Is Relapse in Schizophrenia an Immune-Mediated Effect?
Abstract
Clinical course in schizophrenia is often characterized by recurrent relapses, which are associated with adverse outcomes. Immune system abnormalities, including inflammation, have been one of the more enduring findings in the field, and several recent findings suggest that relapse in some patients with schizophrenia may be an immune mediated effect. These associations raise the possibility of immune-based treatments for relapse (and/or relapse prevention) in a subset of patients with schizophrenia. In this paper, we present a selected review of studies of immune system abnormalities in acute psychosis, in patients with first-episode psychosis and/or relapse of chronic schizophrenia, including cytokines, the acute phase response, leukocyte subsets, autoantibodies, and markers of blood-brain barrier dysfunction. We present a theoretical framework that attempts to integrate these findings and suggest potential mechanisms whereby immune system dysfunction might mediate relapse in some patients with schizophrenia. We also discuss limitations of the current literature and suggest future research directions.
Introduction
Schizophrenia is commonly a chronic, debilitating disorder with life-long consequences for affected individuals. Schizophrenia is heterogeneous with respect to clinical presentation, disease course, and outcome (1). However, the clinical course is often characterized by recurrent relapses, which are associated with adverse outcomes, including treatment-resistant symptoms, cognitive decline, and functional disability. Thus, to better understand relapse in schizophrenia is a compelling opportunity and a public health priority.
Immune system abnormalities in schizophrenia, including inflammation, have been one of the more enduring findings in the field, albeit with significant heterogeneity in the results, including negative studies. Nonetheless, multiple lines of evidence support an association between immune system dysfunction and schizophrenia. Polymorphisms in immune system-related genes, including cytokines (2) and the major histocompatibility complex (3–5) are associated with increased risk of schizophrenia. Prenatal maternal infections with a variety of agents are a replicated risk factor for schizophrenia (6). There is also an increased prevalence of autoimmune disease in both patients with schizophrenia and their first-degree relatives (7).
In this review, we will not focus on the relationship between the immune system and risk of schizophrenia. Rather, we ask a related, but important question that has not been reviewed in the literature: regardless of the underlying etiology, is relapse in schizophrenia an immune mediated effect? A number of recent findings support the plausibility of this association in some patients with schizophrenia. Several randomized, double-blinded trials in relapsed patients found that adjunctive nonsteroidal anti-inflammatory drug (NSAID) treatment significantly improved psychopathology (8–12), and that blood cytokine levels were a predictor of treatment response (10, 13). In a meta-analysis, we found that serum levels of some cytokines are increased in patients with first-episode drug-naïve psychosis (FEP) and acute relapse of schizophrenia, and decreased following treatment for relapse, suggesting a state-related effect that is independent of antipsychotic medications (14). Another recent study found that some patients with FEP have potentially pathogenic autoantibodies to central nervous system (CNS) antigens in the absence of overt signs of encephalitis (15). These associations raise the possibility of immune-based treatments for relapse (and/or relapse prevention) in a subset of patients with schizophrenia.
In this paper, we present a selected review of studies of immune system abnormalities in acute psychosis, in patients with FEP and/or relapse of chronic schizophrenia, including cytokines, the acute phase response, leukocyte subsets, autoantibodies, and markers of blood-brain barrier (BBB) dysfunction. As a comprehensive review of each of these areas of immune function is beyond the scope of the present paper, we will instead focus on replicated positive findings. We present a theoretical framework that attempts to integrate these findings and suggest potential mechanisms whereby immune system dysfunction might mediate relapse in some patients with schizophrenia. We also discuss limitations of the current literature and suggest future research directions.
Cytokines
Cytokines are key regulators of inflammation—the complex response of blood vessels to injury—that involves activation and recruitment of immune cells, and increased blood supply and vascular permeability. They coordinate both innate (e.g. granulocytes, monocytes/macrophages, and natural killer cells) and adaptive (e.g. B- and T-lymphocytes) arms of the immune system. Cytokines are key signaling molecules of the immune system that exert effects by binding specific cytokine receptors on a variety of target cells in the periphery and the brain. Soluble forms of cytokine receptors can either inhibit (e.g. soluble interleukin-2 receptor [sIL-2R]) or enhance (e.g. sIL-6R) the biological activity of cytokines. Endogenous cytokine receptor antagonists (e.g. IL-1 receptor antagonist [IL-1RA]) compete with cytokines for membrane receptors. Cytokines are also key regulators of the acute phase response (detailed below).
