Supportive Oncology: New Models for the Role of Psychiatry in Cancer Care
Abstract
The last 40 years have seen dramatic advances in the scientific understanding of the mechanisms underlying the clinical behavior of cancer and in the effectiveness of cancer prevention, diagnosis, staging, and treatments. Cancer care can now often be truly individualized, based on measurement of tumor biomarkers, advanced imaging technology, and use of targeted anticancer treatments. Treatment advances have resulted in the creation of large numbers of cancer survivors, and to the recognition that cancer is now often a chronic, rather than acutely life-threatening, illness. Evolution of our scientific and clinical understanding of the human dimensions of cancer and cancer care can be encapsulated in the progression of descriptive terms from psycho-oncology, to psychosocial oncology, to supportive oncology. Now a broad range of cancer patient and family services are recognized as being important to providing comprehensive patient-centered cancer care as one component of the patient’s overall care delivery experience. This paper reviews information on psychiatric aspects of cancer and cancer treatment, the role of psychiatry and behavioral health services, best practice organizational models for provision of integrated supportive oncology services within the hospital cancer program environment, and the increasing importance of supportive oncology national quality of care standards.
Introduction
Cancer genetic testing has become an important tool in determining personal genetic risk for some cancers (e.g., members of families with extensive breast and/or ovarian cancer history) which may result in individualized cancer risk reduction interventions (2).
As of January 2012 there were 13.7 million U.S. cancer survivors (defined as individuals previously diagnosed with cancer) (3). As the U.S. population ages, it is anticipated there will be significant increases in the incidence and prevalence of cancer, with the number of cancer survivors expected to grow to 18 million by 2022 (3). This growth will significantly challenge our U.S. cancer care delivery system (4, 5).
Historical Perspective/Current Services Delivery Landscape
The last 40 years has encompassed marked advancements in our understanding of the complexity of the human aspects of cancer care (6). Figure 1 presents one model of the broad range of services included in supportive oncology services. For almost all of the Figure 1 services there now exist national quality of care standards.
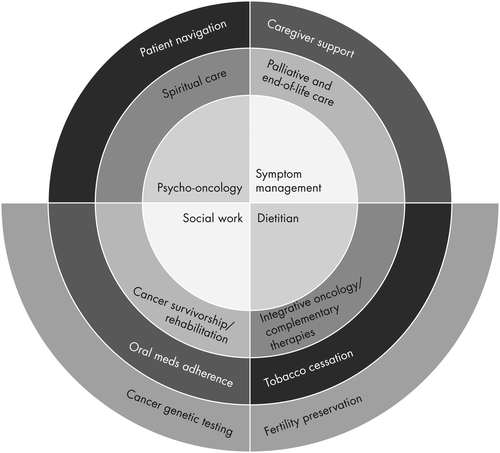
Modern hospital cancer program supportive oncology services are based on the fully integrated multidisciplinary team collaborative care model. The team may include social workers, dietitians, patient navigators, oncology nurses with educational and specialized clinical expertise (e.g., cancer pain management nurses), psychiatrists, psychologists, spiritual care providers, cancer rehabilitation medicine and survivorship services providers, integrative oncology care providers (e.g., acupuncturists, mind-body therapists), and palliative care providers. Integrated supportive oncology teams need to be fully embedded in the hospital cancer program’s cancer care delivery system at the clinical care delivery, quality improvement, and hospital organizational structure levels (such as the Cancer Committee). They need to ensure supportive oncology patient care continuity as patients move between hospital cancer program inpatient and ambulatory, community/facility, and home health and hospice care environments.
National cancer patient/family/caregiver educational and supportive care services provider and advocacy organizations form a very important component of the supportive oncology care system (Table 1) (7). In larger cities there are often local/regional community supportive oncology providers (e.g., Cancer Support Community, WeSpark in Los Angeles). A relatively small number of private practitioners provide psychiatric, psychological, and/or social work services to cancer patients.
| Organization Name | Information/Services | Contact Information |
|---|---|---|
| National Cancer Institute (NCI) | Online Patient Education Documents (OPED), helpline | www.cancer.gov1–800–4-CANCER |
| American Cancer Society (ACS) | National Cancer Information Center, online resources/communities; Local programs/services information | www.cancer.org1–800–227–2345 |
| Cancer Support Community (CSC) | (OPED), helpline, online supportive oncology Services, local physical facilities for direct supportive oncology services, personal webpage creation | www.cancersupportcommunity.org1–888–793–9355 |
| CANCERCare | (OPED), telephone, online, and direct education, counseling, support group, and financial services | www.cancercare.org1–800–813-HOPE (4673) |
| National Comprehensive Cancer Network (NCCN) | NCCN Guidelines for PatientsNCCN Guidelines for Supportive Care (Professionals) | www.nccn.org/patients/default.aspx1–215–690–0300 |
| American Society of Clinical Oncology (ASCO) | ASCO Answers for patients- specific diseases and supportive care- online and printed | www.cancer.net/publications-and-resources/asco-answers-fact-sheets |
| National Coalition for Cancer Survivorship (NCCS) | Cancer survival toolbox, OPED, cancer policy and advocacy | www.canceradvocacy.org1–877-NCCS-YES |
| American Lung Association | Facing lung cancer: interactive library, MD/communication and treatment decisions+general support | www.mylungcancersupport.org |
| Leukemia and Lymphoma Society (LLS) | INFO: Newly diagnosed, treatment decisions, survivorship, caregivers, clinical trials, copay assistance | www.lls/org1–800–955–4572 |
For patients approaching the end of life, home health agency and hospice services providers become critically important components of their personal supportive oncology services delivery system. Given the differing hospital cancer program and hospice financing systems, maintaining supportive oncology care continuity as hospital cancer program patients transition to prehospice or hospice care is a major challenge.
