Stressful Life Events, Genetic Liability, and Onset of an Episode of Major Depression in Women
Abstract
Objective:
This study was undertaken to clarify how genetic liability and stressful life events interact in the etiology of major depression.
Method:
Information about stressful life events and onset of major depressive episodes in the past year was collected in a population-based sample of female-female twin pairs including 2,164 individuals, 53,215 person-months of observation, and 492 onsets of depression.
Results:
Nine “personal” and three aggregate “network” stressful events significantly predicted onset of major depression in the month of occurrence, four of which predicted onset with an odds ratio of >10 and were termed “severe”: death of a close relative, assault, serious marital problems, and divorce/breakup. Genetic liability also had a significant impact on risk of onset of depression. Four severe stressful events, as well as for 10 of the 12 individuals stressful events, the best-fitting model for the joint effect of stressful events and genetic liability on onset of major depression suggested genetic control of sensitivity to the depression-inducing effects of stressful life events. In individuals at lowest genetic risk (monozygotic twin, co-twin unaffected), the probability of onset of major depression per month was predicted to be 0.5% and 6.2%, respectively, for those unexposed and exposed to a severe event. In those at highest genetic risk (monozygotic twin, co-twin affected), these probabilities were 1.1% and 14.6%, respectively. Linear regression analysis indicated significant Genotype by Environment interaction in the prediction of onset of major depression.
Conclusions:
Genetic factors influence the risk of onset of major depression in part by altering the sensitivity of individuals to the depression-inducing effect of stressful life events.
(Reprinted with permission from The American Journal of Psychiatry 1995; 152:833–842)
According as the humour itself is intended or remitted in men [and] their … rational soul is better able to make resistance; so are they more or less affected [by adversity]. For that which is but a flea-biting to one, causeth insufferable torment to another; and which one by his singular moderation and well-imposed carriage can happily overcome, a second is no whit able to sustain, but upon every small occasion of misconceived abuse, injury, grief, disagree [and] loss … yields so far to passion, that … his digestion hindered, his sleep gone, his spirits obscured, and his heart heavy … he himself [is] overcome with melancholy.
There is … a group of depressive illnesses in which the psychological impetus is of prime importance …. These depressive states are constitutionally based: they occur in individuals who from youth onward are inclined to take things badly, whose depressive reaction [to “psychological upsets”] is generally severe and of more than average duration.
The concept that depression commonly arises when a vulnerable individual confronts adversity is not new. However, surprisingly little progress has been made in confirming or refuting this plausible and widely held belief by rigorous hypothesis testing. Although substantial evidence now exists that adversity (3, 4), especially stress life events, and genetic predisposition (5, 6) are important risk factors for major depression, we still know little about how these two risk factors interact. Previous studies of this question have suffered from methodologic limitations, including small sample size, indirect methods of assessment, simplistic analytic strategies, and an inability to separate genetic from familial/environmental effects.
In 1922 Strecker (7) examined the family histories of “mental disease” of patients with manic-depressive psychosis and noted that the proportion of patients with a positive family history was lowest among those whose onset was associated with severe stress. He concluded that “extraneous” factors are of less etiologic importance in cases of manic-depressive illness when the family background is “defective.” Using family history information, Pollitt (8) found that the risk of major depression in relatives was 50% lower when the patient's onset was associated with a severe stressful life event. Perris et al. (9), however, found no difference in the number of stressful life events prior to a depressive episode among patients with family histories of major depression and those without. Phelan et al. (10) examined depressive symptoms and found that individuals with positive family histories were more sensitive to the depression-inducing effects of certain stressful life events than those who had no family history of major depression. In contrast, McGuffin et al. (11) examined the impact of recent stressful life events and family background on the risk of current depression and found that individuals at high familial risk for major depression were less sensitive to the depressive effects of stressful life events than were individuals at low familial risk.
If both genetic and environmental risk factors are of etiologic importance in major depression, they may interact in disease etiology in potentially complex ways (12, 13). Of the possible models, we emphasize two: additive and genetic control of sensitivity to the environment. In the additive model, the increase in risk associated with exposure to stressful life events is similar for individuals with low-risk and high-risk genotypes; this model predicts that the impact of stressful life events and genetic risk factors on liability to major depression are independent. In genetic control of sensitivity to the environment, the increase in risk of major depression associated with exposure to stressful life events is greater for those with a high genetic risk that for those with a low genetic risk; that is, genes have an impact on the risk of major depression in part by altering the individual's sensitivity to the depression-inducing effect of stressful life events.
In this report we examine how stress life events and genetic vulnerability influence the risk of onset of an episode of major depression in a population-based sample of personally interviewed female twins. We address the following specific questions:
| 1. | What are the magnitude and duration of the impact of stressful life events on the risk of onset of major depression? | ||||
| 2. | What is the magnitude of the impact of genetic factors on the risk of onset of major depression? | ||||
| 3. | What is the nature of the interaction between stressful life events and genetic factors in the risk of onset of major depression? Specifically, can we find evidence in support of an additive model or a model of genetic control of sensitivity to the environment? | ||||
METHOD
We studied female-female Caucasian twin pairs ascertained from the population-based Virginia Twin Registry (6, 14, 15) whose mean age at first interview was 30.1 years (SD = 7.6). Twin pairs were enrolled if both individuals responded to a mailed questionnaire, the individual response rate to which was 64%. Personal interviews were then conducted with 93% of this group (N = 2,164 individuals) (92% face to face and 8% by telephone), which included both members of 1,033 pairs. Follow-up interviews were then conducted with 2,002 of the twins initially interviewed (93% of the 2,164), including both members of 938 pairs. Unlike the initial interviews, nearly all of the follow-up interviews were conducted by telephone. After an explanation of the research procedure, written informed consent was obtained from all subjects before the personal interviews. For telephone interviews, verbal assent was obtained.