In a meta-analysis of 29 studies, we found that effect sizes for differences in serum cytokine levels between patients and controls were similar in magnitude and direction for relapse of chronic schizophrenia and FEP, suggesting an association that is independent of antipsychotic medications (14). IL-1β, IL-6, and TGF-β appeared to be state markers of acute psychosis, as levels were increased in acute psychosis, and decreased with antipsychotic treatment for relapse. In contrast, IL-12, interferon-γ (IFN-γ), tumor necrosis factor- α (TNF-α), and sIL-2R appeared to be trait markers, as levels remained elevated in acute psychosis and following antipsychotic treatment.
The findings in acute psychosis were most replicated for IL-6, which was significantly increased in 5 of 6 studies of relapsed patients and 4 of 4 studies of FEP, and significantly decreased following antipsychotic treatment in 3 of 5 studies in the meta-analysis. Serum IL-6 levels were significantly positively correlated with total psychopathology scores at baseline and following antipsychotic treatment in two studies (16, 17). A recent study of relapsed patients with schizophrenia replicated the pattern of results from the meta-analysis for serum IL-6 levels (18), and another study found increased IL-6 messenger ribonucleic acid (mRNA) levels in acute psychosis (19). Cerebrospinal fluid (CSF) IL-6 levels are also increased in relapsed patients with schizophrenia, particularly those with delayed response to antipsychotic treatment (20). Interestingly, unaffected adult carriers of a mutation in neuregulin 1 (NRG1) associated with schizophrenia have abnormal immune function, including increased in vitro IL-6 production and IL-6 mRNA (21). Increased cytokine levels were a predictor of response to adjunctive treatment with NSAIDs in two studies (10, 13). Further investigation of these relationships may even pave the way for targeted immune-based therapeutic interventions for acute psychosis, such as anti-IL-6 biopharmaceuticals.
Changes in serum cytokine levels may also precede relapse in schizophrenia. In 36 patients with schizophrenia who underwent weekly assessments for 1 year, in vitro IL-2 production plus antihippocampal immunoglobulin G (IgG) levels from the previous week significantly predicted relapse in three of seven patients (22). Similarly, among 64 male subjects with schizophrenia, increased CSF IL-2 levels following haloperidol withdrawal were a significant predictor of acute psychotic relapse (23). Longitudinal, intraindividual changes in other serum cytokine levels in stable patients have not been explored as a predictor of relapse in schizophrenia. If changes in serum cytokine levels are found to predict relapse, this marker could be evaluated in a variety of relapse prevention efforts.
Acute phase response
The acute phase response is the body’s compensatory reaction to disturbances in homeostasis due to factors such as infection, tissue injury, and even psychosocial stress. At the local site of abnormal homeostasis, the acute phase response includes activation of leukocytes, fibroblasts, and vascular endothelium. These activated cells produce proinflammatory cytokines, including IL-6 and IL-1β, which can mediate a systemic response that may include hypercortisolemia, leukocytosis, complement activation, increased secretion of immunoglobulins, and hepatic synthesis of acute phase proteins (24).
Several replicated findings are consistent with an acute phase response in patients with FEP and/or relapse of chronic schizophrenia. Hypercortisolemia has been reported in patients with FEP (25, 26). Following antipsychotic treatment of patients with FEP, one study reported a significant decrease in serum cortisol levels (26), and another found a significant positive correlation between decreases in serum cortisol levels and improvements in psychopathology (27). Importantly, in this study there was no correlation between perceived stress and serum cortisol levels, suggesting that the findings are not due to potential increased stress associated with acute psychosis.
Increased peripheral blood white blood cell (WBC) counts have been reported in acute psychosis compared with control subjects (28, 29), and in one of these studies there was a significant decrease in WBC counts following antipsychotic treatment for relapse. Increased serum levels of complement C3 (30-32) and C4 (31, 32) have also been reported. One study found that C3 levels were significantly positively correlated with Positive and Negative Syndrome Scale (PANSS) negative subscale scores (33). Three studies have reported increased total serum IgM and IgG levels (34–36), and lower IgG levels may be a predictor of greater clinical improvement (34). One study found increased erythrocyte sedimentation rate (ESR) with no known cause in 17% of patients with acute psychosis (37). Following eight weeks of antipsychotic treatment for relapse, ESR normalized in two-thirds of these patients, concomitant with decreased psychopathology.