Many U.S. cancer patients receive some or most of their cancer care in the physician office or free-standing cancer treatment facility environment. The provision of excellent, comprehensive, and accessible supportive oncology care to all U.S. cancer patients in all treatment environments throughout their illness is a serious challenge.
Many informational and educational/support group services to cancer patients and their family members are now available on the web, via social media, or by using telecommunications technology (Table 1) (8). In spite of the diversity of this supportive oncology services delivery system, many U.S. cancer patients and their family members do not have timely access to the supportive oncology services they want and need (9).
As we look to the future impact of health care reform on models for the provision of supportive oncology to cancer patients, we can build on the cancer-specific application of current best-practice models for the role of psychiatry in collaborative care of patients with chronic medical diseases (10).
Provision of Clinical Psychiatric and Psychological Services to Cancer Patients
Psychiatric Assessment and Care of Cancer Patients
The epidemiology of psychological distress and psychiatric disorders in cancer patients was one of the first areas of systematic research in psycho-oncology. Clinically important psychological concepts include stress/trauma and distress, individual coping strengths and resources, social and family support, the patient’s life cycle phase, medical and psychosocial illness phases, resilience and posttraumatic growth, loss and grief, existential and spiritual dimensions of the patient’s cancer care experience, and caregiver support and stresses. The financial and vocational/social role function impacts of cancer and its treatment often profoundly adversely affect the patient and his/her family members (4, 11).
Routine, early post-diagnosis patient distress screening and multidisciplinary supportive oncology team clinical assessment and triage are critical to the provision of optimal supportive oncology care. Interventions include the provision of individualized cancer and cancer treatment education, access to educational and support groups, individual supportive psychotherapy, CBT, and existential/meaning and dignity therapies. Other often personally important services include spiritual care, mind/body stress management training, and exercise/physical therapy/rehabilitation services (12, 13)
In smaller communities that have small hospital cancer programs, the psychiatrist may often be a privative practitioner who performs inpatient consultations and sees cancer outpatients in a private practice model. In larger hospital cancer programs, the psychiatrist may receive salary support to provide direct clinical services and clinical and administrative leadership to the supportive oncology service.
Core Psychiatric Oncologist Clinical Services Role
Fundamentally based on core psychiatric and psychosomatic medicine specialty skills, the psychiatric oncologist provides psychiatric diagnostic and pharmacological management services to cancer patients with confusional states and delirium, depression, anxiety, substance abuse disorders, and insomnia. Cancer patients may also present with significant character pathology that may interfere with successful cancer treatment unless psychiatrically managed. Psychiatrist, psychologist, and social worker team members can closely collaborate in the assessment and care of these patients.in the inpatient and ambulatory cancer center settings (14–21).
The clinical judgment concerning the discrimination between normal (if intense) levels of situational distress and symptoms and diagnosable psychiatric disorder remains an often difficult challenge. These judgments can be aided by the use of validated scales such as the PHQ-9 and the MDFI 20.
The related clinical judgments concerning the use of psychopharmacologic agents in the management of symptoms of anxiety, depression, and insomnia are often complicated by the potential neuropsychiatric toxicity of a wide variety of oncologic active treatment medications and of radiation therapy. The potential for adverse psychopharmacologic drug interactions with oncologic agents and for adverse drug/disease interactions is an ongoing concern for psychiatrists prescribing to cancer patients (22–27). For example paroxetine and other antidepressant medications, which interfere with the hepatic metabolism of tamoxifen to endoxifen, may adversely impact the risk of disease recurrence in breast cancer patients (18).
Extended Psychiatric Oncologist Role in Cancer Patient Physical Symptom Management
A host of physical symptoms may be associated with cancer and its treatment, particularly at later disease stages. Pain and fatigue are especially common and often debilitating, and unless effectively treated can greatly contribute to anxiety, depression, and insomnia. The psycho-oncologist psychiatrist would ideally develop significant expertise in the assessment and pharmacologic management of cancer pain (32–34), fatigue (35), decreased appetite with weight loss/cachexia (36), hot flashes (37, 38), chemotherapy-induced peripheral neuropathy (31), and the cognitive impairments associated with cancer treatment including chemotherapy (“chemobrain”) (11, 39–41).
