Psycho-Oncology: A Review for the General Psychiatrist
Abstract
This article provides an overview of psycho-oncology, including epidemiology of common psychiatric conditions in cancer, effects of cancer and chemotherapy on the brain, and effects of coping styles and other psychosocial factors on cancer treatment. It describes the assessment, differential diagnosis, and treatment of adjustment disorders, anxiety, depression, delirium, chemotherapy- and radiotherapy-induced cognitive dysfunction, character disorders, substance disorders, and major mental illness in oncology patients. Survivorship and bereavement are addressed, as are future directions for this growing field.
DEFINITION
Psycho-oncology is a multidisciplinary subspecialty of oncology concerned with the emotional responses of patients, their families, and staff at all stages of disease. It also examines the psychological, social, and behavioral variables that influence cancer risk, prevention, and survival (1).
The formal history of psycho-oncology began in the mid-1970s in the United States. Only at this juncture had the stigma of cancer diminished to the point that most patients were told their diagnosis, allowing the study of their emotional responses. Social workers and nurses initially addressed the psychosocial problems of patients with cancer and their families, with early research in the field conducted mainly by consultation liaison psychiatrists. Subsequent investigators included health psychologists (studying theoretic models of coping and cognitive behavior interventions), behavioral psychologists (studying lifestyle changes such as smoking cessation and dietary modification to reduce cancer risk), and nursing researchers (studying symptom management and control, pain management, and psychological support). The field is notably multidisciplinary (2), although our review will highlight aspects of particular relevance to the practicing psychiatrist.
EPIDEMIOLOGY AND NATURAL HISTORY
Depression, anxiety
As one might expect with any severe illness, cancer tends to increase the prevalence of certain psychiatric symptoms and syndromes above baseline population levels. By far the most prevalent conditions are those that are also prevalent among nononcologic populations: depression and anxiety disorders. However, clinicians appear to underrecognize the severity of psychiatric illness in this population. One large study of depression found a concordance of only 13% between oncologist and patient scales in those with severe symptoms; nurses had no greater concordance (3, 4).
In advanced cancer, prevalence estimates of major mental illness vary widely, depending on the criteria used; use of small samples or non-DSM instruments can result in rates up to five times those found using more rigorous criteria. One study with strict criteria found that approximately 12% of patients met the criteria for at least one major mental illness. Rates of depression were 6.8%, panic disorder 4.8%, and generalized anxiety disorder 3.2% (5). Caregivers were equally affected; panic disorder was the most common (8%), followed by major depressive disorder (4.5%), posttraumatic stress disorder (PTSD) (4%) and generalized anxiety disorder (3.5%). If adjustment disorders are included, the prevalence of mental illness may be as high as 50% of all patients with advanced cancer, with high levels of comorbidity (6–8). Subsyndromal disorders are quite prevalent, with high rates of significant anxiety symptoms, especially posttraumatic ones (9).
The prevalence of depression in cancer has possibly decreased in recent years, perhaps due to changes in outcomes, stigma, palliative care, and screening with treatment (10). The validity and prevalence of relevant constructs such as demoralization (11) and conservation-withdrawal (12), which may be mistaken for or overlap with depression, are contested.
Progressive disease may not cause substantially greater rates of illness than less symptomatic disease (7, 13). However, this finding has been disputed by other researchers who found that metastasis increases risk of depression (14, 15). Its effect on severity of symptoms is unclear: nearing death, existential distress increases with increased physical symptom burden, not with closeness to death (16). In terminal phases, some authors report that rates of depressive disorders may actually decrease and anxiety disorders may increase by only 1%–2% absolute prevalence (approximately a 20% relative increase) (7). Patients who are younger or have less social support have a greater risk (8).
Delirium
Delirium is a frequent complication in patients with advanced cancer, occurring in 28%–44% of hospital admissions and in 90% of patients before death (17). In the palliative care setting, hypoactive delirium is most prevalent, constituting up to 86% of delirium, and confers a worse prognosis (18). Hyperactive delirium occurs in 13%–46% of palliative care patients (19). A reversible etiology is discovered in less than 50% of patients with advanced cancer with delirium. The 1-month mortality rate may be up to 83% in this patient population (20).
