Advances in Pharmacotherapy for Pediatric Anxiety Disorders
Abstract
Pediatric anxiety disorders are prevalent, chronic, and often lead to significant impaired functioning that impacts both short- and long-term outcomes for children and adolescents. Treatment options include pharmacotherapy and psychosocial interventions. This presentation will review treatment advances specifically for pharmacotherapy. Current research supports serotonin reuptake inhibitors as the medication class to be the first-line treatment option for pediatric anxiety disorders. Available evidence for the efficacy of other classes of medications will be reviewed, along with the available approaches to manage partial responders and nonresponders. The risks and benefits of pharmacotherapy will also be reviewed. In addition, recent research has shown the potential promise of novel agents that act upon other neural systems implicated in the development of pediatric anxiety disorders. Novel compounds that affect the glutamate system will be discussed.
(Reprinted with permission from Depression and Anxiety 2011; 28:76–87)
INTRODUCTION
Anxiety disorders are the most common type of psychiatric disorders diagnosed in children (1), with a prevalence rate of between 6 and 20% (2). The average age of onset for this type of disorder is 11 years, and age ranges from 6 to 21 years (3). Of course, it is completely normal for children to experience some degree of worry; however, those children who are diagnosed with an anxiety disorder can experience great distress and impairment in academic, social, and family functioning. The rates for child and adolescent obsessive-compulsive disorder (OCD) are between 0.5 and 2% (4), whereas rates of childhood posttraumatic stress disorder (PTSD) may be as high as 24–34.5% in urban areas (5, 6). However, it should be noted that these rates vary depending on population, methodology, and type of traumatic event (7).
Anxiety disorders can be chronic and are highly comorbid with additional anxiety disorders (8), major depression (9), and disruptive behavior disorders (10). Childhood anxiety disorders often lead to difficulties in adolescence and adulthood, such as anxiety and depressive disorders (11), substance abuse and dependence (12), and suicidal behavior (13). As a clinician or researcher focused on child and adolescent anxiety, it is important to know how to recognize clinical presentations of anxiety and what evidence-based treatment options are available.
PHARMACOLOGICAL STUDIES IN PEDIATRIC ANXIETY DISORDERS
Presently, the most effective known treatments for childhood-onset anxiety disorders are cognitive-behavioral therapy (CBT), behavior therapy (BT), and antidepressant medications, specifically the serotonin reuptake inhibitors (SSRIs). The following discussion will focus on randomized clinical trials (RCTs), which investigated the efficacy of treating child and adolescent suffering from a variety of anxiety disorders with medication or medication in combination with CBT.
See Table 1 for study details and medications.
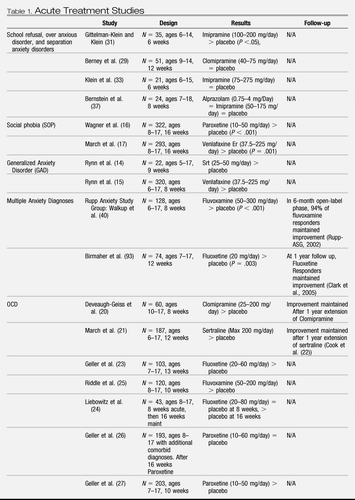 |
Table 1. Acute Treatment Studies
Generalized anxiety disorder
Lana, age 12, has worried about many different things over the past year, including what will happen if her mother gets sick, if her parents cannot afford their house, or if she fails a math test. She has trouble concentrating and becomes easily fatigued. Usually, if she starts worrying about one issue, she starts thinking of others, and often seeks reassurance from her mother. When her mother gets annoyed with her questioning, Lana gets very upset. Her mother describes her “as my little old lady.”
Rynn et al. (14) were the first to examine the effects of medication for children and adolescents with a primary diagnosis of generalized anxiety disorder (GAD) with or without separation anxiety disorder (14). The 9-week study involved 22 children, ages 5–17 years. The efficacy of sertraline was assessed. Sertraline was found to be superior to placebo for reducing anxiety symptoms (see Table 1). In another study of GAD, Rynn et al. (15) compared venlafaxine ER (a serotonin–norepinephrine reuptake inhibitor) to placebo (15). Pooled analysis indicated that 69% of participants responded to medication versus 48% to placebo (see Table 1) (15). The dose was titrated based on weight and response. Adverse events, such as asthenia, pain, anorexia, and somnolence, were experienced at least twice as often by the medication group versus the placebo group. Additionally, significant changes in weight, height, heart rate, blood pressure, and cholesterol level were observed in children in the venlafaxine ER group.
Social phobia
Nick, age 14, has always had difficulty making friends. At school, he sits by himself at the lunch table. Over the years, his teachers have described him as a pleasant, well-behaved child, a little on the shy side. He wants to make new friends, but is not sure how to start conversations with his peers. When he speaks to someone, he starts thinking, “I know they won't like me” and worries about what he looks and sounds like. He knows this is irrational, but describes not feeling comfortable in his “own skin,” and is constantly concerned about embarrassing himself in front of his peers and teachers.
Medication studies.
Wagner et al. (16) found that treatment of pediatric social phobia with paroxetine led to higher response rates than placebo (78% with medication group versus 38% with placebo) (see Table 1) (16). Adverse events (e.g. insomnia, decreased appetite, and vomiting) were experienced at least twice as often in the medication group than the placebo group. Upon discontinuation of paroxetine, nausea, dizziness, and vomiting were experienced twice as much by the medication group than the placebo group.
In another RCT, March et al. examined the efficacy of venlafaxine ER in the treatment of social phobia (17). The study found that participants receiving venlafaxine had a greater reduction in their social anxiety symptoms compared to placebo (response rates in the two groups were 56 versus 37%, respectively) (see Table 1). Both groups reported mild-to-moderate adverse events. Approximately 6% of participants on medication experienced significant weight loss.
Comparative trial.
Beidel et al. compared Social Effectiveness Therapy for Children (SET-C), fluoxetine, and a pill placebo in children and adolescents with social phobia (18). Both the SET-C and the fluoxetine group performed better than the placebo group (see Table 2). However, the SET-C group had significantly higher response rates as compared to fluoxetine and placebo (79 versus 36 versus 6%, respectively). The diagnostic remission rates were 53 versus 21 versus 3%, respectively. Treatment with SET-C was found to be superior to placebo for increasing social skills and social competence. There were no severe adverse events reported. However, some participants on fluoxetine experienced side effects, e.g. diarrhea. Treatment gains were maintained upon follow-up after 1 year.