The mean number of months between these two interviews was 17.3 (SD = 3.8). Both interviews were conducted by individuals with a master's degree or doctorate in social work or psychology or a bachelor's degree and at least 2 years of clinical experience. The same interviewer never interviewed both members of a twin pair. As outlined in detail elsewhere (6), zygosity was determined from a set of interview questions and photographs, and when these were not definitive, from DNA polymorphisms, producing an error rate in zygosity assignment that can be estimated to be under 2%. Final determinations of zygosity yielded 590 monozygotic and 440 dizygotic twin pairs as well as three pairs of unknown zygosity.
Included in both interviews was an identical section that assessed episodes of major depression, defined according to the DSM-III-R criteria, and a series of stressful life events over the month of the interview and the 12 preceding months. These were assessed in different portions of the interview to reduce retrospective reporting bias. Specifically, we assessed the occurrence over the past year of 14 individual symptoms that represented the disaggregated nine “A” criteria for major depression in DSM-III-R (including two items for criterion A4 to assess separately insomnia and hypersomnia). For each symptom reported as present, interviewers probed to ensure that it was not due to physical illness or medication. Respondents then had to aggregate reported symptoms over the past year into co-occurring syndromes. If depressive syndromes occurred, respondents were asked when they occurred, and their onset and end were recorded to the nearest month.
The months of occurrence of all stressful life events were also recorded. At both interviews we assessed nine “personal” events (events that happened primarily to the informant: assault (assault, rape, or mugging), serious marital problems, divorce/breakup (divorce, marital separation, broken engagement, or breakup of other romantic relationship), job loss (being laid off from a job or fired), loss of a confidant (separation from other loved one or close friend), serious illness, major financial problem, being robbed, and serious legal problem. We also assessed 22 “network” events, i.e., events that occurred primarily to, or in interaction with, an individual in the respondent's social network, including death or severe illness of the respondent's spouse, child, parent, co-twin, other sibling, other relative, or “other individual close to you” and serious trouble getting along with the respondent's parent, child, twin, sibling, in-laws, other relative, neighbor, or close friend. Because of the large sample size and limited time in the interview, we assessed neither the contextual aspects nor the putative independence of the life events (3). Interrater reliability for determining the occurrence and dating of our stressful life event categories was assessed for 53 jointly interviewed twins and found to be in the good to excellent range, with kappas equaling 0.93 and 0.82, respectively.
The diagnosis of major depression was made by computer algorithm incorporating the DSM-III-R criteria except for criterion B2 (which excluded depressive syndromes considered to be uncomplicated bereavement). For the 53 jointly interviewed twins, the 1-year prevalence of major depression was assessed with perfect agreement (kappa = 1.00). We assessed the reliability of our dating of depressive episodes of examining rater's agreement on the respondent's having had a depressive episodes in the 689 (53 × 13) assessed months. Reliability was excellent (kappa = 0.97). The details of our statistical analyses are outlined in appendix 1.
RESULTS
Our total sample contained 53,215 person-months of exposure among 2,164 individuals. Four hundred ninety-two separate onsets of major depression were recorded, for an uncorrected monthly risk of 0.92%. These 492 onsets occurred in 312 individuals (or 14% of the entire sample of 2,164). Of those reporting at least one episode of major depression over the period of ascertainment, 189 (61%) had a single episode, 78 (25%) had two episodes, and 45 (14%) had three or more episodes.
Stressful life events
Personal.
Table 1 lists the number of occurrences of each of the nine assessed personal stressful life events and the odds ratio for the onset of major depression in the month in which the event occurred and 1 and 2 months afterward. Serious legal problems were too rare to obtain stable estimates.
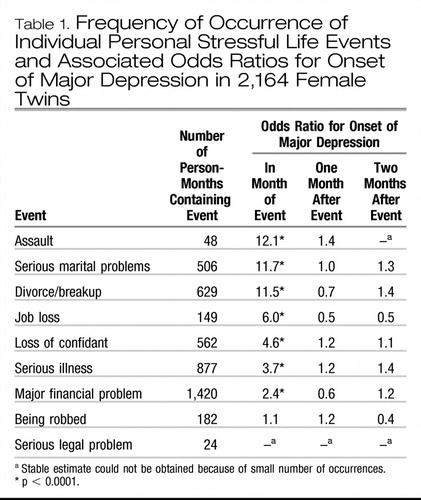 |
Table 1. Frequency of Occurrence of Individual Personal Stressful Life Events and Associated Odds Ratios for Onset of Major Depression in 2,164 Female Twins
The odds ratio for major depression was significantly increased in the month of occurrence of seven of the remaining eight life events; the exception was being robbed. The odds ratios ranged from 2.4 for major financial problems to 12.1 for assault. None of these personal stressful life events significantly increased the risk of major depression 1 or 2 months after their occurrence. Similar results were found for 3 months after occurrence.
Network.