While not common, there are psychiatric oncologists who have significant expertise in the clinical assessment and pharmacologic management of cancer pain and who provide cancer pain management services. The Samuel Oschin Cancer Center at the Cedars-Sinai Medical Center (CSMC) has had a psychiatrist-led cancer pain management service for about 22 years (42). The current psychiatrist and cancer rehabilitation medicine specialist provide cancer pain management physician services to Cancer Center patients upon referral of the patient’s primary oncologist physician, including opiate and adjunctive medication management. This care is supported by dedicated service nurses who have cancer pain management expertise. The physicians have access to Cancer Center examination rooms and infusion center spaces, provide 24/7 physician coverage, and provide limited cancer pain management services to Cancer Center inpatients. The service closely collaborates with the inpatient CSMC Palliative Care Service, which provides most of the inpatient cancer pain/symptom management care. CSMC also has inpatient and outpatient anesthesiologist-led interventional and peri-operative pain management services. Thus, in close collaboration with other institutional pain and symptom management physicians and services the psychiatrist with cancer pain management expertise can play an important role in noninterventional cancer pain management care. Given the often marked clinical overlap between cancer pain, psychological distress, and psychiatric symptoms, this extended role of the psychiatrist can be very helpful in optimizing psychiatric care.
Psycho-Oncologist Psychiatrist Role in Provision of Comprehensive Supportive Oncology Care
The psycho-oncologist psychiatrist ideally works closely with a multidisciplinary supportive oncology team including the full range of supportive oncology providers. As one example the Samuel Oschin Cancer Center at the Cedars-Sinai Medical Center treats about 10,000 patients/year with about 120,000 patient visits. For this population the Oncology Supportive Care Service has one full-time psychiatrist, one full-time cancer rehabilitation medicine specialist, six full-time oncology social workers, three full-time oncology dietitians, two full-time cancer pain management service nurses, and one full-time dedicated chaplain plus administrative support staff. The psychiatrist commonly sees new consults and patients with complex follow-up care needs with the social worker and/or nurse. This team meets briefly each workday morning for a patient review/planning “huddle” and has a weekly extended patient review and care planning meeting. Each dietitian and social worker is assigned to a specific group of oncology practices to optimize continuity of care and typically participates in specific oncology practice or service meetings. Social workers and dietitians are actively involved in a variety of patient education/support groups and wellness/cancer survivorship services. Service handoffs are routine when patients are admitted to or discharged from the inpatient setting. The Service closely follows patients with advanced disease, manages outpatient hospice referrals, and seeks to ensure optimal supportive oncology care continuity for Cancer Center patients referred to hospice. The Service leadership team meets weekly.
The organizational principles of this collaboration are more fully articulated below in the section on the City of Hope Oncology Supportive Care Medicine Service. Fann has reported a model for the collaborative mental health care of cancer patients, which may be especially important in the context of health care reform and the future “oncology accountable care organization” (10).
Psycho-Oncologist Psychiatrist Roles in Palliative and End-of-Life Care
The recent Institute of Medicine (IOM) report on delivering high-quality cancer care has outlined five principles of cancer patient care from diagnosis onward: care planning, palliative care, psychosocial support, prevention and management of long-term and late effects, and family/caregiver support (43). Hospice and palliative medicine physicians have long provided medical leadership to hospice services. Hospice and palliative medicine physicians are increasingly leading not only inpatient palliative care consultation services and inpatient palliative care or hospice units but also leading outpatient palliative care/supportive care medicine clinics.
There is increasing recognition of the importance of the role of psychiatry, behavioral healthcare, and spiritual care services in the care of patients at the end of life. The reader is directed to key chapters and books and multiple recent articles that articulate the scope of the clinical services needs of this population, principles of the clinical assessment and management of psychological distress and psychiatric disorders in this population, and potential roles of psychiatry in palliative care and hospice services delivery (44–62). Many opportunities exist for closer collaboration between psychiatric oncologists/supportive oncology services with palliative care and hospice services to enhance hospital cancer program end-of-life patient care and family/caregiver support. Hopefully future changes in the structure of hospice care and hospice reimbursement will facilitate a broader role for psychiatric oncologist/supportive oncology service in cancer patient hospice care delivery.
Other Major Supportive Oncology Clinical Services Challenges
Many cancer patients utilize a wide variety of complementary therapies in their personal approach to fighting cancer. While mind-body therapies may bring great psychological value, there is concern that ingestion of certain natural substances (supplements, herbs) with antioxidant effects could interfere with the antitumor effectiveness of chemotherapy and/or radiotherapy treatments. The psychiatrist working in the cancer treatment setting needs to be aware of this literature (69–72). Several cancer quality of care standards organizations have set standards that tobacco use should be assessed in cancer patients and that those using tobacco should be offered a cessation intervention program. All hospital cancer programs should offer onsite or by referral tobacco cessation services to their cancer patients who use tobacco (73–75).
Certain chemotherapy agents and many of the newer targeted agents are oral, self-administered medications. Studies have indicated that adherence rates for women with breast cancer on chronic oral hormonal treatments (tamoxifen, aromatase inhibitors) decline quite significantly over the first 3–4 years of treatment (76–78). This finding will likely apply to other chronic oral therapies (79). One of the major challenges for supportive oncology is developing effective mechanisms for determining patients’ risk for nonadherence to oral regimens, and implementing effective educational and adherence support programs to optimize patient adherence to these regimens.