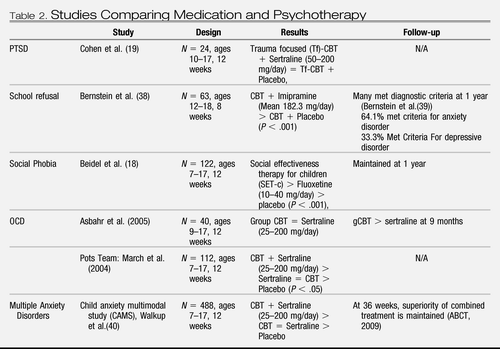 |
Table 2. Studies Comparing Medication and Psychotherapy
Posttraumatic stress disorder (PTSD)
Eliana, age 16, was sexually abused last year by a friend of her older brother. At first, she told herself it was no big deal, but six months ago she started having vivid flashbacks of this incident and thoughts that maybe it was her fault that it happened. She also has nightmares about him running after her and has difficulty falling asleep. She feels irritable, has difficulty concentrating, and has been avoiding going out with her friends because even a boy's smile reminds her of the abuse.
In a study conducted by Cohen et al. the effects of trauma-focused CBT (tf-CBT) combined with sertraline were compared with tf-CBT plus placebo (19). Participants consisted of 24 children, ages 10–17 years, who were experiencing PTSD (92%) or Posttraumatic Stress Syndrome. Both groups experienced significant reduction in PTSD symptoms, and no significant difference was found between treatment groups (see Table 2).
Obsessive-compulsive disorder
Dylan, age 9, has been having trouble finishing his homework because he feels the need to start over if he makes one mistake. His father tried to convince him to just erase, but he feels really nervous unless he starts all over. Lately, when he goes to bed, he cannot fall asleep unless he arranges all of his action figures four times in a specific order and checks that the windows in his room are locked. Once that is done, he silently repeats, “You got everything done” to help him fall asleep.
Medication studies.
DeVeaugh-Geiss et al. compared clomipramine (tricyclic antidepressant, TCA) to placebo (20). Clomipramine provided greater reduction in OCD symptoms versus placebo (37 versus 8%) (see Table 1). During the study, there were four discontinuations of treatment due to anticholinergic effects. However, many participants remained in treatment with clomipramine for 1 year and demonstrated improvement. Among the trials that compared the effects of different SSRIs for treatment of OCD, March et al. found that sertraline led to a greater number of responders to treatment than placebo (42 versus 26%) (see Table 1) (21). Cook et al. conducted a 1-year extension of sertraline (for participants from either group, medication versus placebo) and found that 67% of the sample responded to treatment (see Table 1) (22).
In a study examining the effects of fluoxetine, Geller et al. found that fluoxetine led to a greater number of responders to treatment compared to placebo (49 versus 25% placebo) and symptom improvement (see Table 1) (23). However, Liebowitz et al. found that, after 8 weeks, there were no significant differences between the fluoxetine group versus the placebo group (see Table 1) (24). After another 8 weeks, fluoxetine led to improvement in OCD symptoms and more treatment responders (57 versus 27% placebo). Riddle et al. found that fluvoxamine was significantly effective in reducing OCD symptoms (25 versus 14% placebo) (see Table 1) (25). Adverse events, which were similar across studies with the SSRIs, included headache, nausea, weight loss, insomnia, and agitation.
In a 16-week open trial of paroxetine versus placebo followed by a blinded 16-week randomized discontinuation phase, Geller et al. found that the presence of additional diagnoses decreases the response rate in the open-label treatment phase (see Table 1) (26). The population in this study had the primary diagnosis of OCD; however, 57.6% of the participants had at least one additional psychiatric disorder and 30.4% had multiple other diagnoses. The overall response rate to the open-label treatment phase was 71%; however, the response rates in participants with the following comorbid diagnoses were significantly less than those with only an OCD diagnosis (P < .05): attention deficit hyperactivity disorder (56%), tic disorder (53%), and oppositional defiant disorder (39%). Additional comorbid diagnoses led to greater relapse rates as follows: 46% for one or more comorbid disorders (P = .04) and 56% for two or more comorbid disorders (P < .05) compared to 32% without any comorbidity.
In a 10-week study that randomized entrants to paroxetine or placebo at the beginning of the study, Geller et al. found that paroxetine led to greater improvement in OCD symptoms than placebo (and greater number of treatment responders, 65% with paroxetine versus 41% with placebo) (see Table 1) (27). Upon discontinuation of medication, nausea and vomiting were twice as likely in the paroxetine group than in the placebo group.
Comparative trial.
The Pediatric OCD Treatment Study (POTS) compared CBT alone (including cognitive training and exposure and response prevention), sertraline alone, their combination, and pill placebo (POTS Team) (28). The combined treatment was found to be more effective in reducing symptoms than the other three conditions (P = .008 CBT alone, P = .007 sertraline alone, P = .001 placebo) (see Table 2). Both CBT alone and sertraline alone proved to be more effective than placebo (P = .003 CBT, P = .007 sertraline). In terms of number of participants demonstrating superior response to treatment, the combination treatment and CBT did not differ significantly (54 and 39%, respectively). Furthermore, medication did not differ from placebo.
School refusal
Jessica, age 7, has been complaining of daily stomach aches in school. She often visits the school nurse and frequently calls home, asking her parents to pick her up because her stomach hurts. She also has difficulty getting ready for school in the morning and lately she has been trying to convince her mother to let her stay home. Going to school makes her feel really shaky and she feels like she can't take a deep breath when she walks up the front steps of the school building.
Medication studies.
Four medication RCTs have focused on treating school refusal in children and adolescents with TCAs and have shown mixed results (29–32). These inconsistencies are likely caused by different diagnoses/comorbidities, varying medication dosages, small sample sizes, and the inclusion of participants in additional nonstandardized psychotherapies (33). Gittelman-Klein and Klein found that medication (imipramine) was significantly better for treating school refusal in children as compared to placebo, with 81% in the treatment group returning to school (as compared to 47% in the control, P < .05) (see Table 1) (34). However, Klein et al. also compared imipramine to placebo for children with a primary diagnosis of separation anxiety disorder (71% with school refusal) and failed to find differences between groups (see Table 1) (35).