Serum levels of acute phase proteins are also abnormal in patients with acute psychosis. In a meta-analysis of 8 studies, we found increased CRP levels in patients with schizophrenia (38). Since IL-6 and, to a lesser extent, IL-1β are known inducers of CRP, we hypothesized that CRP levels would be increased in acute psychosis, which is supported by several studies. Shcherbakova et al (39) found significantly increased CRP levels in acutely relapsed males compared with controls. Ohaeri et al. (40, 41) found significant decreases in CRP levels following resolution of acute psychosis. Mazzarello et al. (42) found higher mean CRP levels among patients with an episodic illness course compared with continuously ill subjects. However, no studies have simultaneously measured blood CRP and cytokine levels in acute psychosis. Although the findings have not been replicated, levels of several other proteins consistent with an acute phase response are also abnormal in acute psychosis, including increased alpha-1-antitrypsin (39), ceruloplasmin (43), and haptoglobin (32), and decreased albumin (44). In another study, ceruloplasmin levels were significantly positively correlated with PANSS negative and general symptom subscales (33). Taken together, many findings in relapse of schizophrenia are consistent with changes seen in the acute phase response.
Leukocyte subsets
In addition to increased WBC counts (28, 29), there are a number of replicated abnormalities of blood leukocyte subsets in FEP and/or relapse of schizophrenia. Increased blood monocytes have been reported in acute psychosis (29, 45), and another study of intraindividual changes in blood monocyte levels found an association between monocytosis and worsening of psychotic symptoms (46). Consistent with these findings, two studies found an increased proportion of (monocyte-derived) macrophages in the CSF during acute psychosis compared with controls (47, 48), that normalized in some patients following antipsychotic treatment for relapse (47). As described above, monocyte activation and cytokine production are part of the acute phase response.
Blood levels of lymphocyte subsets are also abnormal in acute psychosis. Three studies have found increased antibody-producing blood CD19+B-lymphocytes in acute psychosis (49–51), with a significant decrease in levels following antipsychotic treatment for relapse (49, 51). Blood total (CD3+) T-lymphocytes are also increased in acute psychosis (28, 52), and are further increased following treatment for relapse (49, 50, 52). One study found that in patients with clinically significant positive symptoms, a higher proportion of lymphocytes in the WBC differential predicted clinically significant improvement (53). There is also evidence for activated lymphocytes in the CSF during acute psychosis (48). Furthermore, dopamine can directly activate T-lymphocytes by binding T-cell D2 and D3 receptors, thereby potentially modulating T-lymphocytes in the CNS (54).
There are also alterations in T-lymphocyte subsets in acute psychosis. CD4+T-helper lymphocytes (Th cells) initiate and maintain immune system responses, including maturation of B-lymphocytes and activation of cytotoxic T-lymphocytes and macrophages. CD8+cytotoxic T-cells (Tc cells) can destroy virally infected cells and tumor cells. When activated, natural killer T-lymphocytes (NK cells) can perform functions ascribed to both Th and Tc cells
Three studies have found increased blood levels of Th cells in acute psychosis (28, 52, 55), although changes in levels following antipsychotic treatment for relapse have been inconsistent. The ratio of blood CD4+/CD8+T-lymphocytes is also increased in acute psychosis (28, 52). Consistent with this observation, another study found a significantly lower percentage of Tc cells in the CSF of relapsed patients compared with controls, although the authors noted a wide distribution of the proportion of Th and Tc cells in the patient group (56). Increased blood levels of NK cells during acute psychosis have also been found (28, 57), although changes in levels following antipsychotic treatment for relapse have varied. Consistent with these findings, serum levels of neopterin, a marker of cell-mediated immunity, including activation of macrophages and NK cells, are increased in patients with acute psychosis (58, 59), and decreased following antipsychotic treatment in one study (58). Few studies have simultaneously measured blood cytokines and leukocyte subsets (an important source of serum cytokines), which limits the ability to make broader inferences regarding immune function.
Autoantibodies
Both patients with schizophrenia and their first-degree relatives have an increased prevalence of autoimmune disease, which are associated with autoantibodies (7). Even in the absence of comorbid autoimmune disease, there is evidence for increased blood autoantibody levels in acute psychosis, consistent with increased levels of antibody-producing blood CD19+B-lymphocytes described above. Serum levels of platelet autoantibodies (PAA) are increased in both younger and adult patients with acute psychosis (60, 61). There is also a case report of a patient with chronic schizophrenia treated with the immunosuppressant azathioprine who had significant clinical improvement preceded by a decrease in PAA levels (62).
Although not replicated, there is evidence for increased serum antinuclear (ANA), smooth muscle (SMA; 63), thyroid peroxidase (TPO; 64), and heat shock proteins 70 kDa (HSP70; 65) autoantibodies in relapse of schizophrenia. HSP70 antibody titers significantly decreased after 6 weeks of antipsychotic treatment, and patients with higher baseline titers had higher baseline Brief Psychiatric Rating Scale scores and greater clinical improvement. Interestingly, patients with schizophrenia and a history of obstetric complications have a significantly higher prevalence of serum autoantibodies, including ANA, SMA, and TPO (66). Furthermore, unaffected carriers of the NRG1 mutation described above also had an increased prevalence of ANA, HSP, and TPO autoantibodies (21). Another study found that patients with positive ANA or rheumatoid factor titers were more likely to have clinically significant negative symptoms (53).