There exist many challenges in implementing sustainable financing/business models for the provision of psychiatric oncology/supportive oncology services in the hospital cancer program environment. Given the current reimbursement environment, these services are not financially self-sustaining and require hospital financial support. The senior author for many years led the corporate development of comprehensive hospital ambulatory cancer center supportive oncology services at multiple hospital sites throughout the United States. In general, the total net costs of providing supportive oncology services ranged between 1.5%−2.5% of net Cancer Center operating revenues. The current business rationale for hospital financial support for these services includes contribution to patient care quality and patient service satisfaction, meeting national supportive oncology care quality standards, contributing to optimal patient treatment adherence, contributing to oncologist physician/nurse/patient visit productivity, and the ability to attract private philanthropy to support certain elements of supportive oncology services.
Particularly in collaboration with inpatient and outpatient palliative care services, supportive oncology services can contribute to optimizing patient end-of-life care, decreasing avoidable utilization of hospital emergency department and inpatient/ICU resources for cancer patients at the end of life, and supporting earlier referrals of patients with advanced disease to hospice from the ambulatory cancer center setting.
In the future, as we move toward the potential “oncology accountable care organization” (away from the current fee-for-service model to the bundled care reimbursement model), and as cost minimization becomes a more important component of hospital cancer program financial sustainability, it is likely that the role of supportive oncology services will become more highly valued.
Cancer Survivorship and Rehabilitation Services
The creation of cancer active treatment summaries and cancer survivorship care plans for cancer patients completing initial active cancer treatment is a standard for hospital cancer programs established by the American College of Surgeons Commission on Cancer (ACoS CoC) as of January 2015. Cancer survivors face many ongoing cancer recurrence prevention, physical/rehabilitation, financial/vocational function, symptom management, psychosocial, and physical and mental health quality of life challenges. Providing effective, timely, accessible, affordable, and sustainable supportive oncology services to cancer survivors is another major challenge to the U.S. cancer care delivery system (4, 5, 80, 81).
Some cancer patients develop personally significant deterioration in often higher executive cognitive functions due to the neuropsychiatric toxicity of cancer medications and possibly radiation therapy. The concept of “chemo brain” has definitely entered the consciousness and vocabulary of many cancer patients. Clinical experience suggests that neurocognitive rehabilitation programs and in some cases psychopharmacologic treatments can be therapeutically helpful (5, 39–41).
Cancer rehabilitation medicine services provide great value to hospital cancer programs whether as an organizationally separate service or as an integrated component of a supportive oncology service. Clinically safe, oncologist physician-sanctioned physical exercise programs/physical therapy programs provide important rehabilitation and fatigue management value to many cancer patients at varying disease stages.
Major Informational/Educational Resources Available to the Psychiatrist or Psychologist Working with Cancer Patients
This section presents some of the important professional organization, textbook, and journal resources valuable to the clinical psychiatrist or psychologist working with cancer patients. The major organizations and resources include the American Psychosocial Oncology Society, Association of Oncology Social Workers, and the Center to Advance Palliative Care. A second textbook on psycho-oncology has recently been published (82). The major journals include Psycho-Oncology, Journal of Psychosocial Oncology, Palliative and Supportive Care, Journal of Cancer Survivorship: Research and Practice, Journal of Pain and Symptom Management, and the Journal of Palliative Medicine.
Organizational Structure of Supportive Oncology
For a comprehensive discussion of creating oncology supportive care programs please see the work of Loscalzo and colleagues (83, 84). The Department of Supportive Care Medicine at the City of Hope is a nationally leading model of a fully integrated multidisciplinary department with the capability of sharing a specific departmental vision/mission, supporting a high level of internal care delivery integration, and supporting comprehensive patient-centered care provided by a large, NCI-designated comprehensive cancer center. This single departmental model can also support optimal health care professional education and productive collaborative research activities. While other successful models exist for the organizational structure of supportive oncology in large academic cancer centers, the fully integrated single departmental model for supportive oncology services appears to hold the most promise for providing high-value supportive oncology care.
For smaller hospital cancer programs with smaller supportive oncology services, the principle of close psychiatrist collaboration with oncology social workers, dietitians, and psychologists can be highly effective. Close collaboration with local regional supportive oncology community service provider organizations, and helping guide patients to national educational and supportive oncology services providers (Table 1) is especially important in the small hospital cancer program environment.
Biopsychosocial Screening: Distress is Never Enough
It is evident from the amount of professional publications that distress screening is of great interest. The influential 2008 IOM Report and subsequent key articles have demonstrated benefits, shared effective implementation strategies, and have been well described elsewhere (83–86). The focus of this section will be to extend the original implications of distress screening (which is now seen as too limited) within the realities of cancer care (84, 85). The Distress Thermometer has been widely influential and is the instrument of choice wherever an automated tool is not available. Because it is paper based, it does not naturally link to specific professionals or to electronic health records, which is increasingly the standard for clinical care.
The more comprehensive automated biopsychosocial screening that is in place in a number of settings automates a series of processes that bring obvious increased institutional value (quality+cost): identification (physical symptoms, psychological problems, social concerns, spiritual challenges, practical barriers), requests (tailored education, resources, clinical trials), criteria driven triage to multispecialists, data storage (program development, research), and tailored reports. Biopsychosocial screening processes are achieved in real-time before or during the clinical encounter and are integrated with electronic medical records and tailored to specific populations (85–93). We can now turn to why biopsychosocial screening is strategically relevant to supportive care programs.