Fatigue
More than 80% of outpatients undergoing chemotherapy or radiation treatment experience fatigue, which is also associated with stem cell or bone marrow transplantation and hormonal therapy. In up to 33% of patients, fatigue can persist for months or years after the conclusion of cancer treatment (21).
Bipolar disorder
Bipolar disorder has a prevalence of 0.4%–1.6% in the general population, which should be similar for cancer patients. Antidepressants, corticosteroids, stimulants, and interferon can all induce or exacerbate mania in patients with bipolar disorder (7).
Character disorders
Narcissistic, histrionic, and borderline personality disorders tend to cause the most problems in medical settings. The prevalence rate in patients with cancer is about the same as that in the general population, i.e., 2%–3% each for borderline and histrionic personality disorders and 1% for narcissistic personality disorder (6).
Schizophrenia
Prevalence rates of schizophrenia are 0.5%–1.5% worldwide. Despite some evidence that patients with schizophrenia have an increased overall cancer risk (16, 22), these rates should be similar in cancer patients. Any increased risk is probably attributable to specific lifestyle factors, particularly tobacco smoking and alcohol consumption.
Substance use disorders
Substance abuse diagnoses such as tobacco and alcohol dependence substantially increase the risk of lung and head/neck cancer. Prevalence rates of alcohol dependence among patients with cancer with pain may be elevated above baseline, perhaps as high as 28% (23). Alcoholism is associated with increased need for opioids (23, 24) and worse outcome in some cancers (25). The highest rates of alcohol dependence (33.6% in one study) are found in head/neck cancers (26).
Suicidality
Thoughts of suicide may be present in up to 17% of patients with cancer (27). Hopelessness is at least as strong a contributor as depression to suicidal ideation (28). Rasic et al. (29) found that among those aged 54 and younger, there was no increase in suicidal ideation with cancer, but for those older than 54, there was an odds ratio of 5.07 (which disappeared with adjustment for sociodemographic factors and mental disorders). The differential diagnosis of suicidal ideation in this population must always include encephalopathic conditions; the presence of hallucinations or delusions is a risk factor for suicide attempts (30).
Suicidality differs from a desire for hastened death. Ten to 30% of terminally ill persons with cancer report a desire for hastened death. This desire has two strong independent contributors, depression and hopelessness (31). An unknown but substantial frequency of patients with cancer demonstrate contingent suicidality, a situation in which patients link their suicidal intent to a future time point or particular life circumstance (32) (for example, severe pain or dyspnea as natural death approaches). Early reports suggest that a minority of family members of cancer patients who struggle with severe anticipatory grief are also at risk for contingent suicide upon the death of their loved one (33). Cancer is the only nonpsychiatric condition independently associated with completed suicide (odds ratio 2.3), and metastatic disease further raises the risk (34). Adjusted rates of suicide are 31.4/100,000 person-years among those with cancer, compared with 16.7 in the general population (35). Nonetheless, completed suicide is rare: only 0.03% of deaths in patients receiving palliative care and 0.2% of all deaths in patients with cancer are due to suicide (36).
Risk factors for suicide among those with cancer include male sex, white race, and unmarried status; lung cancer is associated with the highest rates, followed by stomach and oropharyngeal/laryngeal cancers (35). The risk is highest in the first 5 years after diagnosis, but remains elevated for at least 15 years (35). A mood disorder is present in 80% of completed suicides among those with cancer (37).
BIOPSYCHOSOCIAL UNDERPINNINGS
Biological effect of cancer and cancer treatments on psychiatric syndromes
Brain metastases.
During the course of their illness, 8%–10% of cancer patients will develop brain metastases. The frequency appears to be rising because of improved imaging modalities as well as longer survival after a cancer diagnosis due to improved treatment of systemic disease. The majority of brain metastases arise from one of three primary tumors: lung cancer (45%–50%), breast cancer (15%–25%), and melanoma (5%–20%). Of patients with brain metastases, 65% present with cognitive impairment. Other common presenting symptoms include headaches, seizures, neurologic deficits (38), and anxiety/depression (39).