In a sample of 46 patients with FEP, Zandi et al. (15) retrospectively found four patients with either NMDA receptor or voltage-gated potassium channel autoantibodies, which are associated with limbic encephalitis. However, all four cases fulfilled DSM-IV criteria for schizophrenia, and none of these patients had any neurological signs or symptoms. These findings, particularly if replicated, have important potential implications for the evaluation and treatment of a subset of patients with FEP. Indeed, in conditions outside of schizophrenia other autoantibodies are associated with psychosis, such as antiribosomal P antibodies in lupus psychosis (67). Interestingly, patients with active (versus inactive) systemic lupus erythematosus (including central nervous system disease) have significantly higher blood IL-6 and IFN-γ levels (68).
Markers of blood–brain barrier dysfunction
Some serum cytokines, particularly IL-1RA and IL-6 can cross the BBB and bind target cells and affect CNS function (69). Serum S100B is a marker of astrocyte activation and BBB dysfunction (70). Elevated serum levels of S100B have been reported in 5 studies of patients with acute psychosis compared with controls (70–74). S100B levels were significantly positively correlated with PANSS total scores in two studies (71, 73), and PANSS negative subscale scores in one study (71). Also, serum S100B levels significantly decreased after 6 weeks of treatment for acute psychosis in one study (72), and the subgroup of patients with persistently elevated S100B levels had significantly higher PANSS negative subscale scores. Furthermore, increased CSF levels of S100B have been reported in acute psychosis (73, 75). Another marker of BBB disruption is an elevated CSF/serum albumin ratio, which was in three studies was abnormal in 19% (76), 22% (77), and 53% (78) of patients with acute psychosis, respectively.
Soluble intracellular adhesion molecule 1 (sICAM-1) is a marker of BBB damage and intrathecal immune activation. Paradoxically, three studies by the same group found decreased sICAM-1 in relapse of schizophrenia (77, 79, 80), although there are several caveats to these findings. In one study, patients with elevated HSP 60 kDa antibody titers had higher levels of sICAM-1 and sIL-2R (80). Another study found a significant positive correlation between sICAM-1 levels and PANSS negative subscale scores and a trend for higher sICAM-1 levels in patients with BBB impairment based on lumbar puncture (elevated CSF/serum albumin; 77). Lastly, a third study found higher levels of lymphocyte-function-associated antigen 1 (LFA-1+) Th cells, which binds sICAM-1, in patients with relapse of schizophrenia and BBB impairment (76). Thus, these findings are largely consistent with other evidence for BBB dysfunction in a subset of patients with acute psychosis.
Potential mechanisms of immune-mediated relapse
In order to summarize the studies reviewed above, Figure 1 presents a theoretical framework that attempts to integrate findings and relate them to potential mechanisms whereby immune system dysfunction might mediate relapse in some patients with schizophrenia. Replicated findings, as well as immune parameters that are significantly correlated with psychopathology in acute psychosis and/or “normalize” following treatment for relapse, are highlighted. This model is not intended to be comprehensive, but rather to serve as a catalyst for critical thinking and to suggest potential areas for future research. In brief, abnormal homeostasis results in cellular activation and proinflammatory cytokine production, which in turn stimulates an acute phase response. In the setting of increased BBB permeability, increased autoantibodies may directly cross-react with CNS antigens, or cytokine abnormalities may directly modulate dopaminergic neurotransmission or indirectly modulate glutamatergic neurotransmission through tryptophan catabolism, resulting in acute psychosis.
