustering, and a tendency to be nocturnal. Any sleep-related behavior, even events such as apnea, stridor, coughing, laryngospasm, chest pain, arrhythmias, paroxysmal flushing, and localized hyperhidrosis may be caused by unconventional seizures. They should be considered in the differential diagnosis of any sleep-related behavior that is recurrent, inappropriate, and most importantly, stereotyped (4, 146).
Psychiatric Disorders Presenting as Parasomnias
Panic Disorder
Some primary psychiatric disorders may include symptoms that occur prominently or exclusively in association with the sleep period. Though favored or precipitated by sleep, they must be distinguished from primary sleep parasomnias. Panic disorder is well known in its diurnal form. Up to 69% of individuals with this disorder have had a sleep-related panic attack and 33% report recurrent sleep-related spells. Patients with panic disorder also complain of more middle and terminal insomnia than do control subjects (147, 148). Clinical features of these nocturnal spells resemble those of diurnal attacks, and they arouse the patient who rapidly achieves full wakefulness with anxiety and subsequent difficulty returning to sleep. Patients retain full recall of these events. Published PSG studies have documented increased sleep onset latency, decreased sleep efficiency (decreased time asleep during monitored time in bed), and increased duration between sleep onset and the occurrence of the first REM period (increased REM latency), contrary to what has been reported for major depression. When observed in the laboratory, sleep-related panic episodes tend to occur during transitions from lighter (stage N2) to deeper (stage N3) non-REM sleep (149–152). Diagnostic caution must be emphasized because of a myriad of other disorders which can masquerade as nocturnal panic, such as SW/ST, RBD, seizures, gastroesophageal reflux, OSA, bruxism, nocturnal asthma, and nocturnal cardiac arrhythmias.
Posttraumatic Stress Disorder
Sleep disturbances have figured prominently in descriptions of the disorder and have been designated as “hallmark[s]” (153). Sleep complaints include initial insomnia, sleep discontinuity with increased arousal, limb movements, night terrors, nightmares, and even purposeful behavior; sometimes dream enactment that can result in injury to a bed partner who is misperceived as a threat. Earlier PSG studies describe poor sleep continuity as increased percentages of “lighter” non-REM stages 1 and 2 and decreased deeper stages 3 and 4. Nightmares tend to occur less frequently in the laboratory than in the naturalistic environment (154–156). Conflicting claims have appeared concerning the timing of rapid eye movement (REM) sleep, with reports of diminished REM-latency and a lengthening of this interval (157–162). Nightmares, occurring during REM and NREM sleep, tend to be recurrent with repetitive imagery of the traumatic event (156). No study characterizes any definitive, descriptive, polysomnographic pattern that would ultimately have therapeutic implications. In only two reports, motor activation during REM sleep suggests the possibility of RBD (155, 163, 164). Repetitive body movements seen in other stages may resemble those seen in non-posttraumatic stress disorder (PTSD) patients with SW/ST and rhythmic movement disorder (165). Kardiner, in his original descriptions of war neurosis, presents a case that may represent nocturnal dissociative disorder (166).
Some studies of Vietnam combat veterans with PTSD have noted a paucity of any specific findings. As in patients with conditioned, psychophysiological insomnia, sleep laboratory findings can be substantially milder than would be predicted by the history of sleep in the usual home environment (167–169). Indeed, careful review of sleep-related symptoms of PTSD in Vietnam theater veterans in the National Vietnam Veterans Readjustment Study has confirmed that frequent nightmares are hallmark symptoms in those with current PTSD and correlate more with the level of combat exposure than insomnia symptoms (170). It is very possible that objective studies of sleep in PTSD may underreport nightmare frequency and severity because more severely affected subjects may avoid participation (171).
A striking finding in the PTSD sleep literature is the elevation of auditory arousal thresholds in affected subjects during NREM as well as REM sleep (172–175). Kramer interprets these findings as evidence that in chronic PTSD there is a heightened responsiveness to internal events while individuals are less arousable by external stimuli (175). Increased depth of sleep may represent a chronic adaptation to trauma. Kaminer and Lavie describe the diminished dream recall of their better-adjusted Holocaust survivors compared with those less well adjusted (176). The common subjective sleep complaints of patients may reflect a breakdown of this adaptation but with enough resilience to allow intact sleep in a safe, neutral environment such as a sleep laboratory.