The frequency of individual network stressful life events varied widely (Table 2). While death of a spouse, co-twin, child, or other sibling was very rare, serious illness in individuals in the respondent's social network and serious trouble getting along with them were considerably more common. The impact of network events on the odds ratios for major depression in the month of the events' occurrence is also shown in Table 2. For death of major network figures, only in the case of parents, other relatives, and “other individuals close to you” were there enough events to obtain a stable estimate. The odds ratio for major depression upon the death of a parent (21.3) was the highest noted for any stressful life event. A serious illness of a parent, co-twin, child, other sibling, other relative, or “other individual close to you” significantly but modestly increased the odds ratio for major depression in the month of its occurrence. Trouble getting along with others significantly increased the odds ratio for major depression in the month of its occurrence in all categories of the social network except other sibling. For the seven most common individual network events, where we could obtain stable estimates, we examined the odds ratios for major depression 1–3 months after their occurrence. Only three of these 21 individual analyses yielded significant results (none below p = 0.02), with no evident pattern.
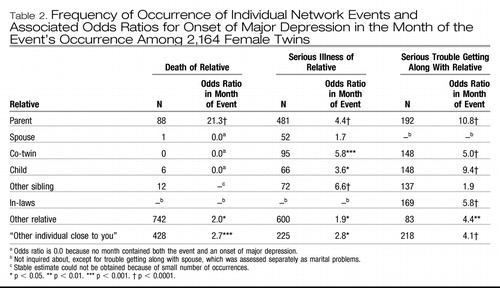 |
Table 2. Frequency of Occurrence of Individual Network Events and Associated Odds Ratios for Onset of Major Depression in the Month of the Event's Occurrence Among 2,164 Female Twins
To produce more stable estimates and reduce the number of categories for subsequent analyses, we created the categories death of, serious illness of, and serious trouble getting along with a close relative. For death and serious illness, a close relative was defined as a parent, spouse, child, co-twin, or other sibling. For trouble getting along, a close relative was defined as a parent, child, co-twin, or other sibling.
Table 3 shows the results for these combined network event categories, all three of which strongly predicted the risk of major depression in the month of their occurrence. The odds ratio for major depression within a month of the death of a close relative (20.5) was higher than that found for illness of or trouble getting along with a close relative. With the exception of serious illness in a close relative, these stressful life events did not increase the risk of major depression 1, 2, or 3 months after their occurrence.
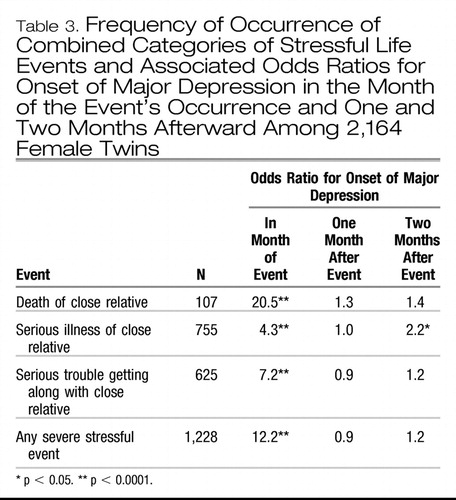 |
Table 3. Frequency of Occurrence of Combined Categories of Stressful Life Events and Associated Odds Ratios for Onset of Major Depression in the Month of the Event's Occurrence and One and Two Months Afterward Among 2,164 Female Twins
Severe.
We created a post hoc category of severe stressful life events that included the four events that produced odds ratios for major depression in excess of 10: death of a close relative, assault, serious marital problems, and divorce/breakup. A total of 1,228 person-months contained a severe stressful life event, and the odds for onset of a depressive episode in the month in which the event occurred were increased 12.2-fold (Table 3). No significant increased risk of major depression 1–3 months after the severe stressful event was found.
Genetic risk factors
Subsequent analyses were restricted to members of the 1,030 twin pairs of known zygosity both members of which were assessed at the first personal interview. This subsample contained 51,268 person-months at risk and 476 onsets of major depression. By logistic regression, controlling for age and zygosity, we found that the rates of onset of major depression and severe life events were similar in the twins included in this subsample and those excluded from it (χ2 = 0.60, df = 1, n.s., and χ2 = 1.66, df = 1, n.s., respectively).
The impact of genetic factors on onset of an episode of major depression in this sample was assessed using a single variable termed “genetic risk” (appendix 1). Using all control variables, we found that this index highly significantly predicted onset of an episode of major depression (χ2 = 65.85, df = 1, p < 0.001). If we assign the lowest risk group (monozygotic twin with co-twin unaffected) a risk of 1.0, then the odds ratio for major depression in any given month is 1.3 when there is an unaffected dizygotic co-twin, 2.0 when there is an affected dizygotic co-twin, and 2.6 when there is an affected monozygotic co-twin.
Stressful life events and genetic risk factors
Table 4 presents results of the analyses that examined jointly the main effects of environmental risk factors (stressful life events), genetic risk factors, and their interaction in the prediction of onset of major depression in the month of the stressful life events. We examined the individual personal stressful life events that had previously been shown to be significant predictors of major depression, the three aggregate network events, and the combined category of any severe stressful life event. In all of these analyses, both the main effect of stressful life events and the main effect of genetic risk factors on the onset of major depression were highly significant. However, in these 11 separate analyses, the interaction term was significant only once, and in seven of them, the chi-square value of the interaction term was <1.0. Furthermore, in the category of any severe stressful life event, despite an extremely high level of statistical significance for the main effect of stressful events (environmental factors), the interaction term between stressful life events and genetic risk factors was nonsignificant.