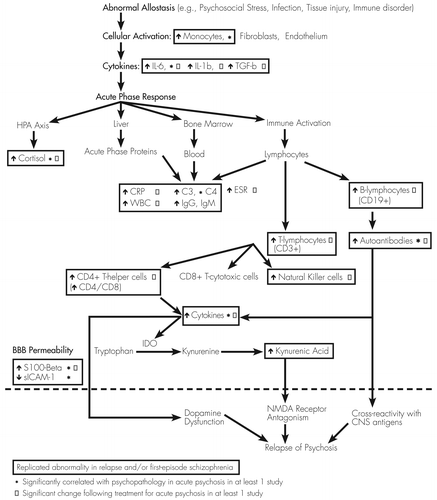
The potential for cross-reactivity between autoantibodies with CNS antigens has already been described above. Cytokines may also mediate acute psychosis. Proinflammatory cytokines can modulate neurotransmitter function, as systemic increases in serum IL-6 in adult rodents modulate dopamine turnover and sensitization to amphetamine-induced locomotion (81–83). By contrast, indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme in tryptophan catabolism, is also expressed in astrocytes and microglia, and its activity can be modulated by cytokines. IDO induction results in increased production of kynurenine, which is converted in astrocytes to the NMDA receptor antagonist kynurenic acid (KYN-A). NMDA receptor hypofunction has been implicated in the pathophysiology of schizophrenia (84, 85). Previous studies have found increased blood (86), CSF (87), and postmortem brain (88) levels of KYN-A, as well as increased IDO activity (89) in patients with schizophrenia.
Limitations and future research
An important consideration in this field is, “Acute psychosis is stressful, and stress can alter immune function, so are the observed abnormalities due to increased stress?” Furthermore, a related concern is, “Many individual studies did not control for potential factors known to influence various immune parameters, including antipsychotic medications, body mass index, and smoking (90), so is the observed immune dysfunction due to confounding by these factors?” Several lines of evidence argue against this reasoning. Many of the immune abnormalities described in this review have been found in drug-naïve patients with FEP, suggesting an effect that is independent of antipsychotic medications. Furthermore, some studies have found immune abnormalities in acute psychosis after controlling for multiple potential confounding factors, for example increased IL-6 (91) and CRP (92). Several studies reviewed here also found significant correlations between immune abnormalities and psychopathology, particularly total and negative symptoms, and we have reviewed putative mechanisms by which immune dysfunction could impact on psychopathology. Longitudinal studies described here also suggest that immune abnormalities normalize to some extent following antipsychotic treatment for relapse, implying that certain immune parameters may be state-dependent markers of acute psychosis. An intriguing study found potentially pathogenic CNS autoantibodies in a subset of patients with FEP, suggesting the potential for immune-based treatments in these patients (15). Lastly, adjunctive NSAIDs have been efficacious in improving psychopathology in relapsed patients (8–12). Taken together, these findings support the plausibility that acute psychosis in some patients with schizophrenia may be an immune mediated effect.
Despite the plausibility of our hypothesis, the literature in this field is fraught with significant heterogeneity, including contradictory findings. As an example, one study found increased CD3+T-lymphocytes that decreased with treatment in patients with FEP (28), whereas two other studies found decreased CD3+T-lymphocytes that increased with treatment (49, 51). How then do we reconcile and interpret these discrepant results? Importantly, it should be emphasized that schizophrenia is a very heterogeneous disorder. The extant literature suggests that immune dysfunction may mediate relapse in a subset of patients with schizophrenia; thus, future research should aim to further characterize this group of patients, which can reduce the “signal-to-noise” ratio in future analyses. Several approaches will facilitate accomplishing this task. Future studies of immune function in schizophrenia must control for potential confounding factors, including age, race, sex, BMI, smoking, duration of illness, and psychotropic medications. Additional studies in drug-naïve patients with FEP will also be indispensable. Most existing studies have focused on a single immune parameter (i.e. cytokines, acute phase reactants, or leukocyte subsets only). Future studies should concomitantly measure multiple immune parameters in individual patients, to begin to explore the validity of potential causal pathways. Longitudinal studies with serial measurements of immune parameters in stable patients (to explore potential relapse-predictive markers) as well as patients with acute psychosis (as a marker of response to treatment) are also needed.
Bilbo and Schwarz (93) hypothesized that maternal inflammation during critical periods of neurodevelopment may sensitize neural substrates and permanently alter the “set-point” of the immune system in adult offspring with schizophrenia. Indeed, a rodent prenatal maternal inflammation model found age-dependent increases in serum levels of IL-2, IL-6, and TNF-α, in the exposed offspring, which were decreased with haloperidol treatment (94, 95). Birth cohorts with archived maternal serum and other prenatal data—and the ability to identify cases of schizophrenia through register linkage—would afford unique opportunities to characterize patients in which relapse may be immune mediated. Future studies could also investigate the effects on immune function of genes associated with both schizophrenia and the immune system, including brain-derived neurotrophic factor, V-akt murine thymoma viral oncogene homolog 1, methylenetetrahydrofolate reductase, and phosphodiesterase 4B (2).