Factitious Parasomnia
Rarely, an individual may present for evaluation with a factitious parasomnia. There is no reason to assume that such a disorder could not be chosen for this purpose. Malingering might be expected in situations involving litigation, correctional institutions, military settings, and many others. This must be very carefully considered as a diagnosis by exclusion supported by observations, reliable reports, or other factors that would support the likelihood of malingering. The forensic literature includes numerous examples of sleep-related violence, some of which result in exculpation because of the presence of a sleep disorder (177–179).
Assessment and Differential Diagnosis
The most typical parasomnias are benign and require no evaluation or treatment. The concern they arouse can be addressed through education and reassurance. Any injurious or more distressing parasomnia warrants careful evaluation and may require consultation with an experienced sleep medicine specialist. Some characteristics that distinguish various typical parasomnias are listed in Table 1.
| Confusional arousal | Sleepwalking | Sleep terrors | Nightmare | RBD | |
|---|---|---|---|---|---|
| Age of onset | Any | Childhood | Childhood | Any | Late adult |
| Time of night | Early, any | Early | Early | Late | Late |
| Sleep stage related | N3, N2 | N3, N2 | N3, N2 | REM | REM |
| Duration | Minutes | Momentary | 1–20 minutes | 5–20 minutes | Brief |
| Ambulation | No | Yes | May occur | No | May occur |
| Behavior wakening | Fight/Protect | Minimal | Explore/Flight | Vocal/Fearful | Abrupt |
| Autonomic arousal | No | May occur | Yes | Yes | No |
| Amnesia | Yes | Yes | Yes | No | No if awakens |
| Dream recall | No | Variable/vague | Variable/vague | Vivid | Vivid |
| Treatment | N/A | BZ, TCA, hypnosis | BZ, paroxetine, hypnosis | Prazosin, Dream Rehearsal | BZ, Melatonin |
An initial history should include age of onset, estimations of frequency of spells at various periods in the course of the disorder, any changes over time, medical and psychiatric history, family history, general sleep history with reference to sleep hygiene and observations of respiration, sleep disruption and body movements during sleep, apparent precipitating factors including drug/alcohol abuse or dependence, and prior responses to treatment. Description of the spells must include a reference to the typical time of onset in the sleep period, recent frequency, specific behaviors with special regard for dangers posed to self or other, dream recall, and degree of amnesia for either dream content or actual behavior. If available, a bed partner should be queried to provide a description of the nocturnal behaviors. Psychiatric assessment should focus especially on the presence or absence of mood disorders as well as drug and/or alcohol abuse or dependence.
When behavioral spells are observed during a PSG study, the diagnosis can be confirmed. In a report of 100 consecutive adult patients with sleep-related injury, diagnoses were established by PSG in 65% of the cases (42.6% of SW/ST, 100% of RBD, 42.9% of dissociative disorders, 100% of nocturnal seizures, and 100% of PLMS). Diagnoses were strongly supported in another 26% (48.1% of SW/ST), accounting for an overall positive yield in PSG studies of 91%. Confirmatory findings include behaviors such as moaning, somniloquy, yelling, limb movements, gesturing including violent movements, finger pointing, sitting up abruptly, looking around in a confused manner, apparently hallucinated behavior, and leaving the bed during particular stages of sleep or arousals. Suggestive findings include excessive abrupt, spontaneous arousals from stages of NREM sleep in cases of SW/ST (5, 180).
While behavioral manifestations of many parasomnia disorders can lead to diagnostic confusion, proper evaluation can lead to gratifying therapeutic results with a reduction in the risk of sleep-related injury for sufferers and their bed partners, careful clinical and polysomnographic evaluations can frequently clarify the nature of the most difficult and dangerous of these disorders. Most importantly, the presence of RBD signals the need for careful neurological assessment and follow-up for the detection of emergent neurodegenerative disorders, in particular, Parkinson’s disease. In the future, when neuroprotective therapies become available, the diagnosis of RBD may permit the application of truly preventive medicine (Table 1).