Finally, Berney et al. tested clomipramine, whereas Bernstein et al. evaluated alprazolam and imipramine in comparison to placebo for school refusal (36, 37). Neither study had positive findings (see Table 1). Both Bernstein et al. and Gittleman-Klein and Klein reported dry mouth, drowsiness, dizziness, constipation, and nausea, with most occurring in the Imipramine groups. Klein et al. (35) reported more adverse effects, including mild-to-moderate dry mouth and irritability, and outbursts of anger in the moderate-to-severe range (35). Significantly, more side effects occurred in the treatment than in the placebo group.
Comparative trial.
Bernstein et al. compared CBT plus imipramine to placebo plus CBT for school refusal (38). They focused on adolescents with comorbid anxiety and depressive disorders, and found that at posttreatment, the imipramine plus CBT group's weekly attendance was significantly more improved (70 versus 28% of the time, P < .01) and there were significantly more participants considered “remitted” (weekly attendance of 75% or more school hours) than in the CBT plus placebo group (54 versus 17%, P < .01) (see Table 2). Depressive symptoms were also significantly more reduced in the combination group as compared to the group with the placebo (P < .05). Medication seemed to be well tolerated, as only one child dropped out after having a side effect related to treatment (i.e. manic symptoms). However, many children in both groups were still symptomatic at posttreatment. At the 1-year follow-up, 64% of those who had participated still met the criteria for an anxiety disorder, whereas 33% met criteria for a depressive disorder (39). In addition, 78% were in outpatient therapy and 68% had been prescribed psychotropic medication.
Summary of randomized control trials
The data from these studies have shown that there are efficacious options for the range of pediatric anxiety disorders: CBT, medication, and their combination. Based upon present available data, SSRIs are the first choice of medication if indicated given the side effect profile for this class of medication. There is preliminary evidence for using venlafaxine ER for pediatric GAD and SAD. Due to the safety profile of TCAs, they are not used as first-line and the efficacy evidence does not support its use. The CAMS (Walkup et al. (40)) and POTS (28) studies have demonstrated efficacy in combining CBT and SSRIs as well as CBT or an SSRI alone for treating certain pediatric anxiety disorders (28, 40). In both studies, the combined approach proved to be more effective than monotherapy with either CBT alone or SSRI alone. However, in a study conducted by Beidel et al. (18) it was found that a group on BT/SET-C performed better in treating SAD in children than an SSRI (18). Recent large-scale trials for social phobia and OCD did not demonstrate long-term benefit of adding antidepressant medications to exposure-based therapy (41, 42); similar findings were obtained for studies that intend to augment exposure-based therapy with benzodiazepines (43).
Certainly, there have been major advances in the available evidence for the use and efficacy of medication treatment for childhood anxiety. However, limitations exist. We need to determine how to enhance treatments for partial and nonresponders to treatment. Most studies discussed here did not compare follow-up samples to controls, in order to assess if findings were a result of treatment or maturity. We do not know the long-term impact of chronic medication treatment for children and adolescents.
NOVEL PSYCHOPHARMACOLOGICAL TREATMENTS
Although SSRI medications and CBT have both demonstrated efficacy in the treatment of pediatric anxiety disorders, approximately 20–35% of individuals receiving these treatments do not benefit (28, 40). Moreover, children and adolescents are frequently averse to engaging in the anxiety-provoking exposures that define CBT for anxiety. Consequently, the identification of new treatments that may enhance the treatment of pediatric anxiety is a much needed area of research.
Over the last decade, a number of novel compounds have garnered attention as potential pharmacological treatments for anxiety. Several of these compounds influence the N-methyl-d-aspartate system (NMDA), which has recently been associated with emotional learning and fear extinction (44). In this section, we will review six compounds—D-cycloserine (DCS), N-Acetylcysteine, Memantine, Riluzole, anticonvulsant medications, and Propanolol—and outline research indicating their efficacies in treating anxiety disorders in both adults and children.
D-cycloserine
Of the glutamatergic compounds being considered in the treatment of anxiety, DCS has been the most studied. DCS is a partial receptor agonist of the N-methyl-d-aspartame system that is believed to consolidate the learning that occurs during exposure to anxiety-provoking situations (45). The ability of DCS to facilitate “exposure learning” was first supported by animal research. Walker et al. conditioned rats to fear a light, by pairing the light with a shock in the rats' feet (46). The rats were then administered either DCS or saline, and half were provided with extinction training to the light while the others did not. Only those rats who received both DCS and extinction training exhibited a reduced fear response to the light. The fact that rats receiving DCS, but not extinction training, did not exhibit a reduced fear response suggested that DCS facilitates the extinction that occurs during prolonged exposure, but does not itself cause a reduction in fear. A subsequent animal study by Ledgerwood et al. (45) found that DCS administration was associated with a reduced fear response when administered after extinction training, suggesting that DCS may influence memory consolidation.
Subsequent research has examined the efficacy of DCS as an adjunct to exposure treatment in humans. Double-blinded clinical trials have demonstrated that exposure therapy augmented by DCS was superior to exposure therapy augmented by placebo in the treatment of specific phobia of heights (47), social phobia (48, 49), and panic disorder (50). Moreover, two studies, examining the additive effects to exposure and response prevention for OCD in adults, found that the DCS and placebo groups did not differ in OCD severity at posttreatment, but the DCS group exhibited gains more quickly during treatment than did the placebo group (51, 52). Other studies have found that the augmentation of CBT did not lead to greater treatment gains than CBT by itself or augmented with a placebo in the treatment of OCD (53) or spider fear (54).
A recent meta-analysis by Norberg et al. (55) suggested several factors that may impact the efficacy of DCS as an adjunct to CBT. First, they proposed that a ceiling effect may exist in which the added benefit of DCS decreases with milder cases of anxiety. That is, cases of mild or moderate anxiety may receive such a large benefit from exposure treatment that there is little added gain to be made with DCS augmentation. This argument is supported by the failure of DCS to improve the efficacy of exposure treatment in subclinical spider-fearing individuals (54) or in individuals trained to exhibit a fear response (56). Second, they argued that DCS is most efficacious when administered immediately before or after exposure therapy. Finally, they proposed that DCS' major contribution may be to increase the speed or efficiency of exposure treatment. This is consistent with the findings of Wilhelm et al. (51) and Kushner et al. (52) that DCS was associated with quicker gains, but not greater gains at posttreatment.