Comprehensive biopsychosocial screening supports the provision of timely comprehensive integrated biopsychosocial care, which thereby supports the ability of patients and their families to fully benefit from medical care, to cope effectively, and to make positive meaning of their experience. Comprehensive biopsychosocial screening also provides the opportunity for psychiatric leadership in the creation and implementation of institutional multidisciplinary psychosocial care triage and care algorithms. When done correctly, this process is much more than a set of mechanistic or operational processes to organize clinical care but rather enables open and honest communication about which professional is licensed and qualified to provide specific services. All too often these essential ongoing conversations do not happen because they are emotionally charged, and in general health care professionals are conflict aversive with each other. This is ironic given that the same health care professionals (including psychiatrists, psychologists, and social workers)—who may be highly skilled in end-of-life and other difficult communications—seldom create environments where these conversations can occur.
Ultimately, even highly trained professionals do not realize that conflict itself is not the problem–not managing the inherent conflicts that are endemic to team work is a serious problem that undermines quality of medical care, teamwork and patient safety (84). Because effective biopsychosocial screening is dependent on coordination and communication among team members there is an opportunity and an acute need for leadership by psychiatrists.
Leveraging the System
Data are always necessary for successful programs but is never enough. Leadership and teamwork is always essential. Supportive care programs, even when integrated within standard cancer care, are frequently seen as both having value and competing for resources. In the present cost-cutting environment, supportive care is increasingly seen as a way to reduce costs, but administrators still struggle to understand the benefits of cost savings in a model that has traditionally focused solely on revenue.
Ultimately supportive care services have never had such a high profile and awareness of potential benefits that go well beyond the finances of a health care system in extremis. NCI Guidelines, NCCN Distress Management Guidelines, the recent IOM report (43), increased emphasis on patient and family centered care, and consumer advocacy groups are all creating a more sustainable base of support for the importance for mental health interventions in cancer settings. Private philanthropy can also support innovative supportive care programs (the American Psychosocial Oncology Society’s website includes a robust video program on fund raising: http://www.apos-society.org/professionals/meetings-ed/webcasts.aspx#2011).
In addition to the biopsychosocial screening and related service benefits for cancer settings, two additional ways that supportive care programs can leverage their influence is by partnering with patients, families, and local communities and pushing all professionals to the top of their licenses. When professionals practice at the top of their license (i.e., oncologists focusing on disease-directed care and not psychosocial problems for which they do not have the time or the training, or psychiatrists seeing patients who are depressed and not simply sad) there are resource efficiencies seen and potentially higher levels of provider and patient satisfaction.
Biopsychosocial screening instruments overall are able to rule-out depression in cancer patients (87). There are major shortages of psychiatric oncologists in hospital cancer program settings. There are also shortages of community psychiatrists willing to see cancer patients, skilled in working with patients and their families affected by cancer, and able to take on people with poor health insurance and few financial resources. These realities make it essential that psychiatrists are used wisely in cancer patient care.
Supportive Oncology Clinical Quality Standards of Care
Efforts to promote greater awareness of the need for supportive care of cancer patients and its benefits received a major boost in 2008 with the publication of an IOM report titled, “Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs” (94). The report summarized the considerable evidence about the deleterious effects of unmet psychosocial needs as well as the beneficial effects of providing psychosocial services. Among the report’s major conclusions was that, despite evidence of the effectiveness of supportive care services, many patients do not receive help for problems that would benefit from this type of care. To address this problem, the report included a list of recommended actions. The first and foremost recommendation is that all entities establishing or using standards for the quality of cancer care adopt a standard that calls for the provision of appropriate supportive care services.
To date, standards for supportive care have been put forward mostly by organizations and committees consisting of members of the supportive care community. Accordingly, there are concerns about the extent to which the wider community of oncology professionals is cognizant of these initiatives. Recent actions undertaken by the American College of Surgeons Commission on Cancer (ACoS CoC) bode well for the wider awareness and acceptance of standards for supportive care. The ACoS CoC is a consortium of 47 professional organizations that establishes cancer care standards and monitors quality of care at the more than 1,500 hospitals it accredits. In 2012, the ACoS CoC released several new standards for patient-centered care that will phase in for the 2015 accreditations (95). Among the new standards is one which specifies that a local oversight committee develop and implement a process for psychosocial distress screening and referral for the provision of psychosocial care. The release of this guideline has generated considerable attention, including recommendations for how it should be met (96, 97).
Clinical practice guidelines are more specific than standards and are meant to provide information that can be of assistance to health care providers in making evaluation, management, and treatment decisions. Numerous organizations have issued clinical practice guidelines that include recommendations for the supportive care of people with cancer. The National Comprehensive Cancer Network (NCCN) has been particularly active in this regard. Among other topics, NCCN has issued guidelines for the management of pain, emesis, anemia, fatigue, distress, and venous thromboembolic disease (98).