Safety Measures for All Parasomnias
Because sleep-related behaviors pose risk of injury to the affected individual and others, consideration should be given to:
| 1. | Maintenance of good sleep hygiene, such as avoidance of sleep deprivation, appropriate sleep schedule, avoidance of stimulants such as caffeine after noon, and avoidance of evening alcohol. | ||||
| 2. | Keep obstacles such as bedroom furniture at a safe distance from the bed and cushioned if possible. | ||||
| 3. | Consider sleeping on a ground floor if there is danger of falling on stairs. | ||||
| 4. | Consider placing mattress on the floor if there is danger of falling from bed. | ||||
| 5. | Keep weapons securely stored. | ||||
| 6. | Consider sleeping separately from bed partner. | ||||
| 7. | Keep windows closed, locked, and cushioned with shades or curtains if possible. | ||||
| 8. | Keep doors, especially with exterior access, closed and locked. | ||||
1
2 ;
3
4 : Things that go bump in the night: The parasomnias revisited. J Clin Neurophysiol 1990; 7:119–143Crossref, Google Scholar
5 : A polysomnographic and clinical report on sleep-related injury in 100 adult patients. Am J Psychiatry 1989; 146:1166–1173Crossref, Google Scholar
6 : Disorders of arousal and epilepsy during sleep, in Sleep and Epilepsy. Edited by Sterman MShouse MPassouant P. New York, Academic Press, 1982, pp 513–531Google Scholar
7 : Sleepwalking precipitated by treatment of sleep apnea with nasal CPAP. Chest 1991; 99:750–751Crossref, Google Scholar
8 : Arousal reactions in sleepwalking and night terrors in adults: The role of respiratory events. Sleep 2002; 25:871–875Crossref, Google Scholar
9 : Migraine and somnambulism. Neurology 2005; 65:1334–1335Crossref, Google Scholar
10 : Sleepwalking associated with hyperthyroidism. Endocr Pract 2005; 11:5–10Crossref, Google Scholar
11 : Forensic sleep medicine: Nocturnal wandering and violence. Sleep 1995; 18:740–748Crossref, Google Scholar
12 : Sleep-related violence. Sleep 1995; 18:731–739Crossref, Google Scholar
13 : Sleepwalking and night terrors related to febrile illness. Am J Psychiatry 1979; 136:1214–1215Crossref, Google Scholar
14 : Sleep-related violence. Curr Neurol Neurosci Rep 2005; 5:153–158Crossref, Google Scholar
15 : Hypnosedative-induced complex behaviours: Incidence, mechanisms and management. CNS Drugs 2008; 22:1021–1036Crossref, Google Scholar
16 : Restless nocturnal eating: A common feature of Willis-Ekbom Syndrome (RLS). JCSM 2012; 8:413–419Google Scholar
17 : Review of nocturnal sleep-related eating disorders. Int J Eat Disord 1994; 15:343–356Crossref, Google Scholar
18 : Amnestic sleep-related eating disorder associated with zolpidem. Sleep Med 2002; 3:323–327Crossref, Google Scholar
19 : Zolpidem, somnambulism, and nocturnal eating. Gen Hosp Psychiatry 2008; 30:90–91Crossref, Google Scholar
20 : Report of two cases where sleep related eating behavior occurred with the extended-release formulation but not the immediate-release formulation of a sedative-hypnotic agent. J Clin Sleep Med 2008; 4:155–156Google Scholar
21 : Clinical and electrophysiological correlates of sleep disorders in children, in Sleep: Physiology and Pathology. Edited by Kales A. Philadelphia, JB Lippincott, 1969, pp 109–118Google Scholar
22 : Sleep problems in children and their relationship with early disturbances of the waking-sleeping rhythms. Sleep 1983; 6:47–51Crossref, Google Scholar
23 : Prevalence of sleep disorders and sleep behaviors in children and adolescents. J Am Acad Child Psychiatry 1982; 21:383–388Crossref, Google Scholar
24 : Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry 1979; 136:1257–1262Crossref, Google Scholar
25 : Enuresis, sleepwalking and nightmares: an epidemiological survey in the republic of San Marino, in Sleep/Wake Disorders: Natural History, Epidemiology, and Long-Term Evolution. Edited by Guilleminault CLugaresi E. New York, Raven Press, 1983, pp 237–241Google Scholar
26 : Prevalence and genetics of sleepwalking: A population-based twin study. Neurology 1997; 48:177–181Crossref, Google Scholar