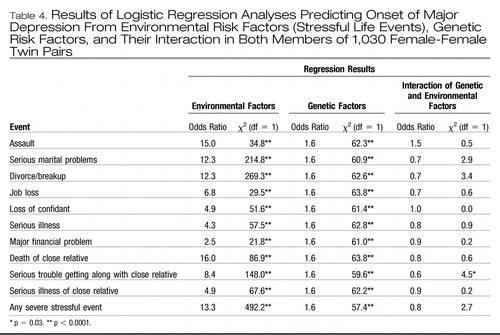 |
Table 4. Results of Logistic Regression Analyses Predicting Onset of Major Depression From Environmental Risk Factors (Stressful Life Events), Genetic Risk Factors, and Their Interaction in Both Members of 1,030 Female-Female Twin Pairs
We then calculated the risk of illness in the eight relevant categories of twins (four levels of genetic risk with and without exposure to the stressful life event) predicted from the best-fitting model for the various categories of stressful life events. Except for “serious trouble getting along with a close relative,” all of these models included only the main effects of stressful life events and genetic risk factors.
Figure 1 shows the results of these calculations for the category of severe stressful life events. While differing in quantitative terms, the figures looked similar for the individual stressful life events. Three major conclusions can be reached from these results. First, even in the presence of high genetic risk and severe stressful life events, the majority of individuals do not develop an episode of major depression. Second, genetic factors are important in influencing the risk of major depression both in the presence and in the absence of stressful life events. Put another way, environmental risk factors influence the risk of major depression in individuals at both low and high genetic risk. Third, the impact of environmental factors on the risk of major depression is substantially greater for individuals at high genetic risk than those at low genetic risk. For example, in those at lowest genetic risk (monozygotic twins whose cotwins are unaffected), the risk of onset of an episode of major depression in a month without a severe stressful life event is estimated at 0.5%. In the month of a severe stressful life event, the risk of onset of major depression in these individuals is predicted to be 6.2%. That is, in individuals at low genetic risk, the predicted increase in risk of a depressive episode associated with a severe stressful life event is 5.7%. However, for those at highest genetic risk (monozygotic twins whose co-twins are affected), the risk of onset of an episode of major depression in the absence and in the presence of a severe stressful life event is predicted to be 1.1% and 14.6%, respectively. That is, for genetically predisposed individuals, exposure to a severe stressful life event is associated with a 13.5% increase in the risk of a depressive episode. As predicted by the model of genetic control of sensitivity to the environment, the increased risk of onset of major depression with exposure to a severe stressful life event is 2.4 times greater for those at highest genetic risk than for those at lowest genetic risk.

a Genetic liability is reflected by both the zygosity of the twin and the lifetime history of major depression in her co-twin. The results presented here are those predicted by the best-fitting logistic regression equation that contains the control variables outlined in the text and the main effects of the life event and genetic risk factors. A severe life event is defined as assault, serious marital problems, divorce/breakup, or death of a close relative.
Using logistic regression, we found no evidence of an interaction between stressful life events and genetic factors in predicting the onset of an episode of major depression. However, what is additive on a logistic scale should be approximately multiplicative on the scale of probabilities (appendix 1 includes a brief discussion of scales of measurement). To test this hypothesis, we conducted a discrete-time survival linear regression predicting onset of major depression. Consistent with the results of the logistic regression, both severe stressful life events (beta = 0.095, t = 12.47, df = 48117, p < 0.0001) and genetic risk factors (beta = 0.004, t = 6.88, df = 48117, p < 0.0001) had highly significant main effects. However, in contrast to the results with logistic regression, the interaction term was both positive and statistically significant (beta = 0.020, t = 2.32, df = 48115, p = 0.02). On the scale of probabilities, as predicted by the model of genetic control of sensitivity to the environment, the increased risk of onset of major depression associated with exposure to a stressful life event was significantly greater for those at high genetic risk than for those at low genetic risk for major depression.
DISCUSSION
We sought to clarify in this report the interaction between stressful life events and genetic predisposition in the etiology of major depression in a population-based sample of female twins. We discuss our major results in turn.
Stressful life events
In this sample, all personal and aggregate network stressful life events except being robbed were significantly related to the onset of episodes of major depression in the month of the events' occurrence. The odds ratio for major depression given these events ranged from 2.4 to 20.5. For our category of severe events, the odds ratio was 12.2. The magnitude of the effect of stressful life events on onset of major depression in our data is toward the upper range of that found by previous researchers (3, 16–19), most of whom examined the impact of stressful life events over longer time periods (e.g., 3–6 months).
We consistently found that the odds ratio for major depression was increased only in the month of occurence of the stressful life event. In their influential report, Brown and Harris (3) noted a large increase in the rate of severe stressful life events in the first 3 weeks prior to onset of major depression, with much smaller increases seen in the second and third 3-week periods prior to onset. They concluded that “severe events usually lead fairly quickly to depression-most often within nine weeks.” Other researchers have also noted that the depression-inducing impact of stressful life events tends to occur quickly (18, 20).
There have been several previous attempts to describe the class of stressful life events that is potent in precipitating major depression, with descriptions containing terms such as “loss,” “exit,” “assault on self-esteem,” and “long-term contextual threat” (3, 4, 21–23). Three of the six stressful life events with an odds ratio of >5 for onset of depression were directly related to “loss”: death of a close relative, divorce/breakup, and job loss. However, the other three events (assault, serious marital problems, and serious trouble getting along with a close relative) are probably more accurately seen as threatening and/or reflecting loss of self-esteem and support.