Details about NCCN Guidelines for Distress Management are provided to illustrate the format and content. This NCCN guideline, updated annually since 1999 (99, 100), consists primarily of recommendations that represent a uniform consensus among experts from NCCN member institutions; in general, they are based on lower-level evidence, such as clinical experience, as opposed to higher-level evidence, such as randomized controlled trials (RCTs). The guideline includes specific recommendations for psychosocial screening, evaluation, treatment and follow-up presented primarily in the form of clinical pathways. For example, recommendations for management of mood disorders (e.g. depression) specify that all patients should undergo brief psychosocial screening, with patients found to have moderate to severe distress referred to psychosocial care professionals. If patients are displaying signs and symptoms of a mood disorder, the initial recommendation is further evaluation, diagnostic studies, and modification of factors potentially contributing to the symptoms, such as concurrent medications and pain. Based on findings, subsequent recommendations may include initiation of psychotherapy and antidepressant medication, possibly in combination with anxiolytic medication. Consideration of referral to social work or chaplaincy services is also recommended before follow-up and reevaluation. A notable strength of these guidelines is the clear guidance they provide for the organization and delivery of psychosocial services given their clinical pathway format. A notable weakness is the limited use made of higher-level evidence to provide care recommendations. For example, the pathways regarding management of mood disorders do not include recommendations for use of specific forms of psychotherapy or pharmacotherapy even though there is a body of evidence on this topic for people with cancer (101).
Moving forward, two considerations should guide future development of guidelines in this area. First, it is important to insure that procedures used to formulate and present guideline recommendations are consistent with recommended methods (102). To address this issue, the quality of existing guidelines addressing supportive care should be evaluated using accepted methods such as the AGREE (Appraisal of Guidelines for Research & Evaluation) instrument (103). The results are likely to suggest ways that existing guideline efforts in this area could be improved or could lead to the formation of new guideline efforts if improvements are not forthcoming. Second, greater effort needs to be devoted to embedding guidelines for supportive care into broader sets of cancer treatment guidelines. The Children’s Oncology Group Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers (104) are a notable example of how consideration of the psychosocial dimension of care was melded into a comprehensive set of guidelines addressing late-effects of cancer treatment. Similar approaches should be considered in developing guidelines for adult-onset cancers, especially those for which supportive care issues are frequently encountered.
1 : Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29Crossref, Google Scholar
2 : Genetic susceptibility to breast/ovarian cancer, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 105–109Crossref, Google Scholar
3 American Cancer Society: Cancer Treatment & Survivorship Facts and Figures: 2012-2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveillance/documents/document/aspc-033876.pdf. Accessed Sept 5, 2013Google Scholar
4 : Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 2012; 21:2108–2117Crossref, Google Scholar
5 : Cancer survivorship and cancer rehabilitation: revitalizing the link. J Clin Oncol 2012; 30:904–906Crossref, Google Scholar
6 : History of psycho-oncology, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 3–12Crossref, Google Scholar
7 : The Wellness Community’s integrative model of evidence-based psychosocial programs, services, and interventions, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 473–478Crossref, Google Scholar
8 Stanton AL, Thompson EH, Crespi CM, Link JS, Waisman JR: Project connect online: randomized trial of an internet-based program to chronicle the cancer experience and facilitate communication. J.Clin Oncol 2013; 31:1–109Google Scholar
9 : The state of psychosocial services in cancer care in the United States. Psychooncology 2013; 22:699–703Crossref, Google Scholar
10 : Integrating psychosocial care into cancer services. J Clin Oncol 2012; 30:1178–1186Crossref, Google Scholar
11 : Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv 2009; 3:223–232Crossref, Google Scholar
12 : Principles of Psychotherapy, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 397–401Crossref, Google Scholar
13 : Group Psychotherapy for persons with cancer, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 408–414Crossref, Google Scholar
14 : Oncology, in The American Psychiatric Association Textbook of Psychosomatic Medicine: Psychiatric Care of the Medically Ill, 2nd ed. Edited by Levenson JL. Arlington, VA, American Psychiatric Publishing, Inc, 2012, pp 525–549Google Scholar
15 : Patients with Cancer, in The Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th ed. Edited by Stern TAFricchione GLCassem NHJellinek MSRosenbaum JF. Philadelphia, PA, Saunders/Elsevier, 2010, pp 371–382Crossref, Google Scholar
16 : Delirium, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 332–339Crossref, Google Scholar
17 : Depressive Disorders, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 311–318Crossref, Google Scholar
18 Strouse TB: Psychopharmacologic treatment of depression in patients with cancer: a 2013 update. Focus 2013; 11:450–459Google Scholar
19 : Anxiety Disorders, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 324–331Crossref, Google Scholar