27 : Somnambulism in childhood—prevalence, course and behavioral correlations. A prospective longitudinal study (6–16 years). Acta Paediatr Scand 1982; 71:495–499Crossref, Google Scholar
28 : Night terrors, sleepwalking, and confusional arousals in the general population: Their frequency and relationship to other sleep and mental disorders. J Clin Psychiatry 1999; 60:268–276, quiz 277Crossref, Google Scholar
29 : Somnambulism psychiatric interview studies. U S Armed Forces Med J 1956; 7:1143–1153Google Scholar
30 : Somnambulism, its clinical significance and dynamic meaning in late adolescence and adulthood. Arch Gen Psychiatry 1963; 9:400–413Crossref, Google Scholar
31 : Somnambulism: Psychophysiological correlates. II. Psychiatric interviews, psychological testing, and discussion. Arch Gen Psychiatry 1966; 14:595–604Crossref, Google Scholar
32 : Somnambulism. Clinical characteristics and personality patterns. Arch Gen Psychiatry 1980; 37:1406–1410Crossref, Google Scholar
33 : Night terrors. Clinical characteristics and personality patterns. Arch Gen Psychiatry 1980; 37:1413–1417Crossref, Google Scholar
34 SCL-90: an outpatient psychiatric rating scale–preliminary report, Psychopharmacol Bull 1973, 9:13–28Google Scholar
35 : Night terrors in adults: Phenomenology and relationship to psychopathology. J Clin Psychiatry 1992; 53:392–394Google Scholar
36 : Prevalence and comorbidity of nocturnal wandering in the U.S. adult general population. Neurology 2012; 78:1583–1589Crossref, Google Scholar
37 : Parasomnia among psychiatric outpatients: A clinical, epidemiologic, cross-sectional study. J Clin Psychiatry 2008; 69:1374–1382Crossref, Google Scholar
38 : Sleepwalking in psychiatric patients: Comparison of childhood and adult onset. Aust N Z J Psychiatry 2009; 43:426–430Crossref, Google Scholar
39 : Somnambulism due to probable interaction of valproic acid and zolpidem. Ann Pharmacother 2003; 37:1429–1433Crossref, Google Scholar
40 : Amnesia possibly associated with zolpidem administration. Pharmacotherapy 1996; 16:687–689Google Scholar
41 : Amnestic syndrome induced by zoplclone. Eur J Clin Pharmacol 1996; 50:509Crossref, Google Scholar
42 : Zolpidem tartrate and somnambulism. Mil Med 1999; 164:669–670Crossref, Google Scholar
43 : Zaleplon overdose associated with sleepwalking and complex behavior. J Am Acad Child Adolesc Psychiatry 2004; 43:927–928Crossref, Google Scholar
44 : Drug-facilitated sexual assault and analytical toxicology: The role of LC-MS/MS A case involving zolpidem. J Clin Forensic Med 2005; 12:36–41Crossref, Google Scholar
45 : One rare side effect of zolpidem—sleepwalking: A case report. Arch Phys Med Rehabil 2005; 86:1265–1266Crossref, Google Scholar
46 : Compulsive activity and anterograde amnesia after zolpidem use. Clin Toxicol (Phila) 2007; 45:179–181Crossref, Google Scholar
47 : Somnambulism secondary to olanzapine treatment in one patient with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:581–582Crossref, Google Scholar
48 : Olanzapine-induced somnambulism. Am J Psychiatry 2001; 158:1158Crossref, Google Scholar
49 : Two cases of somnambulism induced by quetiapine. Prim Care Companion J Clin Psychiatry 2007; 9:313Crossref, Google Scholar
50 : Violent parasomnia associated with a selective serotonin reuptake inhibitor: A case report. J Clin Psychiatry 2008; 69:1982–1983Crossref, Google Scholar
51 : Paroxetine-induced somnambulism. J Clin Psychiatry 2003; 64:483Crossref, Google Scholar
52 : Sleepwalking associated with reboxetine in a young female patient with major depression—a case report. Pharmacopsychiatry 2004; 37:307–308Crossref, Google Scholar
53 : Bupropion-induced somnambulism. Addict Biol 2003; 8:359–362Crossref, Google Scholar
54 : Somnambulistic-like behaviour in patients attending a lithium clinic. Int Clin Psychopharmacol 1999; 14:173–175Crossref, Google Scholar
55 : Lithium-induced somnambulism. Can J Psychiatry 1998; 43:957–958Google Scholar
56 : NREM sleep-arousal parasomnias, in Principles and Practice of Sleep Medicine, 4th ed. Edited by Kryger MRoth TDement W. Philadelphia, Elsevier Saunders, 2005, pp 889–896Crossref, Google Scholar
57 : Topiramate induced somnabulism and automatic behaviour. Indian J Med Sci 2003; 57:508–510Google Scholar