Leptomeningeal carcinomatosis.
This complication is seen in 5%–8% of cancer patients, with the highest frequency in breast cancer, lung cancer, melanoma, leukemia, and lymphoma. It is usually a late occurrence in cancer and associated with active systemic disease. The clinical manifestations are protean, including headaches, nausea/vomiting, cranial nerve palsies, and spinal cord symptoms. Relevant to psychiatrists, somnolence and confusion may be the only presenting symptoms (40).
Chemotherapy.
Delirium may occasionally be observed as an adverse effect of several cytotoxic chemotherapies (41) (Table 1). Certain biological response modifiers can also cause acute neuropsychiatric toxicities (Table 2).
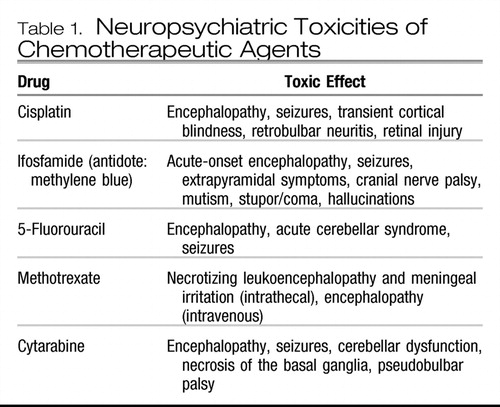 |
Table 1. Neuropsychiatric Toxicities of Chemotherapeutic Agents
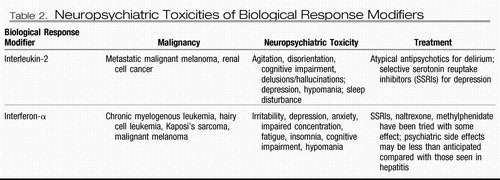 |
Table 2. Neuropsychiatric Toxicities of Biological Response Modifiers
During and after chemotherapy, some patients experience more insidious difficulties with memory, attention, and executive function, a phenomenon known commonly as “chemo brain.” Cognitive dysfunction has been reported in as many as 50% of women undergoing chemotherapy for breast cancer (42) and has also been observed in patients after stem cell transplantation (43) and after chemotherapy for other solid tumors (44). In a minority of patients, chemo brain is still perceptible and psychologically distressing 10 years after treatment (45).
Radiation therapy
Whole brain radiotherapy (WBRT) for metastatic disease offers tumor shrinkage and palliation, which has been associated in some studies with better preservation of neurocognitive functioning (46). However, WBRT itself is associated with cognitive impairment, specifically preferential adverse effects on hippocampal functions of learning, memory, and spatial information processing (47). Certain quality-of-life parameters, specifically fatigue, drowsiness, and appetite, may worsen after WBRT (39).
Paraneoplastic limbic encephalitis.
This rare autoimmune neuropsychiatric complication may either herald a malignancy or follow a cancer diagnosis. It presents with subacute memory loss and other limbic symptoms such as depression, seizures, hallucinations, and personality change (48). Typically associated malignancies are small cell lung cancer in approximately 40% of patients and seminoma in approximately 25% of patients, with nearly any other tumor associated at a lower rate (49). Clinical suspicion should prompt a lumbar puncture to rule out herpes simplex viral infection or leptomeningeal cancer spread and brain magnetic resonance imaging to rule out metastatic disease. If these studies are unrevealing, antineuronal antibodies (anti-Hu, anti-Ma, anti-Ta, and others) should be used as these are required for definitive diagnosis.
Effect of coping style/psychosocial factors on cancer
Mortality and survival.
How important are psychosocial factors to the mortality and survival time outcomes of cancer treatment? The evidence is strongest for ethnosocioeconomic factors, which affect treatment and outcome in many areas of health. African Americans have a lower 5-year survival rate for all cancers than whites (57% versus 68%) (50).