20 : Sleep and Cancer, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 258–269Crossref, Google Scholar
21 : Psychotropic Medications in Cancer, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 378–385Crossref, Google Scholar
22 : Breast cancer recurrence risk related to concurrent use of SSRI antidepressants and tamoxifen. Acta Oncol 2010; 49:305–312Crossref, Google Scholar
23 : Antidepressants and seizures: emphasis on newer agents and clinical implications. Int J Clin Pract 2005; 59:1435–1440Crossref, Google Scholar
24 : Clinical impact of selective serotonin reuptake inhibitors therapy with bleeding risks. J Intern Med 2007; 261:205–213Crossref, Google Scholar
25 : QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13Crossref, Google Scholar
26 : Drug interactions and pharmacogenomics in the treatment of breast cancer and depression. Am J Psychiatry 2008; 165:1251–1255Crossref, Google Scholar
27 : The serotonin syndrome. N Engl J Med 2005; 352:1112–1120Crossref, Google Scholar
28 : Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7:327–340Crossref, Google Scholar
29 : Neurotoxicity of immunosuppressive drugs. Liver Transpl 2001; 7:937–942Crossref, Google Scholar
30 : Posterior reversible encephalopathy syndrome. Psychosomatics 2013; 54:205–211Crossref, Google Scholar
31 : Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 2008; 44:1507–1515Crossref, Google Scholar
32 : Assessment and Management, 2nd ed. Edited by Bruera EDPortenoy RK. New York, NY, Cambridge University Press, 2010Google Scholar
33 : Pain, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 215–228Crossref, Google Scholar
34 : Assessing the quality of pain care in ambulatory patients with advanced stage cancer. J Pain Symptom Manage 2012; 43:1072–1081Crossref, Google Scholar
35 : Fatigue, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 236–244Crossref, Google Scholar
36 : Weight and Appetite Loss in Cancer, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 270–274Crossref, Google Scholar
37 : Managing the toxicities of the aromatase inhibitors. Curr Opin Obstet Gynecol 2010; 22:56–60Crossref, Google Scholar
38 : Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295:2057–2071Crossref, Google Scholar
39 : Neuropsychological Impact of Cancer and Cancer Treatments, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 251–257Crossref, Google Scholar
40 : Brain vulnerability to chemotherapy toxicities. Psychooncology 2012; 21:1141–1148Crossref, Google Scholar
41 : Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil 2011; 90(Suppl 1):S16–S26Crossref, Google Scholar
42 : Managing cancer pain in a 24-hour outpatient cancer center. J Support Oncol 2006; 4:351–354 [Also at http://www.SupportiveOncology.net]Google Scholar
43 Levit L, Balogh E, Nass S, Ganz PA (Eds): Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population; Board on Health Care Services, Institute of Medicine. (2013) Pre-publication. National Academies Press at http://www.nap.edu/catalog.php?record_id=18359. Accessed on Sept 10, 2013)Google Scholar
44 : Palliative Care, in The American Psychiatric Association Textbook of Psychosomatic Medicine: Psychiatric Care of the Medically Ill, 2nd ed. Edited by Levenson JL. Arlington, VA, American Psychiatric Publishing, Inc, 2012, pp 1053–1084Google Scholar
45 : Care at the end of life, in The Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th ed. Edited by Stern TAFricchione GLCassem NHJellinek MSRosenbaum JF. Philadelphia, PA, Saunders/Elsevier, 2010, pp 555–563Crossref, Google Scholar
46 Care P: Transforming the Care of Serious Illness. Edited by Meier DE, Isaacs SL, Hughes RG. San Francisco, CA, Jossey-Bass A Wiley Imprint, 2010. (Robert Wood Johnson Series on Health Policy)Google Scholar
47 Walsh DCaraceni ATFainsinger RFoley KGlare PGoh CLloyd-Williams MOlarte JNRadbruch L (ed): Palliative Medicine. Philadelphia, PA, Saunders an Imprint of Elsevier, Inc., 2009Google Scholar
48
49 Fairman N, Irwin SA: Palliative care psychiatry: Update on an emerging dimension of psychiatric practice. Curr Psychiatry Rep 2013;15 (Suppl):374 (1-9)Google Scholar
50 : Undetected cognitive impairment and decision-making capacity in patients receiving hospice care. Am J Geriatr Psychiatry 2012; 20:306–316Crossref, Google Scholar
51 : Psychiatry and palliative care: 2 sides of the same coin. Can J Psychiatry 2008; 53:711–712Crossref, Google Scholar
52 : Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. J Palliat Med 2011; 14:275–279Crossref, Google Scholar
53 : Psychiatric issues in palliative care: recognition of depression in patients enrolled in hospice care. J Palliat Med 2008; 11:158–163Crossref, Google Scholar
54 : The opportunity for psychiatry in palliative care. Can J Psychiatry 2008; 53:713–724Crossref, Google Scholar
55 : Improving the quality of spiritual care as a dimension of palliative care: the report of the Consensus Conference. J Palliat Med 2009; 12:885–904Crossref, Google Scholar
56 : American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012; 30:880–887Crossref, Google Scholar
57 : Palliative care in the outpatient oncology setting: evaluation of a practical set of referral criteria. J Oncol Pract 2011; 7:366–370Crossref, Google Scholar
58 : Palliative cancer care a decade later: accomplishments, the need, next steps — from the American Society of Clinical Oncology. J Clin Oncol 2009; 27:3052–3058Crossref, Google Scholar
59 : Palliative care—a shifting paradigm. N Engl J Med 2010; 363:781–782Crossref, Google Scholar
60 : Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 2013; 309:470–477Crossref, Google Scholar
61 : Conceptual models for integrating palliative care at cancer centers. J Palliat Med 2012; 15:1261–1269Crossref, Google Scholar
62 : Impact of a palliative care consultation team on cancer-related symptoms in advanced cancer patients referred to an outpatient supportive care clinic. J Pain Symptom Manage 2011; 41:49–56Crossref, Google Scholar