In terms of safety, DCS is approved by the US Food and Drug Administration (FDA) at chronic doses of 1,000–2,000 mg/day for pediatric tuberculosis treatment. At this dosing, there are more serious infrequent adverse events, such as confusion, tremor, vertigo, paraesthesias, and seizures (57). However, dose range for augmentation treatment for psychiatric disorders ranges shows mild adverse events of sedation, increased anxiety, restlessness, and headache (57).
In the pediatric population, there is one preliminary study of CBT augmented with DCS for OCD in children ages 8–17 who were randomized to receive CBT plus DCS (N = 15) versus CBT plus placebo (N = 15). Study participants received the DCS or pill placebo 1 hr before the exposure sessions which were held at least 5 days apart. The two treatment arms were found not to be statistically different, but the CBT plus DCS group showed small-to-moderate treatment effects (d = .31–.47) on the primary outcome measures (58) and no reported adverse events. However, the animal and adult literature provides promise that DCS may serve as a potentially efficacious adjunct to CBT for children and adolescents with anxiety disorders, especially considering the favorable side effect profile and the benefit of not requiring daily dosing. Animal studies suggest chronic dosing of DCS does not provide a significant improvement for learning or extinction as compared to acute administration (59). Based on the studies reviewed above, DCS may be most beneficial for cases in which CBT by itself has been unsuccessful or when there is a need for rapid treatment gains (e.g. anxiety leading to school refusal).
Riluzole
Riluzole is a potent antiglutamatergic agent that reduces glutamatergic neurotransmission in several ways, including inhibition of glutamate release, inactivation of voltage-dependent sodium channels in cortical neurons, and blockade of GABA reuptake (60, 61). Because of its antiglutamatergic effects, Riluzole has been targeted as a potential treatment for anxiety disorders. A number of adult case studies have demonstrated the efficacy of Riluzole in the treatment of OCD (62) and trichotillomania (63), as well as disordered eating and skin-picking behavior (64). In a series of case studies, Pittenger et al. (65) administered Riluzole 50–100 mg BID to 13 adults with treatment-refractory OCD. Seven of 13 participants exhibited a greater than 35% reduction in the YBOCS after 12 weeks of treatment augmented by Riluzole, and Riluzole was well-tolerated by all participants. Similarly, an open-label study by Coric et al. (66) found that Riluzole augmentation led to a greater than 35% decrease in the YBOCS for 7 of 13 adults receiving treatment for treatment-refractory OCD.
Riluzole has also demonstrated some efficacy as an augmenting agent in the treatment of GAD. In an open-label trial, Mathew et al. (67) administered Riluzole 100 mg/day to 18 adults with GAD. No other treatments were administered during the 8-week study period. At posttreatment, 12 participants exhibited improved symptoms according to the Hamilton Anxiety Rating Scale. In parallel to this study, 14 participants and 7 healthy controls for comparison completed an imaging protocol involving 3 1H MRS scans at baseline, 24 hr following medication administration, and at the end of treatment. Mathew et al. (68) demonstrated that improvement in GAD symptoms while receiving Riluzole treatment was associated with increases in hippocampal N-acetylaspartate (NAA), a putative marker for neuroplasticity, whereas, hippocampal NAA decreases were observed in Riluzole nonresponders at the end of treatment. The presence of increased NAA suggests modulation of the glutamatergic system, which for anxiety disorders may provide neuroprotection and enhancement of neural processes (69). Additionally, Ginsberg (70) completed a similar open-label study in which 18 adults with GAD were administered Riluzole 50 mg BID with no other treatments for 8 weeks. Approximately half the participants exhibited remission of GAD symptoms at 8 weeks (44% of all participants, 53% of completers), and a higher percentage demonstrated clinically significant symptom improvement (67% of all participants, 80% of completers). One study, examining Riluzole's efficacy and safety in a pediatric population, has also yielded preliminary promising results. Six children meeting criteria for OCD (ages 8–16) were administered Riluzole daily (maximum daily dose = 120 mg) for 12 weeks during an open-label trial by Grant et al. (71) At posttreatment, four of six participants exhibited decreased OCD symptoms, according to the CYBOCS, and improved overall functioning, according to the CGI. All participants demonstrated adequate tolerance of Riluzole, with no serious adverse events reported. Presently, the NIMH Pediatric Developmental Neuroscience branch is conducting a double placebo-controlled study comparing Riluzole versus placebo in pediatric OCD youth, which allows for comorbid autism spectrum disorders.
In general, Riluzole seems to be well-tolerated by both adults and children. In one study, common reported side effects included headache, nausea, and fatigue (65). Elevations in transaminases were reported for some participants in three studies (65, 66, 71), but none of these elevations led to discontinuation of Riluzole.
Memantine
Memantine is an NMDA receptor antagonist that is believed to provide neuroprotection against glutamatergic neurotoxicity (72). It received FDA approval for the treatment of Alzheimer's disease; but, like other agents that impact the glutamatergic system, memantine has recently been tested in the treatment of anxiety disorders, OCD in particular. Memantine, in combination with fluvoxamine, was found to reduce compulsive scratching in mice more so than fluvoxamine alone (73). In humans, Stewart et al. (74) conducted a single-blinded study, in which memantine (mean final dose = 18 mg/day) was added to the treatment regimens of 50% of participating individuals at an intensive residential treatment program for OCD. They found that the memantine group exhibited greater decrease in OCD symptoms and greater increase in overall functioning than did the control group. Published case studies in which memantine was used as an augmenting agent have yielded mixed results. Pasquini and Biondi (75) reported a clinically significant decrease in OCD symptoms for one individual, but not another receiving memantine augmentation (15 mg/day), and Aboujaoude (76) found that 6 of 14 individuals receiving memantine augmentation (20 mg/day) were considered responders (25% or greater decrease on CYBOCS) after a 12-week trial. Finally, Poyurovsky found a positive effect of memantine augmentation (20 mg/day) on an adult OCD patient after 3 weeks (77). Memantine has not yet been formally studied in children or adolescents with OCD, although Hezel et al. (78) reported considerable improvement when memantine (5 mg/day) was added to citalopram and CBT for a 15-year-old female with severe OCD symptoms (CYBOCS = 36). The participant exhibited maintained improvement without any negative side effects, after 9 months of continuous memantine augmentation. All the above studies reported good tolerance of memantine, and a study of memantine in the treatment of mania (79) also reported tolerance, with the most common side effects being relatively minor (e.g. nausea, constipation, headache).