Depression may predict progression and mortality, although this theory is disputed (10, 51, 52). There is evidence that many psychological and social factors that appear to affect outcome are mediated by adherence to treatment (53, 54). For coping style, stress, and social support, constructs that are not widely validated or consistently used, evidence of an independent effect is mixed or inadequate (55, 56). Even for psychosocial interventions evidence of an effect is only weak or inconsistent apart from an effect on adherence (53, 54).
However, psychosocial interventions can be a key to increased adherence and improved outcomes: “meta-analyses show that adherence reduces the risk of a poor treatment outcome by 26% … and the odds of nonadherence is three times higher if comorbid depression exists.” (53) The PROSPECT trial showed that patients with major depression in primary care practices with depression care algorithms were less likely to die from cancer than those in other practices (this algorithm included citalopram with or without interpersonal therapy) (57).
Health-related quality of life at baseline does appear to be of some prognostic value. However, despite the clear effect of many psychosocial interventions on quality of life, they do not appear to affect survival (55, 58).
Other outcomes: distress and quality of life.
The effect on quality of life and other outcomes is more robust. Cancer diagnosis, treatment, and disease progression are potentially severely impairing. According to Block (30), “Psychological distress impairs the patient's capacity for pleasure, meaning, and connection; erodes quality of life; amplifies pain and other symptoms; reduces the patient's ability to do the emotional work of separating and saying good-bye; and causes anguish and worry in family members and friends.”
Defense mechanisms.
Less adaptive defense mechanisms such as denial and distortion are frequently brought to the forefront in discussions of cancer, based on the belief that facing death inevitably brings out primitive defenses. This belief has been challenged in recent years, as treatment advances have disentangled a cancer diagnosis from certain death, and the growing hospice and palliative care movement has enabled patients to discuss mortality and the death process openly and with some control.
However, denial is not infrequently present: a review of the literature found denial of diagnosis in 4%–47%, denial of impact in 8%–70%, and denial of affect in 18%–42% of patients with cancer (59). Denial itself is not a necessary precondition for hope, and the will to live is not necessarily reduced by the acknowledgment of mortality. Most patients with metastatic cancer say that they have both hope and the will to live, without denial or minimization (36, 60).
Idealization and devaluation of treatment team members is common; often idealized is the surgeon or oncologist in whose hands the patient imagines his or her life. Isolation of affect and intellectualization are commonly transitional defenses, especially in patients who have been wary of affect at baseline or who fear that their emotions might add to the burden on their loved ones. A surprising number of cancer patients and survivors prioritize altruism and humor, mature defenses that may assume greater prominence in their lives than before diagnosis.
Avery Weisman discussed two major features of patients' experience of cancer: coping and vulnerability. In his study, so-called “good copers” are self-fulfilling, in the sense that their key characteristic is the persistence of belief that they can cope; they are practical and address issues directly. “Bad copers” are characterized by self-pity and pessimism (61, 62). Vulnerability is divided into dysphoric and dispositional types, the former being based on patient reports of current experience and symptoms (resembling an axis I depression). Dispositional vulnerability instead reflects underlying character dynamics, including one's sense of the plight of being a cancer patient, and includes subdispositions of annihilation, alienation, endangerment, infringement, and denial (63).
Phases of illness.