Another major issue in life event research has been the characterization of stressful life events as putatively independent versus dependent on the respondent's behavior (3, 4). We did not assess the independence/dependence of individual stressful life events. Of the six events most potent in precipitating major depression, only one (death of a close relative) is unambiguously independent. Depending on individual circumstances, two other event categories (assault and job loss) might be either independent or dependent. However, in most circumstances, the three remaining potent events (serious marital problems, divorce/breakup, and serious trouble getting along with a close relative) would be considered, at least in part, dependent on the respondent's own behavior. These results are consistent with previous findings that most depression-inducing stressful life events are in part dependent on the individual's behavior (4). Because the single stressful life event category that most strongly predicted the onset of major depression, death of a close relative, was clearly independent of the respondent's own behavior, our results suggest that the association between stressful life events and major depression cannot be entirely due to individuals precipitating their own stressful life events.
Genetic risk factors
Using discrete-time survival analysis, we found that genetic risk factors strongly predicted onset of an episode of major depression. The individuals at highest risk (monozygotic twins with affected co-twins) had a risk of depression that was 2.6 times greater than that of those with the lowest genetic risk (monozygotic twins with unaffected co-twins). These results are consistent with the results in most, but not all, of the previous twin and adoption literature (5), as well as with results obtained in this twin sample with the use of more traditional analyses of both lifetime (6) and 1-year (14) prevalence of major depression.
Interaction of genetic and environmental risk factors
Our major goal was to clarify how genetic and environmental (stressful life events) risk factors interact in the etiology of major depression. Our initial analyses, using logistic regression, found strong evidence for a main effect of stressful life events and a main effect for genetic risk factors in influencing onset of episodes of major depression, but no evidence for an interaction. Because logistic regression utilizes the logarithm of the probability of a depressive episode, the absence of an interaction indicates that the impact of genetic and environmental risk factors on major depression was multiplicative in nature. When we examined the predicted risks from the best-fitting logistic models, we found that the impact of stressful life events on the risk of onset of depression was much greater for individuals at high genetic risk that for those at low genetic risk. These results were consistent with the expectations of the model of genetic control of sensitivity to the environment and not with the additive model.
To test formally for the presence of an interaction on the scale of probabilities (rather than the log of probabilities), we used linear regression to examine the impact of severe stressful life events and genetic factors on onset of major depression. These analyses showed statistically significant evidence of positive interaction between genotype and environment. That is, the net increase in risk of onset of major depression associated with a severe stressful life event was significantly greater for individuals with a high genetic liability to major depression than for those with a low genetic liability. These results suggest that genetic factors influence the risk of major depression in part by influencing the susceptibility of individuals to the depressive effect of stressful life events (12).
However, in all of the regression analyses examining interactions, both genetic effects and stressful life events continued to have substantial and significant main effects. These results suggest that 1) even in the absence of the assessed stressful life events, the risk of onset of depression was influenced by genetic factors, and 2) even in the absence of high genetic risk, stressful life events continued to be significant predictors of onset of major depression.
Previous attempts to rigorously examine the interrelation of genetic and environmental factors in the etiology of psychiatric illness have been rare and restricted to adoption studies of alcoholism (24, 25), antisocial personality (26, 27), and schizophrenia (28). All of these studies examined the interaction between genetic risk factors and the rearing environment in the adoptive home. To our knowledge, no previous study has examined the interrelation between genetic factors and environmental risk factors that occur close to the onset of an episode of psychiatric illness in adulthood.
We are aware of five studies that have examined the relation between family history of major depression and exposure to stressful life events in governing the risk of major depression or depressive symptoms. Of these studies, three (7, 8, 10) found results broadly consistent with those reported here—that individuals with a familial “loading” for major depression are more sensitive to the depression-inducing effects of stressful events—while two (9, 11) did not.
Issues of scale
All statistical interactions are scale dependent. In this study, with the use of a logistic scale, no statistical interaction was found between stressful life events and genetic predisposition in the prediction of onset of major depression. However, when these results are examined on the scale of true probability, a substantial interaction is evident. Which scale is correct?
Unfortunately, no definitive answer to this question is available. In the biological and social sciences, there is rarely strong a priori evidence for the validity of one scale of measurement over another. Some persons have argued that a logarithmic scale is more appropriate and statistically convenient (29). We choose to emphasize the scale of true probability for three reasons. First, most clinicians think in terms of probabilities and not logarithms of probabilities. Second, Rothman (13) argued persuasively that causal assumptions about the independence of the effect of multiple risk factors can only be rigorously evaluated by using the scale of true probabilities. Biological effects are, he argued, more likely to increase the probability of onset rather than the odds of onset. Third, public health officials implicitly use the scale of probabilities in their efforts to identify individuals at increased risk for a disorder because of exposure to a given risk factor. Only by using this scale is it possible to identify individuals who, when exposed to a given environmental agent, have a particularly large absolute increase in risk of illness.
Implications and limitations
Etiologic models for psychiatric illness in which genetic and environmental risk factors act additively are easy to understand and to analyze statistically. However, the results of this and other studies (24–27) suggest that despite their intuitive appeal, such models are unlikely to be correct. While our findings complicate the task of understanding the etiology of mental illness, they are exciting because they force us to confront the dynamic nature of our genetic endowment. Gene expression as it influences risk of psychiatric illness is not static but, rather, reacts to and interacts with environmental experiences.
The results of this study should be interpreted in the context of six potentially significant methodologic limitations. First, the analyses assume that observations in twin pairs are independent. This is not precisely true. However, the tetrachoric correlation (0.17) in all twin pairs for onset of major depression in a given month is so small that correcting for it (30) would produce no substantial change in the findings. Second, our logistic regression discrete-time survival analysis modeled genetic effects in a way that is less elegant and immediately interpretable than it would be with structural equations (31). However, under most realistic conditions, this approach produces little distortion in the estimation of genetic effects (32).