Anticonvulsant medications
Because of their purported effects on the glutamate and GABA systems, anticonvulsant medications, including topiramate, gabapentin, and pregabalin, have been the focus of some study in the treatment of anxiety disorders. Whereas these medications seem to have several mechanisms of action, it is believed that the blockade of L-type calcium channels and inhibitory effects on GABA-ergic receptors may be related to the reduction of anxiety (80). In adults, open-trial and case studies have demonstrated some support for anticonvulsant medications in the treatment of anxiety disorders (80). The strongest study of anticonvulsant medications was a double-blind fixed-dose study of pregabalin (150 or 600 mg daily) in the treatment of adults with GAD (81). Individuals receiving pregabalin at either dose exhibited greater improvement in anxiety symptoms, as measured by the Hamilton Anxiety Rating Scale, than individuals receiving placebo, and pregabalin was well-tolerated. These findings were replicated by another double-blind trial of pregabalin in the treatment of GAD (82). However, anticonvulsants have been associated with a number of negative psychiatric side effects, including depression, aggressive behavior, and psychosis (83, 84). Because of this side effect profile, as well as the lack of research demonstrating the efficacy of anticonvulsants in pediatric populations, the use of anticonvulsants for pediatric anxiety is currently not a feasible treatment option.
Propanolol
Propanolol is a -adrenergic blocker that inhibits memory consolidation caused by the -adrenergic system (85). Based on this discovery, propanolol has been studied as a potential method to prevent and treat PTSD. Pitman and Sanders conducted a double-blind trial comparing propanolol (40 mg) four times daily to placebo in the treatment of PTSD (86). PTSD severity, as measured by the Clinician Administered PTSD scale (CAPS), did not differ between the two groups 1 month after treatment, which may be attributed to an outlier with an extremely high CAPS score in the propanolol group. However, the percentage of individuals continuing to meet criteria for PTSD was lower in the propanolol group (18%) than the placebo (30%) 1 month after treatment, and the propanolol group demonstrated reduced physiologic response compared to the placebo group when exposed to traumatic imagery. There was no difference reported between the groups at the 3-month follow-up, although the propanolol group, compared to the placebo group, continued to display reduced physiologic response to traumatic imagery. Case studies (87, 88), analog studies (89), and open trials (90) have also suggested that propanolol may treat PTSD and possibly prevent its onset. Of note, propanolol has not been studied in children who have experienced trauma or who meet criteria for PTSD. Whereas propanolol may be a promising treatment for PTSD in both adults and children, its use in modifying an individual's memories has raised some ethical concerns (see Bell (91)).
Summary
Medications influencing the glutamatergic, GABA-ergic, and -adrenergic systems hold promise as potential treatments for anxiety disorders. Most of the compounds mentioned above were well-tolerated in clinical trials and provide potential additional treatment options to consider. To this point, however, most research on the compounds listed above has been case studies or open trials, with the exception of several double-blind studies examining DCS. Furthermore, none of these compounds have been studied sufficiently in pediatric populations. Additional research is needed to determine the efficacy and safety of these medications in the treatment of pediatric anxiety disorders.
PRESENT PRACTICE FOR PEDIATRIC MEDICATION TREATMENT AND MONITORING
Although there is evidence supporting a combination of CBT and medication for treating child and adolescent anxiety, both treatments alone are also empirically supported options. This is useful, as combined treatment may not be feasible or accepted by a child and family. Potential benefits and risks of each treatment should be discussed to help families make an educated decision. Medication treatment is recommended when a child's symptoms are in the moderate-to-severe range, where the child cannot function (e.g. cannot eat or sleep, cannot start CBT) or when the child has been in CBT for 8–12 weeks, but is not improving.
Currently, SSRIs are considered first-line treatment, as these medications have few side effects and laboratory testing is not indicated. Children or adolescents who do not respond to an SSRI may benefit from venlafaxine ER, which is considered a second- to third-line medication that should involve monitoring of a child's weight, vital signs, and cholesterol based upon the available data that suggests some children may experience weight loss, increases in cholesterol, and vital sign changes.(15) It has been suggested that medication treatment may also be augmented for complicated and treatment-resistant cases with buspirone, benzodiazepines, stimulants, a second SSRI, atypical antipsychotics, and TCAs; however, there is little to no empirical evidence to support these options (7, 92, 93). Beginning with the lowest dosage of medication for the first 7 days is recommended. However, children tend to metabolize medications quickly and often require the same dosages as adults. Children and families should be told what to expect in terms of side effects, what to do when they occur, and how the clinician will manage potential adversities. Addressing the child directly is also essential, as parents are not always aware of side effects (i.e. an adolescent may not reveal that medication has reduced their sex drive). If after 1 week the child is tolerating the medication with minimal side effects, the dose can slowly be titrated upwards over several weeks until a treatment response occurs and the child is taking a dose known to be clinically efficacious. After 8–12 weeks, the medication treatment should be reevaluated to determine if it is being tolerated, reducing the target anxiety symptoms, and improving functioning. Systematic evaluation of a child's progress over time helps to create an evidence-based treatment plan, to review the child's progress over time, and to make treatment modifications as needed.
The FDA recommends making weekly appointments with the child for 4 weeks before switching to every 2 weeks for 4 weeks (94). Medication management appointments should then occur at 12 weeks and then every month. This guideline may be difficult to implement due to lack of physician availability, insurance coverage, and families' schedules. A clinician could use a phone session to monitor clinical progress and the development of adverse events. When a child or adolescent on an SSRI has a significant reduction in anxiety or depressive symptoms that has been maintained for more than 1 year, a medication-free trial during a low-stress time period (e.g. vacation) should occur. However, if the child begins to relapse, the clinician should immediately reinstate the medication (95).