Recently, an increased understanding of the complex interactions between immune dysfunction and the brain in other chronic diseases has better informed this relationship in schizophrenia. There is growing empirical support that immune dysfunction may mediate relapse and response to treatment in some patients with schizophrenia. These findings also fit into the broader context of how a better understanding of the interface between immunology and chronic disease can guide and drive the delivery of clinical care, the advent of monoclonal antibody therapies for several human cancers being a prime example (96). The characterization of a subset of patients with schizophrenia in whom relapse is immune-mediated could be used to assess treatment effectiveness, advance relapse prevention efforts, and potentially even lead to future immune-based therapeutic interventions, thereby reducing the burden of relapse and improving clinical care of patients with schizophrenia.
1 : Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am 2007; 30:323–338Crossref, Google Scholar
2 : Using bioinformatic tools. Am J Psychiatry 2009; 166:854Crossref, Google Scholar
3 : Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460:748–752Crossref, Google Scholar
4 : Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 2009; 460:753–757Crossref, Google Scholar
5 : Common variants conferring risk of schizophrenia. Nature 2009; 460:744–747Crossref, Google Scholar
6 : Prenatal infection and schizophrenia: a review of epidemiological and translational studies. Am J Psychiatry 2010; 167:261–280Crossref, Google Scholar
7 : Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry 2006; 163:521–528Crossref, Google Scholar
8 : Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry 2002; 159:1029–1034Crossref, Google Scholar
9 : Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res 2007; 90:179–185Crossref, Google Scholar
10 : Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2010; 71:520–527Crossref, Google Scholar
11 : Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res 2010; 121:118–124Crossref, Google Scholar
12 : COX-2 inhibitors as antidepressants and antipsychotics: clinical evidence. Curr Opin Investig Drugs 2010; 11:31–42Google Scholar
13 : COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci 2004; 254:14–22Crossref, Google Scholar
14 : Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011; 70:663–671Crossref, Google Scholar
15 : Disease-relevant autoantibodies in first episode schizophrenia. J Neurol 2011; 258:686–688Crossref, Google Scholar
16 : Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci 1997; 247:228–233Crossref, Google Scholar
17 : Antipsychotic treatment may alter T-helper (TH) 2 arm cytokines. Int Immunopharmacol 2006; 6:666–671Crossref, Google Scholar
18 : Increased interleukin-6 level in Taiwanese schizophrenic patients. Chang Gung Med J 2011; 34:375–381Google Scholar
19 : Expression of anti-cardiolipin antibodies and inflammatory associated factors in patients with schizophrenia. Psychiatry Res 2011; 187:341–346Crossref, Google Scholar
20 : Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology 2003; 28:1515–1520Crossref, Google Scholar
21 : In vivo and in vitro genetic evidence of involvement of neuregulin 1 in immune system dysregulation. J Mol Med (Berl) 2010; 88:1133–1141Crossref, Google Scholar
22 Ganguli R, Gubbi A: Clinical and immunological characteristics of a subgroup of patients suffering from schizophrenia. In: Henneber AE, Kaschka WP (eds): Immunological alterations in psychiatric diseases. Adv Biol Psychiatry, Basel, Karger, 1997, 18, 35-43.Google Scholar
23 : Increases in CSF levels of interleukin-2 in schizophrenia: effects of recurrence of psychosis and medication status. Am J Psychiatry 1995; 152:1291–1297Crossref, Google Scholar
24 : Interleukin-6 and the acute phase response. Biochem J 1990; 265:621–636Crossref, Google Scholar
25 : Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res 2010; 175:47–53Crossref, Google Scholar
26 : Effect of antipsychotic treatment on Insulin-like Growth Factor-1 and cortisol in schizophrenia: a longitudinal study. Schizophr Res 2010; 119:131–137Crossref, Google Scholar
27 : Cortisol and dehydroepiandrosterone-sulphate levels correlate with symptom severity in first-episode psychosis. J Psychiatr Res 2011; 45:249–255Crossref, Google Scholar
28 : T-cell subsets in schizophrenia: a comparison between drug-naive first episode patients and chronic schizophrenic patients. Schizophr Res 1999; 38:61–70Crossref, Google Scholar
29 : Leukocytes and organ-nonspecific autoantibodies in schizophrenics and their siblings: markers of vulnerability or disease? Biol Psychiatry 1996; 40:825–833Crossref, Google Scholar