Third, the discrete-time survival model, as well as all continuous-time survival models, treats each individual's history as if it were a set of independent observations (33). Violations of this assumption will result in standard error estimates that are biased downward, while parameter estimation is not affected. However, we included control variables in our model that indexed the prior occurrence of stressful life events and episodes of major depression during the period of observation. Furthermore, only a small proportion of the twins with major depression in our sample reported multiple episodes over the period of ascertainment. It is therefore unlikely that this problem produced substantial distortions in our pattern of results.
Fourth, time was too limited in our interviews to assess contextual information for each individual life event that would have allowed judgments of severity or “degree of threat” (3). Therefore, the life events analyzed in each category are probably heterogeneous with respect to contextual severity. Such heterogeneity would more likely attenuate than exaggerate effects.
Fifth, since both stressful life events and onsets of major depression were assessed to the nearest month, we assumed that when both occurred in the same month, the stressful life event preceded and influenced the onset of major depression. We tested the validity of this key assumption in two ways. First, in a section of the interview separate from that which evaluated stressful life events, respondents with a depressive syndrome in the past year were asked, “Did something happen to make you feel that way or did the feeling just come on you ‘out of the blue’?” There wee 96 twins who reported a severe stressful life event and onset of depression occurring in the same month and who answered this question in sufficient detail. In 81 (84%) of these cases, the twins answered this question with the same life event they had previously reported as occurring in the same month and which we had scored as “severe.” In 11 cases (11%), two or more stressful life events had occurred together in a single causal sequence in 1 month, and one was considered by us to be “severe” while the twin listed another as “causing” the major depression. In only four cases (4%), did the twin state that her major depression was “caused” by something other than the severe stressful life event that occurred in the same month as the onset of the depression. Validating the assumptions of our analysis, our results suggest that in 96% of cases, when a severe stressful life event and onset of depression occurred in the same month, the respondent considered the stressful life event to precede and either directly or indirectly contribute to the onset of major depression.
We then examined whether onset of depression significantly predicted stressful life events in the subsequent month. Of the 11 categories of stressful life events examined in table 4 (including the aggregate measure of any severe stressful life event), we could obtain stable estimates for nine (no onset of serious marital problems or death of a close relative occurred in the month following onset of a depressive episode). In none of these analyses did the onset of major depression significantly predict the occurrence of stressful life events in the succeeding month. These findings further support the hypothesis that the tendency for onset of major depression and stressful life events to co-occur in the same month is due mostly to stressful life events predisposing to onset of depression.
Sixth, in this sample, both the risk of major depression (6, 14) and the probability of experiencing certain stressful life events (34) were influenced by genetic factors. We have not considered here whether these genetic factors are correlated whether genes that increase the risk of major depression might also predispose individuals to stressful life events. In a standard linear regression, this phenomenon, termed “genotype-environment correlation” or “genetic control of exposure to the environment” (12), would not influence estimates of genotype-environment interaction. We further addressed this issue by assessing, in two different ways, the strength of the possible genetic correlation between major depression and stressful life events. First, adding to our model the co-twin's history of stressful life events in the past 2 years neither significantly predicted onset of major depression nor changed any of the other parameter estimates. Second, we examined how much of the impact of stressful life events on major depression might be noncausal and mediated through common genetic influences. Examining severe stressful life events, we found that the odds ratio in predicting onset of depression declined 5% when the co-twin's history of major depression was added to the model. These analyses suggest that if any genetic correlation exists between major depression and stressful life events, it is modest and explains very little of the impact of stressful life events on onset of major depression. Our assessment of genotype-environment interaction in this sample was, therefore, unlikely to be substantially biased by a correlation between genetic risk factors for major depression and stressful life events.
APPENDIX 1. STATISTICAL ANALYSIS
We conducted an event history analysis using a discrete-time approach (35, 36), examining each “person-month” of observation. This usually meant 26 months of observation for individuals who were interviewed initially and at follow-up, and 13 for those not successfully interviewed at follow-up. Each person-month record contained information about whether an episode of major depression started in that month, whether a given stressful life event occurred in that month, or whether that stressful life event occurred in the previous 3 months. Each observation record also showed the relevant covariates, which included age, previous history of that stressful life event or major depression within the time frame of the study, and the year of the study (first versus second). The hazard rate for major depression for that month (as measured from the time of the interview) was also included as a control variable, since episodes of major depression were not uniformly distributed over the period of study. When a twin experienced an episode of major depression, she was censored from the sample until she was again at risk, having recorded an end to that depressive episode. She then re-entered the sample with the variable “previous history of major depression” updated. We had, in our sample, a number of women who reported chronic depressions that began prior to our period of ascertainment. These individuals were not considered to have an onset of major depression in the first study month and were censored from the sample for the entire period they reported meeting criteria for major depression. Our discrete-time survival model assumed that if the same individual experienced multiple onsets of major depression within the study period, the same underlying processes operated in all onsets. Analyses that included history of major depression prior to the observation period as a covariate were also completed. Little change in results was found.
These analyses treat each individual person-month as an independent observation. The risk of having an onset of major depression is modeled as the dependent variable in a logistic regression. A weighted sum of the independent variables is transformed to a predicted risk (or probability of onset) by a logistic function. The effects of independent variables are therefore linear on the logistic scale but usually nonlinear on the scale of probability. Allison (35) has shown that under the assumption of independence of the individual observations, such a model produces the true maximum-likelihood estimators and artifactually inflates neither the sample size nor the test statistic.