Unfortunately, we do not yet know moderators or predictors of treatment response, and so we must often rely on educated trial and error. First, it is important to make sure that the child has been on adequate dose of the medication for a sufficient amount of time. If before 12 weeks, results should be reassessed at the end of this time period. Second, dosage should also be assessed. If a child has no benefit or adverse side effects, increasing the dose slowly over time is appropriate. Third, it is important to determine if the child is actually taking the medication. In cases of medication non-compliance, it is important to discuss this issue with the child and family to determine the root of the problem and evaluate potential solutions (e.g. alarm reminder, more psychoeducation, and alternative treatment options). If medication is still not effective, another trial of a different medication can be attempted.
As discussed, a combination of CBT and medication treatment can help significantly more children and adolescents suffering from an anxiety disorder. Even before large multisite RCTs, researchers and clinicians have advocated for the combination of psychosocial and pharmacological treatments. Arguments have been put forth to support the use of combination treatments as follows (96): (1) for children with severe anxiety, two treatments provide greater dosage, which may result in a quicker positive outcome; (2) comorbid diagnoses or various outcomes may respond to different treatments. For example, a child with separation anxiety disorder may show great symptom reduction from medication, but needs to participate in exposure therapy to return to school, whereas a child with comorbid ADHD and anxiety may also benefit from a combination of psychostimulant medication and CBT, such as classroom behavior management and parent training; and (3) when children partially respond, augmenting the first treatment with another intervention can increase the effect in one or more symptom areas, such as when a child already in CBT for OCD is prescribed an SSRI for comorbid depression. Combining psychosocial and psychopharmacological approaches to treatment also engenders a multidisciplinary team effort that provides mental health professionals with a variety of opportunities to learn from those with different backgrounds, fostering innovative ideas and improved clinical care.
CONCLUSION
The field of pediatric psychopharmacology for anxiety disorders has made great strides in examining medication treatment in terms of efficacy, safety, and its use in combination with psychotherapy. The evidence supports the use of medication monotherapy for anxiety disorders with SSRIs being first-line choice. However, cognitive behavioral therapy is an efficacious treatment for all anxiety disorders. In the case of pediatric OCD, excellent responder status was achieved by CBT monotherapy and combination treatment, whereas the medication arm did not differ from placebo. There are children who will benefit from starting with combination treatment from depending on illness severity, presence of comorbid diagnoses, and the child's ability to participate in treatment.
The field continues to be informed by the development and use of new compounds in adult populations suffering from the same disorders. However, safety concerns and the limited knowledge about the impact of mediation exposure on brain development, especially for long-term exposure, necessitate continued research of potential effects from presently used medications and cautious exploration with new compounds. In reviewing animal studies and the development of anxiety disorders in humans, Leonardo and Hen (91) discuss the importance of studying critical brain developmental windows where the interaction between environmental stressors and genetic makeup shape the formation of the anxiety neural circuitry (97). The manipulation of the serotonergic system in animal models during these periods seem to impact the baseline anxiety levels seen in the mature animal. As these processes are better understood in animal models, this will lead to a better understanding of the potential impact of various classes of medication on the developing brain (98).
At this time, there is limited information on the treatment approach for partial and nonresponders to first-line treatments. Research is needed to evaluate the use of second- and third-line treatments in order to determine the best augmentation and sequence strategies. Treatment strategies may be informed by the identification of clinical or biological traits that may help predict the best treatment selection for a specific child. An example of this is the finding that greater activity in the left amygdala predicts medication response (99). As the fields of clinical therapeutics and biological research continue to integrate to pursue these lines of inquiry, there is hope for continued advancement for the development of targeted treatments for those children who do not respond to the present available treatments.
1 Anderson JC, Williams S, McGee R, Silva PA. DSM-III disorders in preadolescent children. Prevalence in a large sample from the general population. Arch Gen Psychiatry 1987;44:69–76.Crossref, Google Scholar
2 Costello EJ, Egger H, Angold A. 10-year research update review: the epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. J Am Acad Child Adolesc Psychiatry 2005;44:972–986.Crossref, Google Scholar
3 Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593–602.Crossref, Google Scholar
4 Barrett PM, Farrell L, Pina AA, Peris TS, Piacentini J. Evidence-based psychosocial treatments for child and adolescent obsessive-compulsive disorder. J Clin Child Adolesc Psychol 2008;37:131–155.Crossref, Google Scholar
5 Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry 1991;48:216–222.Crossref, Google Scholar
6 Berman SL, Kurtines WM, Silverman WK, Serafini LT. The impact of exposure to crime and violence on urban youth. Am J Orthopsychiatry 1996;66:329–336.Crossref, Google Scholar
7 Connolly SD, Bernstein GA. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2007;46:267–283.Crossref, Google Scholar
8 Keller MB, Lavori PW, Wunder J, Beardslee WR, Schwartz CE, Roth J. Chronic course of anxiety disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry 1992;31:595–599.Crossref, Google Scholar
9 Brady EU, Kendall PC. Comorbidity of anxiety and depression in children and adolescents. Psychol Bull 1992;111:244–255.Crossref, Google Scholar
10 Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am 2005;14:631–648.Crossref, Google Scholar
11 Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 1998;55:56–64.Crossref, Google Scholar
12 Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 2007;64:566–576.Crossref, Google Scholar