Weisman (1979) also proposed a model of coping with cancer, taking into account the existential vulnerabilities associated with a diagnosis: annihilation, alienation, endangerment, and denial. These correspond, respectively, with emotional responses of anxiety, depression, anger, and inappropriate (elevated or dissociated) affect. He found four phases:
| I. | Existential Plight, involving impact of diagnosis, confrontation with mortality, and anxieties about the future. Denial is most common during this phase; hopes for cure are often present. | ||||
| II. | Accommodation and Mitigation, involving variable levels of psychosocial impairment, focus on the pragmatic aspects of treatment and side effects, and a sense of being changed but neither moribund nor well. Surveillance is the characteristic activity; denial slips into the background. Patients who are cured may not progress beyond this phase. | ||||
| III. | Relapse or Recurrence, in which existential concerns reawaken, a sense of optimism may be harder to come by, and denial is rare. A sense of control is often the patient's goal. | ||||
| IV. | Deterioration and Decline, involving significant impairment in function, a sense of limited time, and a variable attitude, but often with a letting-go of details and a focus on palliation (62). | ||||
ASSESSMENT AND DIFFERENTIAL DIAGNOSIS
General issues
Several aspects of the initial psycho-oncology evaluation distinguish it from an intake assessment in general adult psychiatry. Asking about patients' religious or spiritual preferences may tap into crucial internal strengths and sources of external support or reveal existential distress (64). Referral to a chaplain may be indicated (65). A longitudinal history of the individual's coping style is useful, particularly as it relates to prior medical illnesses. Much information can be gleaned from the patient's account of events just before the cancer, including themes such as anger over a missed or delayed diagnosis or unresolved grief over recent losses. Asking about family members, friends, or acquaintances with cancer may expose the patient's preconceived ideas or fears about the illness. Because cancer often involves a loss of physical and sexual vitality, patients may find themselves sensitized to previously buried memories of prior deaths or losses. It is also essential to inquire into how cancer has affected marital/romantic relationships and dependent children (66). Finally, including in the outpatient setting, a comprehensive Mini-Mental State Examination (MMSE) and some measure of executive function (e.g., the Clock Drawing Test) are useful to obtain at baseline, given the high incidence of cognitive dysfunction seen over time. Neurocognitive decline should prompt consultation with oncologists regarding a CNS workup.
Depression
Diagnosis of depression in oncologic settings is vital, but not straightforward. Several of the diagnostic criteria for major depressive disorder are confounded by symptoms resulting from cancer or its treatment, specifically low energy, poor appetite, and impaired concentration. In addition, several other constructs have been proposed to describe psychological symptoms of low mood, interest, or rumination; some authors differentiate grief, demoralization, hopelessness, or conservation-withdrawal from depressive disorders overall.
Demoralization, in particular, deserves clarification. As Kissane et al. have proposed, it includes the following features, which can be assessed with the Demoralization Scale (11, 68):
| •. | complaints of life's meaninglessness, pointlessness, or loss of purpose | ||||
| •. | sense of pessimism, helplessness, and stuckness in the predicament | ||||
| •. | loss of hope for improvement or recovery | ||||
| •. | associated isolation, alienation, or lack of support | ||||
| •. | potential to develop suicidal thoughts and plans | ||||
| •. | phenomena persisting over more than 2 weeks | ||||
In assessing major depression in this population, criteria of particular interest include anhedonia, early morning awakening, disability out of proportion to condition, and somatic preoccupation with intractable symptoms. The six-item Brief Edinburgh Depression Scale, initially developed for post-partum settings, may be useful as a screening tool for oncology practices (67). Even briefer, two questions (“Are you depressed?” and “Have you lost interest in things you usually enjoy?”) are reasonably accurate in excluding depression, although they are inadequate for confirming depression (69).
Suicide/requests for hastened death
Suicidal ideation and/or requests for physician-assisted suicide require careful psychiatric evaluation. Contributing psychodynamic factors may include a need for control, ambivalence (i.e., communicating desires to the physician may reflect an underlying wish for rescue), or a split in the experience of the self (in which the medically ill self is experienced as an intrusive entity that needs to be eradicated for the good self to survive). Other possibilities include suicide as a means of revenge against loved ones, atonement for guilt (especially if the cancer was initially attributed to bad deeds and unacceptable emotions), and actualizing a “felt experience” of already being dead (70).
Anxiety
The differential diagnosis of anxiety in patients with cancer is broad and includes biological, psychological, and spiritual factors. Categories of particular importance include DSM-IV anxiety spectrum disorders, other DSM-IV disorders of which anxiety is merely an associated symptom, medical conditions (especially pulmonary), and social/spiritual factors (Table 3).