30 : Hyperactivation of the alternative complement cascade in schizophrenia. Dokl Biochem Biophys 2008; 419:56–57Crossref, Google Scholar
31 : Classical pathway complement activity in schizophrenia. Neurosci Lett 2005; 374:35–37Crossref, Google Scholar
32 : Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res 1997; 66:1–11Crossref, Google Scholar
33 : Acute phase proteins as biological markers of negative psychopathology in paranoid schizophrenia. Actas Esp Psiquiatr 2007; 35:249–252Google Scholar
34 : Immunoglobulins and improvement in acute schizophrenic reactions. Arch Gen Psychiatry 1973; 28:673–677Crossref, Google Scholar
35 : Immunoglobulin profile in acute psychiatric disorders. Indian J Med Res 1990; 92:101–104Google Scholar
36 : Gamma globulin levels in psychiatric patients. Can Psychiatr Assoc J 1972; 17:93–97Crossref, Google Scholar
37 : Erythrocyte sedimentation rate in patients with schizophrenia. Can J Psychiatry 2000; 45:938Google Scholar
38 : C-Reactive Protein in Schizophrenia: A Review and Meta-Analysis. Clinical Schizophrenia and Related Psychoses. (in press)Google Scholar
39 : The possible role of plasma kallikrein-kinin system and leukocyte elastase in pathogenesis of schizophrenia. Immunopharmacology 1999; 43:273–279Crossref, Google Scholar
40 : Tissue Injury-Inducing Potential of Unmodified ECT: Serial Measurement of Acute Phase Reactants. Convuls Ther 1992; 8:253–257Google Scholar
41 : The profile of C-reactive proteins in functional psychotic states in a cohort in Nigeria. Acta Psychiatr Scand 1993; 88:252–255Crossref, Google Scholar
42 : Lymphocytes in schizophrenic patients under therapy: serological, morphological and cell subset findings. Ital J Anat Embryol 2004; 109:177–188Google Scholar
43 : Plasma copper, iron, ceruloplasmin and ferroxidase activity in schizophrenia. Schizophr Res 2006; 86:167–171Crossref, Google Scholar
44 : Decreased serum albumin levels in Taiwanese patients with schizophrenia. Psychiatry Clin Neurosci 2002; 56:627–630Crossref, Google Scholar
45 : Do systemic inflammation and blood-brain barrier failure play a role in pediatric psychosis? Cleve Clin J Med 2009; 76:S93aCrossref, Google Scholar
46 : Correlation or coincidence between monocytosis and worsening of psychotic symptoms in veterans with schizophrenia? Schizophr Res 2011; 126:306–307Crossref, Google Scholar
47 : Accumulation of macrophages in the CSF of schizophrenic patients during acute psychotic episodes. Am J Psychiatry 1999; 156:1725–1729Google Scholar
48 : Increased frequency of activated lymphocytes in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res 2001; 49:99–105Crossref, Google Scholar
49 : T- and B-lymphocytes in patients with schizophrenia in acute psychotic episode and the course of the treatment. Psychiatry Res 2007; 152:173–180Crossref, Google Scholar
50 : Lymphocyte subsets in schizophrenic disorders. Relationship with clinical, neuromorphological and treatment variables. Schizophr Res 1990; 3:269–275Crossref, Google Scholar
51 : Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci 2010; 260:509–518Crossref, Google Scholar
52 : Cellular immunity in schizophrenic patients before and during neuroleptic treatment. Psychiatry Res 1991; 37:147–160Crossref, Google Scholar
53 : Leukocyte differentials predict short-term clinical outcome following antipsychotic treatment in schizophrenia. Biol Psychiatry 1998; 43:887–896Crossref, Google Scholar
54 : Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates beta1 integrin function. Eur J Immunol 2001; 31:3504–3512Crossref, Google Scholar
55 : T-lymphocyte subpopulations in schizophrenic patients. Eur Arch Psychiatry Neurol Sci 1990; 239:283–284Crossref, Google Scholar
56 : Abnormal distributions of T-lymphocyte subsets in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res 1995; 14:215–221Crossref, Google Scholar
57 : Changes of immunological functions after acute exacerbation in schizophrenia. Biol Psychiatry 1994; 35:173–178Crossref, Google Scholar
58 : Oxidative stress and neopterin abnormalities in schizophrenia: a longitudinal study. J Psychiatr Res 2010; 44:310–313Crossref, Google Scholar
59 : Increased serum neopterin levels in acutely ill and recovered schizophrenic patients. Schizophr Res 1998; 32:63–67Crossref, Google Scholar
60 : Blind verification of elevated platelet autoantibodies in serum of schizophrenic patients—part I: young subjects. Neuropsychobiology 2009; 60:44–48Crossref, Google Scholar
61 : Blind verification of elevated platelet autoantibodies in serum of schizophrenic patients—part II: adult subjects. Neuropsychobiology 2009; 60:49–54Crossref, Google Scholar
62 : Treatment of schizophrenia with an immunosuppressant. Lancet 1994; 344:59–60Crossref, Google Scholar
63 : Autoantibody reactivity in serum of patients with major depression, schizophrenia and healthy controls. Psychiatry Res 2008; 158:83–86Crossref, Google Scholar