58 : REM-Sleep Behavior Disorder, in Handbook of Sleep Disorders. Edited by Thorpy M. New York, Marcel Dekker, Inc., 1990, pp 567–593Google Scholar
59 : NREM sleep parasomnias. Neurol Clin 1996; 14:675–696Crossref, Google Scholar
60 : Somnambulistic-like episodes secondary to combined lithium-neuroleptic treatment. Br J Psychiatry 1979; 135:418–424Crossref, Google Scholar
61 : Two case reports: Night terrors with sleepwalking—a potentially lethal disorder. J Nerv Ment Dis 1983; 171:503–505Crossref, Google Scholar
62 : Somnambulistic-like behaviors induced by lithium taken with or without other pharmacological agents. Sleep Res 1997; 26:407Google Scholar
63 : Sleepwalking associated with zolpidem. J Clin Psychopharmacol 1994; 14:150Crossref, Google Scholar
64 : Late-life somnambulism after therapy with metoprolol. Clin Neuropharmacol 2008; 31:248–250Crossref, Google Scholar
65 : Agitated sleepwalking with fluoroquinolone therapy. Pediatr Infect Dis J 1999; 18:484–485Crossref, Google Scholar
66 : Sleep and Wakefulness, Revised and Enlarged Edition. Chicago, University of Chicago Press, 1963Google Scholar
67 : SPECT during sleepwalking. Lancet 2000; 356:484–485Crossref, Google Scholar
68 : Hypersynchronous delta sleep EEG activity and sudden arousals from slow-wave sleep in adults without a history of parasomnias: Clinical and forensic implications. Sleep 2004; 27:706–710Crossref, Google Scholar
69 : Analysis of polysomnographic events surrounding 252 slow-wave sleep arousals in thirty-eight adults with injurious sleepwalking and sleep terrors. J Clin Neurophysiol 1998; 15:159–166Crossref, Google Scholar
70 : Analysis of postarousal EEG activity during somnambulistic episodes. J Sleep Res 2004; 13:279–284Crossref, Google Scholar
71 : Parasomnias: An updated review. Neurotherapeutics 2012; 9:753–775Crossref, Google Scholar
72 : Adult chronic sleepwalking and its treatment based on polysomnography. Brain 2005; 128:1062–1069Crossref, Google Scholar
73 : A retrospective outcome study and review of hypnosis as treatment of adults with sleepwalking and sleep terror. J Nerv Ment Dis 1991; 179:228–233Crossref, Google Scholar
74 : The treatment of parasomnias with hypnosis: A 5-year follow-up study. J Clin Sleep Med 2007; 3:369–373Google Scholar
75 : Behavioral treatment of night terrors in a child with acute leukemia. J Nerv Ment Dis 1979; 167:182–185Crossref, Google Scholar
76 : Treatment of somnambulism with anticipatory awakening. J Pediatr 1993; 122:426–427Crossref, Google Scholar
77 : Hypnotic treatment of sleep terror disorder: A case report. Am J Clin Hypn 1989; 32:36–40Crossref, Google Scholar
78 : Sleep-terror disorder in children: The role of self-hypnosis in management. Am J Clin Hypn 1992; 34:233–244Crossref, Google Scholar
79 : 10 cases of somnambulism treated with combined acupuncture and medicinal herbs. J Tradit Chin Med 1989; 9:174–175Google Scholar
80 : Treatments for somnambulism in adults: Assessing the evidence. Sleep Med Rev 2009; 13:295–297Crossref, Google Scholar
81 : Diazepam (Valium) treatment in childhood sleep disorders. A preliminary investigation. Dis Nerv Syst 1971; 32:565–566Google Scholar
82 : Treatment of coexistent night-terrors and somnambulism in adults with imipramine and diazepam. J Clin Psychiatry 1987; 48:209–210Google Scholar
83 : A child with severe night terrors and sleep-walking responds to melatonin therapy. Dev Med Child Neurol 2004; 46:789Crossref, Google Scholar
84 : Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med 1996; 100:333–337Crossref, Google Scholar
85 : Somnambulism in adults. Neurology 1990; 40:749–752Crossref, Google Scholar
86 : Adult night terrors and paroxetine. Lancet 1997; 350:185Crossref, Google Scholar
87 : Sleep-related eating disorders: Polysomnographic correlates of a heterogeneous syndrome distinct from daytime eating disorders. Sleep 1991; 14:419–431Crossref, Google Scholar
88 : A review of night-time eating disorders. Sleep Med Rev 2009; 13:23–34Crossref, Google Scholar
89 : Sleep-related eating disorder induced by olanzapine. J Clin Psychiatry 2002; 63:597Crossref, Google Scholar