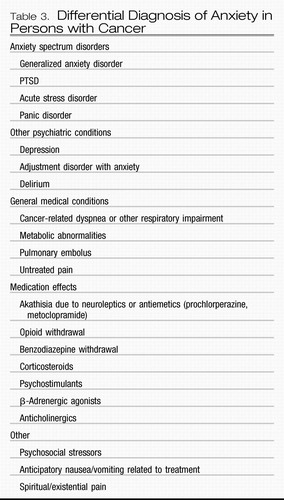 |
Table 3. Differential Diagnosis of Anxiety in Persons with Cancer
Delirium
Risk factors for development of delirium include advanced age, cognitive impairment, low albumin level, bone metastases, and the presence of hematologic malignancy (71). Delirium is reversible in approximately 50% of episodes, with the presence of psychoactive medications (especially opioids) and dehydration associated with reversibility (17). The Memorial Delirium Assessment Scale (MDAS) has been validated in the advanced cancer population and may supersede the MMSE based on its inclusion of both cognitive and neurobehavioral dimensions (72). Although the Clock Drawing Test is a good detector of cognitive impairment, it is not suitable for detection of delirium in elderly medical inpatients (73).
Of patients with cancer who recover from delirium, 54% may recall the symptoms, with a level of recollected distress most strongly predicted by prior delusions (73). In patients who have been in the intensive care unit (ICU), these delusional memories may be associated with higher anxiety and possibly a risk of PTSD after discharge (75). Notably, hypoactive delirium can be equally as distressing to patients as hyperactive delirium (74, 76). Caregivers who report observing delirium in patients may have higher distress and caregiver burden, especially given that they are frequently acting as surrogate decision makers in this setting (77, 78).
Substance use disorders
The differential diagnosis of problematic drug-taking behavior during cancer pain treatment includes pseudoaddiction (aberrant behaviors driven by unrelieved pain), self-medication of depression/anxiety or even of adjustment symptoms (such as boredom due to diminished ability to engage in usual activities), character pathology, and mild delirium (causing confusion about the proper doses) (79).
Fatigue
Contributing factors may include malnutrition, inactivity, sleep disorders, pain, emotional distress, noncancer comorbidities such as infection or hypothyroidism, and anemia (80). Fatigue has been rated as more troublesome and capable of having a more negative impact on quality of life than other cancer-related symptoms, including pain and nausea.
Chemotherapy-induced cognitive dysfunction
Possible mechanisms and confounding factors include direct cytotoxic effects on the nervous system, anemia, menopause, depression, and psychological coping style. Risk factors have not yet been well defined but may include the apolipoprotein E4 allele, advanced age, and dose and type of chemotherapy (with high-dose and cyclophosphamide, methotrexate, and fluorouracil regimens conferring higher risk). Many studies have had methodologic limitations including limited sample sizes, lack of a baseline cognitive assessment, and lack of control for potential confounding factors such as hormonal therapy (81).
TREATMENT AND OUTCOMES
Depression
Treatment of depression is effective for reducing key symptoms (82) as well as the prevalence of the desire for hastened death (83) and improves quality of life (84). Tricyclic antidepressants, selective serotonin reuptake inhibitors, and psychosocial interventions are all evidence-supported interventions for depression in cancer (82, 85).
There is little evidence to recommend one antidepressant agent as being more efficacious than others. Because of interactions and side effects, sertraline, escitalopram, and citalopram are the preferred selective serotonin reuptake inhibitors. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) may have some advantage in augmentation of pain management. Mirtazapine is frequently used because of its side effect profile, which includes increased appetite and a mild hypnotic effect at lower doses. Tolerability to and drug interactions with tricyclic agents limit their use to second-line agents. Often low doses are effective (7).
Psychostimulants are frequently used, although strong evidence to support this practice is lacking. Near the end of life, when waiting weeks for the effects of typical antidepressants is an unavailable luxury, methylphenidate and dextroamphetamine may be agents of choice for low energy and withdrawal; the onset of effects can be only 1–2 days. One option is to start psychostimulants at the same time as a titration of a typical antidepressant and then to taper the psychostimulant when the other agent is therapeutic (30). Modafinil has also been used for fatigue; its effects on mood are questionable (86).
There may also be a role for electroconvulsive therapy, which, although highly effective in older adults with other medical conditions, has never been specifically studied in oncologic settings (87). Space-occupying intracranial lesions are a relative contraindication, although the literature suggests that safe electroconvulsive therapy is possible (88) even in this setting.