13 Boden JM, Fergusson DM, Horwood LJ. Anxiety disorders and suicidal behaviours in adolescence and young adulthood: findings from a longitudinal study. Psychol Med 2007;37:431–440.Crossref, Google Scholar
14 Rynn MA, Siqueland L, Rickels K. Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am J Psychiatry 2001;158:2008–2014.Crossref, Google Scholar
15 Rynn MA, Riddle MA, Yeung PP, Kunz NR. Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: two placebo-controlled trials. Am J Psychiatry 2007;164:290–300.Crossref, Google Scholar
16 Wagner KD, Berard R, Stein MB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry 2004;61:1153–1162.Crossref, Google Scholar
17 March JS, Entusah AR, Rynn M, Albano AM, Tourian KA. A randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry 2007;62:1149–1154.Crossref, Google Scholar
18 Beidel DC, Turner SM, Sallee FR, Ammerman RT, Crosby LA, Pathak S. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry 2007;46:1622–1632.Crossref, Google Scholar
19 Cohen JA, Mannarino AP, Perel JM, Staron V. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry 2007;46:811–819.Crossref, Google Scholar
20 DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder—a multicenter trial. J Am Acad Child Adolesc Psychiatry 1992;31:45–49.Crossref, Google Scholar
21 March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. J Am Med Assoc 1998;280:1752–1756.Crossref, Google Scholar
22 Cook EH, Wagner KD, March JS, et al. Long-term sertraline treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 2001;40:1175–1181.Crossref, Google Scholar
23 Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry 2001;40:773–779.Crossref, Google Scholar
24 Liebowitz MR, Stein MN, Tancer M, Carpenter D, Oakes R, Pitts CD. A randomized, double-blind, fixed-dose comparison of paroxetine and placebo in the treatment of generalized social anxiety disorder. J Clin Psychiatry 2002;63:66–74.Crossref, Google Scholar
25 Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry 2001;40:222–229.Crossref, Google Scholar
26 Geller DA, Biederman J, Stewart SE, et al. Impact of comorbidity on treatment response to paroxetine in pediatric obsessive-compulsive disorder: is the use of exclusion criteria empirically supported in randomized clinical trials? J Child Adolesc Psychopharmacol 2003;13:S19–S29.Crossref, Google Scholar
27 Geller DA, Wagner KD, Emslie G, et al. Paroxetine treatment in children and adolescents with obsessive-compulsive disorder: a randomized, multicenter, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 2004;43:1387–1396.Crossref, Google Scholar
28 March J, Foa EB, Gammon P, et al. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) randomized controlled trial. J Am Med Assoc 2004;292:1969–1976.Crossref, Google Scholar
29 Berney T, Kolvin I, Bhate SR, et al. School phobia: a therapeutic trial with clomipramine and short-term outcome. Br J Psychiatry 1981;138:110.Crossref, Google Scholar
30 Bernstein GA, Garfinkel BD, Borchardt CM. Comparative studies of pharmacotherapy for school refusal. J Am Acad Child Adolesc Psychiatry 1990;29:773.Crossref, Google Scholar
31 Gittelman-Klein R, Klein DF. Controlled imipramine treatment of school phobia. Arch Gen Psychiatry 1971;25:204–207.Crossref, Google Scholar
32 Klein RG, Koplewicz HS, Kanner A. Imipramine treatment of children with separation anxiety disorder. J Am Acad Child Adolesc Psychiatry 1992;31:21.Crossref, Google Scholar
33 King NJ, Bernstein GA. School refusal in children and adolescents: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 2001;40:197.Crossref, Google Scholar
34 Gittelman-Klein R, Klein DF. School phobia: controlled imipramine treatment. Calif Med 1971;115:42.Google Scholar
35 Klein RG, Koplewicz HS, Kanner A. Imipramine treatment of children with separation anxiety disorder. J Am Acad Child Adolesc Psychiatry 1992;31:21–28.Crossref, Google Scholar
36 Berney T, Kolvin I, Bhate SR, et al. School phobia: a therapeutic trial with clomipramine and short-term outcome. Br J Psychiatry 1981;138:110–118.Crossref, Google Scholar
37 Bernstein GA, Garfinkel BD, Borchardt CM. Comparative studies of pharmacotherapy for school refusal. J Am Acad Child Adolesc Psychiatry 1990;29:773–781.Crossref, Google Scholar
38 Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry 2000;39:276–283.Crossref, Google Scholar
39 Bernstein GA, Hektner JM, Borchardt CM, McMillan MH. Treatment of school refusal: one-year follow-up. J Am Acad Child Adolesc Psychiatry 2001;40:206–213.Crossref, Google Scholar
40 Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 2008;359:2753–2766.Crossref, Google Scholar
41 Davidson JR, Foa EB, Huppert JD, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry 2004;61:1005–1013.Crossref, Google Scholar
42 Foa EB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry 2005;162:151–161.Crossref, Google Scholar
43 Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776–787.Crossref, Google Scholar
44 Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry 2002;52:998–1007.Crossref, Google Scholar
45 Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci 2003;117:341–349.Crossref, Google Scholar
46 Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci 2002;22:2343–2351.Crossref, Google Scholar
47 Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61:1136–1144.Crossref, Google Scholar
48 Hofmann SG, Meuret AE, Smits JA, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry 2006;63:298–304.Crossref, Google Scholar
49 Guastella AJ, Richardson R, Lovibond PF, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry 2008;63:544–549.Crossref, Google Scholar
50 Otto MW, Tolin DF, Simon NM, et al. Efficacy of D-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry 2010;67:365–370.Crossref, Google Scholar
51 Wilhelm S, Buhlmann U, Tolin DF, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry 2008;165:335–341.Crossref, Google Scholar
52 Kushner MG, Kim SW, Donahue C, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry 2007;62:835–838.Crossref, Google Scholar
53 Storch EA, Merlo LJ, Bengtson M, et al. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol 2007;22:230–237.Crossref, Google Scholar
54 Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res 2007;41:466–471.Crossref, Google Scholar
55 Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry 2008;63:1118–1126.Crossref, Google Scholar
56 Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther 2007;45:663–672.Crossref, Google Scholar
57 Hofmann SG, Pollack MH, Otto MW. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Rev 2006;12:208–217.Crossref, Google Scholar
58 Storch E, Murphy T, Goodman W, et al. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry, in press.Google Scholar
59 Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry 2006;60:369–375.Crossref, Google Scholar