90 : Sleep-related eating disorder induced by risperidone. J Clin Psychiatry 2004; 65:273–274Crossref, Google Scholar
91 : Zolpidem-induced sleep-related eating disorder (SRED) in 19 patients. Sleep 2005; 28:A259Google Scholar
92 : Defining the borders between sleep-related eating disorder and night eating syndrome. Sleep Med 2012; 13:686–690Crossref, Google Scholar
93 : Additional categories of sleep-related eating disorders and the current status of treatment. Sleep 1993; 16:457–466Google Scholar
94 : Combined bupropion-levodopa-trazodone therapy of sleep-related eating and sleep disruption in two adults with chemical dependency. Sleep 2000; 23:587–588Google Scholar
95 . A pilot double-blind placebo-controlled trial of low-dose pramipexole in sleep-related eating disorder. Eur J Neurol 2005; 12:432–436Crossref, Google Scholar
96 : A study of circadian eating and sleeping patterns in night eating syndrome (NES) points the way to future studies on NES and sleep-related eating disorder. Sleep Med 2006; 7:653–656Crossref, Google Scholar
97 : Efficacy and tolerability of open-label topiramate in the treatment of sleep-related eating disorder: A retrospective case series. J Clin Psychiatry 2006; 67:1729–1734Crossref, Google Scholar
98 : Sleep and sex: What can go wrong? A review of the literature on sleep related disorders and abnormal sexual behaviors and experiences. Sleep 2007; 30:683–702Crossref, Google Scholar
99 : Somnambulistic sexual behaviour (sexsomnia). J Clin Forensic Med 2006; 13:219–224Crossref, Google Scholar
100 : Sexsomnia—a new parasomnia? Can J Psychiatry 2003; 48:311–317Crossref, Google Scholar
101 : Nightmares and bad dreams: Their prevalence and relationship to well-being. J Abnorm Psychol 2000; 109:273–281Crossref, Google Scholar
102 : Prevalence of nightmares and their relationship to psychopathology and daytime functioning in insomnia subjects. Sleep 1997; 20:340–348Crossref, Google Scholar
103 : Type A-B behavior and nightmare types among college students. Percept Mot Skills 1995; 81:15–19Crossref, Google Scholar
104 : Donepezil-induced nightmares in mild cognitive impairment. Psychiatry Clin Neurosci 2006; 60:123–124Crossref, Google Scholar
105 : Clinical management of chronic nightmares: Imagery rehearsal therapy. Behav Sleep Med 2006; 4:45–70Crossref, Google Scholar
106 : The nightmare of returning home: A case of acute onset nightmare disorder treated by lucid dreaming. Isr J Psychiatry Relat Sci 1995; 32:140–145Google Scholar
107 : Posttraumatic stress disorder among survivors of Cambodian concentration camps. Am J Psychiatry 1984; 141:645–650Crossref, Google Scholar
108 : A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry 2007; 61:928–934Crossref, Google Scholar
109 : A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry 2013; 170:1003–1010Crossref, Google Scholar
110 Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): Sustained clonazepam efficacy in 89.5% of 57 treated patients. Cleveland Clin J Med 1990; 57 (supp.):9–23Google Scholar
111 : REM Sleep Behavior Disorder, in Principles and Practice of Sleep Medicine. Edited by Kryger MRoth TDement W. Philadelphia, W.B. Saunders Company, 1989, pp 389–401Google Scholar
112 : Validation of a polysomnographic score for REM sleep behavior disorder. Sleep 2005; 28:993–997Crossref, Google Scholar
113 : Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 1996; 46:388–393Crossref, Google Scholar
114 REM behavior disorder (RBD): Delayed emergence of parkinsonism and/or dementia in 65% of older men initially diagnosed with idiopathic RBD, and analysis of the minimum and maximum tonic and/or phasic electromyographic abnormalities found during REM sleep. Sleep 2003; 26(abstract suppl.):A316Google Scholar
115 : Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: A 16-year update on a previously reported series. Sleep Med 2013; 14:744–748Crossref, Google Scholar
116 : Idiopathic REM sleep behavior disorder: Toward a better nosologic definition. Neurology 2005; 64:780–786Crossref, Google Scholar
117 : Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: A descriptive study. Lancet Neurol 2006; 5:572–577Crossref, Google Scholar