Previous analyses in this sample suggested that the familial aggregation of both lifetime and 1-year prevalence of major depression was due to additive genetic factors (6, 14). Given these results, all twins could be conveniently assigned to one of four categories of increasing “genetic risk” of major depression on the basis of their co-twins' lifetime history of major depression: 1) monozygotic twin and co-twin unaffected, 2) dizygotic twin and co-twin unaffected, 3) dizygotic twin and co-twin affected, and 4) monozygotic twin and co-twin affected. For the present analysis, we assume that the genetic effects in these four groups are proportional to −1.0, −0.5, 0.5, and 1.0, respectively. The magnitude of the estimated regression coefficient therefore reflects the importance of additive genetic effects (37, 38).
This parsimonious model has potential limitations. First, there is no simple relation between the estimated logistic regression coefficient for genetic effects and heritability as determined by the liability-threshold model (39). However, there is a surprising degree of correspondence between the two over the range of parameters utilized in this study (32).
Second, this model does not contain measures of family environment or genetic dominance. In fitting a full twin regression model to our data (38), which include such effects, modest evidence was found for genetic dominance but not for family environment. However, we chose not to include dominance in our final models for three reasons. First, previous analyses indicate that in studies restricted to twins, additive and dominance variance are highly confounded (31). Second, inclusion of dominance would considerably complicate the analyses, requiring the estimation of two separate genotype-environment interactions. Third, the exclusion of genetic dominance leads to a conservative estimate of the impact of genetic effects.
The odds ratio for major depression given stressful life events and a high genetic risk of illness is calculated from the logistic regression coefficient, controlling for all of the covariates outlined above.
Interactions and scale
In these analyses the dependent variable, the presence or absence of onset of major depression in a given person-month, is dichotomous. This fact introduces unavoidable practical and conceptual complexity into our analyses because 1) we are primarily interested in clarifying the nature of the interaction between genetic and environmental risk factors and 2) from a statistical perspective, any interaction is scale dependent. In regression analysis, dichotomous dependent variables have two unattractive properties. First, true probabilities that would be used to predict such variables have floor and ceiling effects (probabilities of illness must range from 0 to 1). Second, such probabilities would not be homoscedastic (have equal variance at any point on the scale). Therefore, the most popular regression method for the analysis of dichotomous dependent variables is now logistic regression (40). In logistic regression, instead of the probability of a given outcome p, the model predicts the logistic function of p, which equals ln p/(1 − p), where ln equals the natural logarithm. The logistic function has a number of important advantages, including homoscedasticity and a range from −∞ to ∞. However, it has one important disadvantage. With the inclusion of a logarithmic function in the dependent variable, the nature of what an interaction means has been dramatically changed. What is a multiplicative interaction in the probability model becomes (to a first approximation) simply additive in the logistic model. What is additive in the probability model usually becomes a negative interaction in the logistic model.
Because of the ease and wide acceptability of logistic regression, most of our analyses used this method. However, we recognized a priori that evidence for an additive model in logistic regression is, in fact, usually evidence for a multiplicative interaction on the scale of simple probabilities. To confirm this, we implemented a cumbersome regression method on the scale of simple probabilities using standard linear regression (40). To correct for unequal error variances due to the dichotomous dependent variable, iterated weighted regressions were carried out (41, 42). For each iteration, each observation was weighted by the inverse of the binomial variance of the observation plus a small adjustment factor that allowed observations with a predicted value of 0 or 1 to be included in the next iteration. Observations with a predicted value of <0 or >1 were excluded from subsequent analyses, resulting in a loss of power. The variance was calculated with the use of the predicted value of the previous regression. Iteration was continued until improvement in heteroscedasticity was no longer obtained.
1 Burton R: The Anatomy of Melancholy, vol 1 ( 1621). New York, EP Dutton, 1932Google Scholar
2 Bonhoeffer K: How far should all psychogenic illnesses be regarded as hysterical? (1909), in Themes and Variations in European Psychiatry: An Anthology. Edited by Hirsch SR, Shepherd M. Charlottesville, University Press of Virginia, 1974Google Scholar
3 Brown GW, Harris T: Social Origins of Depression: A Study of Psychiatric Disorder in Women. New York, Free Press, 1978Google Scholar
4 Thoits PA: Dimensions of life events that influence psychological distress: an evaluation and synthesis of the literature, in Psychosocial Stress: Trends in Theory and Research. Edited by Kaplan HB. New York, Academic Press, 1983Google Scholar
5 Tsuang MT, Faraone SV: The Genetics of Mood Disorders. Baltimore, Johns Hopkins University Press, 1990Google Scholar