60 Jehle T, Bauer J, Blauth E, et al. Effects of riluzole on electrically evoked neurotransmitter release. Br J Pharmacol 2000;130:1227–1234.Crossref, Google Scholar
61 Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci 2000;12:3567–3574.Crossref, Google Scholar
62 Coric V, Milanovic S, Wasylink S, Patel P, Malison R, Krystal JH. Beneficial effects of the antiglutamatergic agent riluzole in a patient diagnosed with obsessive-compulsive disorder and major depressive disorder. Psychopharmacology 2003;167:219–220.Crossref, Google Scholar
63 Coric V, Kelmendi B, Pittenger C, Wasylink S, Bloch MH, Green J. Beneficial effects of the antiglutamatergic agent riluzole in a patient diagnosed with trichotillomania. J Clin Psychiatry 2007;68:170–171.Crossref, Google Scholar
64 Sasso DA, Kalanthi PS, Trueblood KV, et al. Beneficial effects of the glutamate-modulating agent riluzole on disordered eating and pathological skin-picking behaviors. J Clin Psychopharmacol 2006;26:685–687.Crossref, Google Scholar
65 Pittenger C, Kelmendi B, Wasylink S, Bloch MH, Coric V. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a series of 13 cases, with long-term follow-up. J Clin Psychopharmacol 2008;28:363–367.Crossref, Google Scholar
66 Coric V, Taskiran S, Pittenger C, et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry 2005;58:424–428.Crossref, Google Scholar
67 Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry 2005;162:2379–2381.Crossref, Google Scholar
68 Mathew SJ, Price RB, Mao X, et al. Hippocampal N-acetylaspartate concentration and response to riluzole in generalized anxiety disorder. Biol Psychiatry 2008;63:891–898.Crossref, Google Scholar
69 Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry 2005;162:2379–2381.Crossref, Google Scholar
70 Ginsberg DL. Riluzole effective for generalized anxiety disorder. Prim Psychiatry 2005;12:25.Google Scholar
71 Grant P, Lougee L, Hirschtritt M, Swedo SE. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J Child Adolesc Psycho-pharmacol 2007;17:761–767.Crossref, Google Scholar
72 Chipana C, Torres I, Camarasa J, Pubill D, Escubedo E. Memantine protects against amphetamine derivatives-induced neurotoxic damage in rodents. Neuropharmacology 2008;54:1254–1263.Crossref, Google Scholar
73 Wald R, Dodman N, Shuster L. The combined effects of memantine and fluoxetine on an animal model of obsessive compulsive disorder. Exp Clin Psychopharmacol 2009;17:191–197.Crossref, Google Scholar
74 Stewart S, Jenike EA, Hezel DM, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol 2010;30:34–39.Crossref, Google Scholar
75 Pasquini M, Biondi M. Memantine augmentation for refractory obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:1173–1175.Crossref, Google Scholar
76 Aboujaoude E, Barry JI, Gomel N. Memantine augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. J Clin Psychopharmacol 2009;29:51–55.Crossref, Google Scholar
77 Poyurovsky M, Weizman R, Weizman A, Koran L. Memantine for treatment-resistant OCD. Am J Psychiatry 2005;162:2191–2192.Crossref, Google Scholar
78 Hezel DM, Beattie K, Stewart S. Memantine as an augmenting agent for severe pediatric OCD. Am J Psychiatry 2009;166:237.Crossref, Google Scholar
79 Keck Jr PE, Hsu H-A, Papadakis K, Russo Jr J. Memantine efficacy and safety in patients with acute mania associated with bipolar I disorder: a pilot evaluation. Clin Neuropharmacol 2009;32:199–204.Crossref, Google Scholar
80 Mula M, Pini S, Cassano GB. The role of anticonvulsant drugs in anxiety disorders: a critical review of the evidence. J Clin Psychopharmacol 2007;27:263–272.Crossref, Google Scholar
81 Pande AC, Crockatt JG, Feltner DE, et al. Pregabalin in generalized anxiety disorder: a placebo-controlled trial. Am J Psychiatry 2003;160:533–540.Crossref, Google Scholar
82 Feltner DE, Crockatt JG, Dubovsky SJ, et al. A randomized, double-blind, placebo-controlled, fixed-dose, multicenter study of pregabalin in patients with generalized anxiety disorder. J Clin Psychopharmacol 2003;23:240–249.Google Scholar
83 Levinson DF, Devinsky O. Psychiatric adverse events during vigabatrin therapy. Neurology 1999;53:1503–1511.Crossref, Google Scholar
84 Mula M, Trimble MR, Sander JW. Are psychiatric adverse events of antiepileptic drugs a unique entity? A study on topiramate and levetiracetam. Epilepsia 2007;48:2322–2326.Google Scholar
85 Cahill L, Prins B, Weber M, McGaugh JL. β-Adrenergic activation and memory for emotional events. Nature 1994;371:702–704.Crossref, Google Scholar
86 Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002;51:189–192.Crossref, Google Scholar
87 Taylor F. A case of reemergent post-traumatic stress disorder arrested by propranolol intervention: does a stitch in time save nine? [References]. In: Corales TA, editor. Focus on Posttraumatic Stress Disorder Research. Hauppauge, NY: Nova Science Publishers; 2005:37–49.Google Scholar
88 Taylor F, Cahill L. Propranolol for reemergent posttraumatic stress disorder following an event of retraumatization: a case study. J Trauma Stress 2002;15:433–437.Crossref, Google Scholar
89 Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress. J Psychiatr Res 2008;42:503–506.Crossref, Google Scholar
90 Vaiva G, Ducrocq F, Jezequel K, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 2003;54:947–949.Crossref, Google Scholar
91 Bell J. Propranolol, post-traumatic stress disorder and narrative identity. J Med Ethics: J Insitute Med Ethics 2008;34:1–4.Google Scholar
92 Labellarte M, Ginsburg GS, Walkup J, Riddle MA. The treatment of anxiety disorders in children and adolescents. Biol Psychiatry 1999;46:1572–1578.Crossref, Google Scholar
93 Birmaher B, Yelovich K, Renaud J. Pharmacologic treatment for children and adolescents with anxiety disorders. Pediatr Clin North Am 1998;45:1187–1204.Crossref, Google Scholar
94 FDA. Revisions to medication guide medication guide: anti-depressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions; 2007. http://www.fda.gov.downloads/Drugs/DrugSafety/Informationby Drug Class/ucm100211.pdf.Google Scholar
95 Pine DS. Treating children and adolescents with selective serotonin reuptake inhibitors: how long is appropriate? J Child Adolesc Psychopharmacol 2002;12:189–203.Crossref, Google Scholar
96 March. Combining medication and psychosocial treatments: an evidence-based medicine approach. Int Rev Psychiatry 2002;14: 155–163.Crossref, Google Scholar
97 Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology 2008;33:134–140.Crossref, Google Scholar
98 Pine DS, Helfinstein S, Bar-Haim Y, Nelson EE, Fox N. Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology 2009;34:213–228.Crossref, Google Scholar
99 McClure EB, Adler A, Monk CS, et al. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology 2007;191:97–105.Crossref, Google Scholar