118 : Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007; 130:2770–2788Crossref, Google Scholar
119 : Symptomatic REM sleep behavior disorder. Neurol Sci 2007; 28:S21–S36Crossref, Google Scholar
120 : REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 2010; 1184:15–54Crossref, Google Scholar
121 : Rapid eye movement sleep behavior disorder in adults younger than 50 years of age. Sleep Med 2013; 14:768–774Crossref, Google Scholar
122 , Antidepressants and REM Sleep Behavior Disorder: Isolated Side Effect or Neurodegenerative Signal? Sleep 2013; 36:1579–1585Crossref, Google Scholar
123 : Update on the pharmacology of REM sleep behavior disorder. Neurology 2006; 67:742–747Crossref, Google Scholar
124 : Rapid eye movement sleep behavior disorder. Ann Pharmacother 2007; 41:1833–1841Crossref, Google Scholar
125 : Drug treatment of REM sleep behavior disorder: The use of drug therapies other than clonazepam. J Clin Sleep Med 2009; 5:235–239Google Scholar
126 : Issues in the clinical use of benzodiazepines: Potency, withdrawal, and rebound. J Clin Psychiatry 2004; 65(suppl. 5):7–12Google Scholar
127 : A novel therapy for REM sleep behavior disorder (RBD). J Clin Sleep Med 2011; 7:639–644ACrossref, Google Scholar
128 : Melatonin as a therapy in REM sleep behavior disorder patients: An open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov Disord 1999; 14:507–511Crossref, Google Scholar
129 : Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci 2001; 55:267–269Crossref, Google Scholar
130 : Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: Results in 14 patients. Sleep Med 2003; 4:281–284Crossref, Google Scholar
131 : Treatment outcomes in REM sleep behavior disorder. Sleep Med 2013; 14:237–242Crossref, Google Scholar
132 : Effectiveness of pramipexole, a dopamine agonist, on rapid eye movement sleep behavior disorder. Tohoku J Exp Med 2012a; 226:177–181Crossref, Google Scholar
133 : Factors associated with the effect of pramipexole on symptoms of idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord 2013; 19:153–157Crossref, Google Scholar
134 : Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson’s disease. Mov Disord 2012; 27:559–561Crossref, Google Scholar
135 : The effects of pramipexole in REM sleep behavior disorder. Neurology 2003; 61:1418–1420Crossref, Google Scholar
136 : Use of pramipexole in REM sleep behavior disorder: Results from a case series. Sleep Med 2006; 7:418–423Crossref, Google Scholar
137 : REM sleep parasomnias. Neurol Clin 1996; 14:697–720Crossref, Google Scholar
138 : Fluoxetine for isolated sleep paralysis. Psychosomatics 1993; 34:184–187Crossref, Google Scholar
139 : Characteristics of patients with nocturnal dissociative disorders. Sleep Hypn 2001; 3:131–134Google Scholar
140 : Dissociative disorders presenting as somnambulism: Polysomnographic, video and clinical documentation (8 cases). Dissoc 1989; 2:194–204Google Scholar
141 : Vocalization during episodes of prolonged expiration: A parasomnia related to REM sleep. Sleep Med 2001; 2:19–30Crossref, Google Scholar
142 : Catathrenia (nocturnal groaning): A new type of parasomnia. Neurology 2001; 56:681–683Crossref, Google Scholar
143 : Clinical features of the exploding head syndrome. J Neurol Neurosurg Psychiatry 1989; 52:907–910Crossref, Google Scholar
144 : The exploding head syndrome: Polysomnographic recordings and therapeutic suggestions. Sleep 1991; 14:263–266Crossref, Google Scholar
145 : Complex nocturnal visual hallucinations. Sleep Med 2005; 6:363–366Crossref, Google Scholar
146 : Episodic nocturnal wanderings responsive to anticonvulsant drug therapy. Ann Neurol 1977; 2:30–35Crossref, Google Scholar
147 : Electroencephalographic sleep in panic disorder. A focus on sleep-related panic attacks. Arch Gen Psychiatry 1989; 46:178–184Crossref, Google Scholar
148 : Sleep panic attacks: New clinical findings and theoretical implications. Am J Psychiatry 1989; 146:1204–1207Crossref, Google Scholar
149 : Electroencephalographic study of nighttime panic attacks. J Nerv Ment Dis 1985; 173:744–746Crossref, Google Scholar