63 : Effect of dignity therapy on distress and end-of-life experience in terminally ill patients: a randomised controlled trial. Lancet Oncol 2011; 12:753–762Crossref, Google Scholar
64 : Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol 2005; 23:5520–5525Crossref, Google Scholar
65 : Dignity therapy implementation in a community-based hospice setting. J Palliat Med 2011; 14:729–734Crossref, Google Scholar
66 : Pilot randomized controlled trial of individual meaning-centered psychotherapy for patients with advanced cancer. J Clin Oncol 2012; 30:1304–1309Crossref, Google Scholar
67 : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363:733–742Crossref, Google Scholar
68 : Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol 2012; 30:394–400Crossref, Google Scholar
69 Integrative Oncology. Edited by Abrams DI, Weil AT. New York, NY, Oxford University Press, Inc., 2009 (Weil Integrative Medicine Library)Google Scholar
70 : Integrative Oncology, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 447–454Crossref, Google Scholar
71 : Integrative oncology: complementary therapies for cancer survivors. Hematol Oncol Clin North Am 2008; 22:343–353, viiiCrossref, Google Scholar
72 : High prevalence of complementary and alternative medicine use among cancer patients: implications for research and clinical care. J Clin Oncol 2005; 23:2590–2592Crossref, Google Scholar
73 : Tobacco Use and Cessation, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 15–21Crossref, Google Scholar
74 : Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. J Clin Oncol 2006; 24:5125–5131Crossref, Google Scholar
75 : The clinical assessment and treatment of nicotine dependence. Focus 2011; 9:15–24Link, Google Scholar
76 : Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 2008; 26:556–562Crossref, Google Scholar
77 : Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res (Phila) 2011; 4:1360–1365Crossref, Google Scholar
78 : Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 2006; 99:215–220Crossref, Google Scholar
79 : Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 2002; 94:652–661Crossref, Google Scholar
80 : Cancer care and cancer survivorship care in the United States: will we be able to care for these patients in the future? J Oncol Pract 2009; 5:119–123Crossref, Google Scholar
81 : Implementing the Survivorship Care Plan: A Strategy for improving the quality of care of cancer patients, in Psycho-Oncology, 2nd ed. Edited by Holland JCBreitbart WSJacobsen PBLederberg MSLoscalzo MJMcCorkle R. New York, NY, Oxford University Press, 2010, pp 557–561Crossref, Google Scholar
82 Wise TNBiondi MConstantini A (ed): Psycho-Oncology. Arlington, VA, American Psychiatric Publishing, Inc., 2013Google Scholar
83 : Interdisciplinary teamwork in palliative care: Compassionate expertise for serious illness, in Handbook of Psychiatry in Palliative Medicine, 2nd ed. Edited by Chochinov HMBreitbart W. New York, NY, Oxford University Press, 2009, pp 172–185Google Scholar
84 Loscalzo MJ, Bultz BD, Jacobsen PB: Building psychosocial programs: a roadmap to excellence, in Psycho-Oncology, 2nd ed. Edited by Holland JC, Breitbart WS, Jacobsen PB, Lederberg MS, Loscalzo MJ, McCorkle R. New York, Oxford University Press, 2010:, pp 569–574Google Scholar
85 : Successful strategies for implementing biopsychosocial screening. Psychooncology 2011; 20:455–462Crossref, Google Scholar
86 : Implementing screening for distress, the 6th vital sign: a Canadian strategy for changing practice. Psychooncology 2011; 20:463–469Crossref, Google Scholar
87 : Screening for cancer-related distress: when is implementation successful and when is it unsuccessful? Acta Oncol 2013; 52:216–224Crossref, Google Scholar
88 : Implementing touch-screen technology to enhance recognition of distress. Psychooncology 2009; 18:822–830Crossref, Google Scholar
89 : Institute of Medicine, Committee on Psychosocial Services to Cancer Patients/Families in Cancer care for the Whole Patient: meeting psychosocial health needs. Washington, D.C, National Academies Press, 2008Google Scholar
90 : Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol 2012; 30:1160–1177Crossref, Google Scholar
91 ;
92 Kohn LTCorrigan JMDonaldson MS (ed): To Err Is Human: Building a Safer Health System. Washington, National Academies Press, 1999Google Scholar
93 : Acceptability of common screening methods used to detect distress and related mood disorders-preferences of cancer specialists and non-specialists. Psychooncology 2008; 17:226–236Crossref, Google Scholar
94
95 Commission on Cancer: Cancer program standards 2012: ensuring patient-centered care, http://facs.org/cancer/coc/programstandards2012.html, Accessed July 24, 2013Google Scholar
96 Oncology Nursing Society: Oncology Nursing Society issues joint position statement on implementing screening for distress, http://www.ons.org/news.aspx?id=285, Accessed July 24, 2013Google Scholar
97 : Using the science of psychosocial care to implement the new american college of surgeons commission on cancer distress screening standard. J Natl Compr Canc Netw 2013; 11:214–221Crossref, Google Scholar
98 National Comprehensive Cancer Network: NCCN guidelines for supportive care, http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive, Accessed July 24, 2013Google Scholar
99
100 National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology. Distress management. V2.2013, http://www.nccn.org/professionals/physician_gls/PDF/distress.pdf. Accessed July 24, 2013Google Scholar
101 : Evidence-based treatment of depression in patients with cancer. J Clin Oncol 2012; 30:1187–1196Crossref, Google Scholar
102
103
104 Children's Oncology Group: Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancer, http://www.survivorshipguidelines.org/, Accessed July 24, 2013Google Scholar