64 : Neuro- and immunomodulatory steroids and other biochemical markers in drug-naive schizophrenia patients and the effect of treatment with atypical antipsychotics. Neuroendocrinol Lett 2011; 32:141–147Google Scholar
65 : Identification of antibodies to heat shock proteins 90 kDa and 70 kDa in patients with schizophrenia. Schizophr Res 2001; 52:127–135Crossref, Google Scholar
66 : Obstetric complications and autoantibodies in schizophrenia. Acta Psychiatr Scand 1995; 92:270–273Crossref, Google Scholar
67 : Clinical and biological aspects of anti-P-ribosomal protein autoantibodies. Autoimmun Rev 2007; 6:119–125Crossref, Google Scholar
68 : Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J Clin Immunol 1993; 13:58–67Crossref, Google Scholar
69 : Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des 2005; 11:973–984Crossref, Google Scholar
70 : Neuron-specific enolase is unaltered whereas S100B is elevated in serum of patients with schizophrenia—original research and meta-analysis. Psychiatry Res 2009; 167:66–72Crossref, Google Scholar
71 : Plasma S-100B protein in Chinese patients with schizophrenia: comparison with healthy controls and effect of antipsychotics treatment. J Psychiatr Res 2007; 41:36–42Crossref, Google Scholar
72 : Increased S100B blood levels in unmedicated and treated schizophrenic patients are correlated with negative symptomatology. Mol Psychiatry 2001; 6:445–449Crossref, Google Scholar
73 : Glial cell dysfunction in schizophrenia indicated by increased S100B in the CSF. Mol Psychiatry 2004; 9:897–899Crossref, Google Scholar
74 : Glial cell activation in a subgroup of patients with schizophrenia indicated by increased S100B serum concentrations and elevated myo-inositol. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:361–364Crossref, Google Scholar
75 : Increased cerebrospinal fluid and serum levels of S100B in first-onset schizophrenia are not related to a degenerative release of glial fibrillar acidic protein, myelin basic protein and neurone-specific enolase from glia or neurones. J Neurol Neurosurg Psychiatry 2006; 77:1284–1287Crossref, Google Scholar
76 : Increase in expression of adhesion molecule receptors on T helper cells during antipsychotic treatment and relationship to blood-brain barrier permeability in schizophrenia. Am J Psychiatry 1999; 156:634–636Google Scholar
77 : Decreased levels of soluble intercellular adhesion molecule-1 (sICAM-1) in unmedicated and medicated schizophrenic patients. Biol Psychiatry 2000; 47:29–33Crossref, Google Scholar
78 : Blood-cerebrospinal fluid barrier in schizophrenic patients. Eur Arch Psychiatry Neurol Sci 1987; 236:257–259Crossref, Google Scholar
79 : ICAM G241A polymorphism and soluble ICAM-1 serum levels: evidence for an active immune process in schizophrenia. Neuroimmunomodulation 2005; 12:54–59Crossref, Google Scholar
80 : Autoantibodies against 60-kDa heat shock protein in schizophrenia. Eur Arch Psychiatry Clin Neurosci 1998; 248:282–288Crossref, Google Scholar
81 : Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience 1999; 88:823–836Crossref, Google Scholar
82 : Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res 1994; 643:40–49Crossref, Google Scholar
83 : Interleukin-6 increases sensitivity to the locomotor-stimulating effects of amphetamine in rats. Brain Res 1999; 847:276–283Crossref, Google Scholar
84 : Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem 2009; 111:891–900Crossref, Google Scholar
85 : Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004; 174:54–64Crossref, Google Scholar
86 : Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurol India 2000; 48:231–238Google Scholar
87 : Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res 2005; 80:315–322Crossref, Google Scholar
88 : Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull 2011; 37:1147–1156Crossref, Google Scholar
89 : Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol 2009; 23:287–294Crossref, Google Scholar
90 : To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun 2009; 23:887–897Crossref, Google Scholar
91 : Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br J Psychiatry 2009; 194:434–438Crossref, Google Scholar
92 : C-reactive protein serum level in drug-free male Egyptian patients with schizophrenia. Psychiatry Res 2011; 190:91–97Crossref, Google Scholar
93 : Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci 2009; 3:14Crossref, Google Scholar
94 : Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology 2007; 32:1791–1804Crossref, Google Scholar
95 : Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry 2010; 15:372–383Crossref, Google Scholar
96 : Immunotherapy: past, present and future. Nat Med 2003; 9:269–277Crossref, Google Scholar