150 Electroencephalography during sleep of patients with panic disorder. J Neuropsychiatry Clin Neurosci 1989;1(fall):372–376.Google Scholar
151 : Sleep in patients with spontaneous panic attacks. Sleep 1989; 12:323–337Crossref, Google Scholar
152 : Sleep in panic and generalized anxiety disorders, in Neurobiology of Panic Disorder. Edited by Ballenger J. New York, Wiley-Liss, 1990, pp 365–376Google Scholar
153 : Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry 1989; 146:697–707Crossref, Google Scholar
154 : Post-Vietnam syndrome: Recognition and management. Psychosomatics 1981; 22:931–943Crossref, Google Scholar
155 : Nightmares and trauma: A comparison of nightmares after combat with lifelong nightmares in veterans. Am J Psychiatry 1984; 141:187–190Crossref, Google Scholar
156 : Toward rational pharmacotherapy for posttraumatic stress disorder: An interim report. Am J Psychiatry 1988; 145:281–285Crossref, Google Scholar
157 : Biological markers of affective disorders and posttraumatic stress disorder: A pilot study with desipramine. J Clin Psychiatry 1987; 48:366–367Google Scholar
158 : War neuroses and the adaptive function of REM sleep. Br J Med Psychol 1972; 45:27–33Crossref, Google Scholar
159 : Sleep in delayed-stress victims. Sleep Res 1982; 11:113Google Scholar
160 : The Dream Experience in Dream-Disturbed Vietnam Veterans, in Post-Traumatic Stress Disorder: Psychological and Biological Sequelae. Edited by Kolk BAVD. Washington, DC, American Psychiatric Press, 1984, pp 82–95Google Scholar
161 : Sleep patterns in three acute combat fatigue cases. J Clin Psychiatry 1978; 39:546–549Google Scholar
162 : Long-term effects of traumatic war-related events on sleep. Am J Psychiatry 1979; 136:175–178Crossref, Google Scholar
163 : Sleep-related injury in 85 adult patients: A polysomnographic study. Sleep Res 1988; 17:247Google Scholar
164 : Long-term effects of extreme situational stress on sleep and dreaming. Am J Psychiatry 1987; 144:344–347Crossref, Google Scholar
165 : Increased sleep motility and respiration rates in combat neurotic patients. Biol Psychiatry 1979; 14:983–987Google Scholar
166 : The Traumatic Neuroses of War. Washington, DC, Hoeber, 1941Crossref, Google Scholar
167 : Sleep disturbances as the hallmark of PTSD: Where are we now? Am J Psychiatry 2013; AiA:1–11Google Scholar
168 : Polysomnographic sleep is not clinically impaired in Vietnam combat veterans with chronic posttraumatic stress disorder. Biol Psychiatry 1998; 44:1066–1073Crossref, Google Scholar
169 : Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychiatry 1996; 39:411–418Crossref, Google Scholar
170 : Sleep disturbances in the Vietnam generation: Findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry 1998; 155:929–933Crossref, Google Scholar
171 : Self-selection bias in sleep and psychophysiological studies of posttraumatic stress disorder. J Trauma Stress 2007; 20:619–623Crossref, Google Scholar
172 : Subjective and objective characteristics of sleep and dreaming in war-related PTSD patients: Lack of relationships. Sleep Res 1991; 20A:270Google Scholar
173 : Auditory thresholds in the dream disturbed. Sleep Res 1984; 13:102Google Scholar
174 : Elevated awaking thresholds during sleep: Characteristics of chronic war-related posttraumatic stress disorder patients. Biol Psychiatry 1998; 44:1060–1065Crossref, Google Scholar
175 : Developing delayed, chronic PTSD: The contribution of disturbances in sleep, dreams and responsivity. San Antonio, International Society for Traumatic Stress Studies, 1993Google Scholar
176 : Sleep and dreaming in Holocaust survivors. Dramatic decrease in dream recall in well-adjusted survivors. J Nerv Ment Dis 1991; 179:664–669Crossref, Google Scholar
177 : Sleep violence—forensic science implications: Polygraphic and video documentation. J Forensic Sci 1990; 35:413–432Crossref, Google Scholar
178 : Homicidal somnambulism: A case report. Sleep 1994; 17:253–264Google Scholar
179 : Sleep-related violence: A medical and forensic challenge. Sleep 1995; 18:727–730Crossref, Google Scholar
180 : Age-related sleep-wake disorders at a sleep disorder center. J Am Geriatr Soc 1983; 31:364–370Crossref, Google Scholar