6 Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: A population-based twin study of major depression in women: the impact of varying definitions of illness. Arch Gen Psychiatry 1992; 49: 257– 266Crossref, Google Scholar
7 Strecker EA: A preliminary study of the precipitating situation in two hundred cases of mental disease. Am J Psychiatry 1922; 78: 503– 536Crossref, Google Scholar
8 Pollitt J: The relationship between genetic and precipitating factors in depressive illness. Br J Psychiatry 1972; 121: 67– 70Crossref, Google Scholar
9 Perris H, von Knorring L, Perris C: Genetic vulnerability for depression and life events. Neuropsychobiology 1982; 8: 241– 247Crossref, Google Scholar
10 Phelan J, Schwartz JE, Bromet EJ, Dew MA, Parkinson DK, Schulberg HC, Dunn LO, Blane H, Curtis EC: Work stress, family stress and depression in professional and managerial employees. Psychol Med 1991; 21: 999– 1012Crossref, Google Scholar
11 McGuffin P, Katz R, Bebbington P: The Camberwell Collaborative Depression Study, III: depression and adversity in the relatives of depressed probands. Br J Psychiatry 1988; 152: 775– 782Crossref, Google Scholar
12 Kendler KS, Eaves LJ: Models for the joint effect of genotype and environment on liability to psychiatric illness. Am J Psychiatry 1986; 143: 279– 289Crossref, Google Scholar
13 Rothman KJ: Modern Epidemiology. Boston, Little, Brown, 1986Google Scholar
14 Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: A longitudinal twin study of 1-year prevalence of major depression in women. Arch Gen Psychiatry 1993; 50: 843– 852Crossref, Google Scholar
15 Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ: The prediction of major depression in women: toward an integrated etiologic model. Am J Psychiatry 1993; 150: 1139– 1148Crossref, Google Scholar
16 Paykel ES: Contribution of life events to causation of psychiatric illness. Psychol Med 1978; 8: 245– 253Crossref, Google Scholar
17 Costello CG: Social factors associated with depression: a retrospective community study. Psychol Med 1982; 12: 329– 339Crossref, Google Scholar
18 Surtees PG, Miller PM, Ingham JG, Kreitman NB, Rennie D, Sashidharan SP: Life events and the onset of affective disorder: a longitudinal general population study. J Affect Disord 1986; 10: 37– 50.Crossref, Google Scholar
19 Bebbington PE, Sturt E, Tennant C, Hurry J: Misfortune and resilience: a community study of women. Psychol Med 1984; 14: 347– 363Crossref, Google Scholar
20 Bebbington PE, Tennant C, Hurry J: Adversity and the nature of psychiatric disorder in the community. J Affect Disord 1981; 3: 345– 366Crossref, Google Scholar
21 Finlay-Jones R, Brown GW: Types of stressful life events and the onset of anxiety and depressive disorders. Psychol Med 1981; 11: 803– 815Crossref, Google Scholar
22 Gilbert P: Depression: The Evolution of Powerlessness. New York, Guilford Press, 1992Google Scholar
23 Brown GW, Harris TO: Depression, in Life Events and Illness. Edited by Brown GW, Harris TO. New York, Guilford Press, 1989Google Scholar
24 Bohman M, Sigvardsson S, Cloninger CR: Maternal inheritance of alcohol abuse: cross-fostering analysis of adopted women. Arch Gen Psychiatry 1981; 38: 965– 969Crossref, Google Scholar
25 Cloninger CR, Bohman M, Sigvardsson S: Inheritance of alcohol abuse: cross-fostering analysis of adopted men. Arch Gen Psychiatry 1981; 38: 861– 868Crossref, Google Scholar
26 Cadoret RJ, Troughton E, O'Gorman TW: Genetic and environmental factors in alcohol abuse and antisocial personality. J Stud Alcohol 1987; 48: 1– 8.Crossref, Google Scholar
27 Cadoret RJ, Cain CA, Crowe RR: Evidence for gene-environment interaction in the development of adolescent antisocial behavior. Behav Genet 1983; 13: 301– 310Crossref, Google Scholar
28 Tienari P: Interaction between genetic vulnerability and family environment: the Finnish Adoptive Family Study of Schizophrenia. Acta Psychiatr Scand 1991; 84: 460– 465Crossref, Google Scholar
29 Everitt BS, Smith AMR: Interactions in contingency tables: a brief discussion of alternative definitions. Psychol Med 1979; 9: 581– 583Crossref, Google Scholar
30 Kish L, Frankel MR: Inferences from complex samples. J Royal Statistical Society Series B 1974; 36: 1– 37Google Scholar
31 Neale MC, Cardon LR: Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands, Kluwer Academic, 1992Google Scholar
32 Sham PC, Walters EE, Neale MC, Heath AC, MacLean CJ, Kendler KS: Logistic regression analysis of twin data: estimation of parameters of the multifactorial liability-threshold model. Behav Genet 1994; 24: 229– 238Crossref, Google Scholar
33 Allison PD: Event History Analysis. Beverly Hills, Calif, Sage, 1984Google Scholar
34 Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: A twin study of recent life events and difficulties. Arch Gen Psychiatry 1993; 50: 589– 596Google Scholar
35 Allison PD: Discrete-time methods for the analysis of event histories, in Sociological Methodology. Edited by Leinhardt S. San Francisco, Jossey-Bass, 1982Google Scholar
36 Laird N, Olivier D: Covariance analysis of censored survival data using log-linear analysis techniques. J Am Statistical Assoc 1981; 76: 231– 240Crossref, Google Scholar
37 Falconer DS: Introduction to Quantitative Genetics, 3rd ed. New York, John Wiley & Sons, 1989Google Scholar
38 DeFries JC, Fulker DW: Multiple regression analysis of twin data. Behav Genet 1985; 15: 467– 474Crossref, Google Scholar
39 Falconer DS: The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet 1965; 29: 51– 76Crossref, Google Scholar
40 SAS/STAT User's Guide, version 6, 4th ed, vols 1, 2. Cary, NC, SAS Institute, 1990Google Scholar
41 Cox DR: The Analysis of Binary Data. London, Methuen, 1970Google Scholar
42 Fleiss J: Statistical Methods for Rates and Proportions, 2nd ed. New York, John Wiley & Sons, 1981Google Scholar