Therapeutic tactics for contingent suicidality include exploring the motivation for the patient's death wish, sharing the patient's sense of helplessness, and avoiding power struggles or forcing the patient to renounce his or her threats too soon in the therapy (32). Much contingent suicidality in oncology patients resolves with good access to palliative care and aggressive symptom management at the end of life (83) and with a commitment to giving patients a sense of control over the location and circumstances of their death.
Modalities of psychosocial treatments for depression
A review of all psychosocial interventions has found evidence to support use of the following interventions to reduce depression in specific subgroups:
| •. | for new diagnosis: relaxation techniques, psychoeducation, problem-solving therapy | ||||
| •. | for surgery: relaxation, supportive, supportive-expressive, couples counseling | ||||
| •. | for chemotherapy: relaxation, psychoeducation, supportive, supportive-expressive, problem-solving, cognitive therapies | ||||
| •. | for radiotherapy: relaxation, supportive, supportive-expressive. | ||||
| •. | after completion of active treatment: relaxation | ||||
| •. | for metastatic disease: supportive, supportive-expressive, cognitive behavior therapy | ||||
| •. | for terminal illness: relaxation, cognitive behavior, problem-solving | ||||
| •. | for survivors: group treatments | ||||
Psychiatrists may be helpful in setting up the educational interventions, but their implementation will mainly involve the oncology team and an affiliated counselor. Psychoeducation and relaxation training are useful, resource-sparing, preventive interventions that can be made a routine part of postdiagnosis oncologic care. At the other end of treatment, there is moderate evidence to suggest the usefulness of psychotherapy (cognitive behavior therapy, supportive, or problem-solving) for depressive symptoms in terminally ill patients with cancer.
The provision of palliative care to outpatients with cancer does not appear to have significant effect on depression or anxiety (94). However, without adequate symptom management, psychological or psychopharmacologic approaches may be ineffective (95).
Anxiety
Evidence-based psychosocial interventions for anxiety in cancer include cognitive behavior therapy, psychoeducation, preventative psychosocial interventions, relaxation training, and perhaps music therapy. Surprisingly, the effects of relaxation therapy are weaker for anxiety than for depression (92). A review of all psychosocial interventions showed that evidence supports the following interventions to reduce anxiety in specific subgroups (91, 92, 96):
| •. | for new diagnosis: relaxation techniques, psychoeducation | ||||
| •. | for surgery: hypnotherapy, relaxation training, cognitive behavior therapy | ||||
| •. | for chemotherapy: hypnotherapy, relaxation, psychoeducation, supportive, supportive-expressive | ||||
| •. | for radiotherapy: relaxation, supportive, supportive-expressive | ||||
| •. | after completion of active treatment: relaxation | ||||
| •. | for metastatic disease: supportive, supportive-expressive | ||||
| •. | for terminal illness: relaxation | ||||
Pharmacologic mainstays of anxiety treatment are monoamine antidepressants. Up to 2003, there had been no adequate studies for pharmacotherapy of anxiety in palliative care (97). Benzodiazepines are frequently used, with clonazepam being favored for patients without renal impairment or shorter-acting agents such as lorazepam for those with hepatic impairment. Atypical antipsychotics are also used widely for symptoms of anxiety, without definitive evidence of effect in cancer. Progressive muscle relaxation has efficacy equivalent to that of benzodiazepines in patients with good functional status (98).
One major confounder in the presentation of anxiety is the breathlessness or dyspnea that occurs in thoracic, widely metastatic, or end-stage cancers. Anxiety and physiological dyspnea can pair to create a mind-body state of intense distress with complex precipitants and elements of panic attacks (99). Relaxation training can be the basis of psychological and somatic therapies for this state, including biofeedback, breathing training, and vibration of the chest wall (100).
Although anxiety is often associated with insomnia, there have been no randomized clinical trials testing the efficacy of benzodiazepine receptor agonists for insomnia in palliative care (101).
Delirium
Treatment includes correcting dehydration, diagnosing infections, addressing hypoxia and metabolic derangements, discontinuing any unnecessary medications such as benzodiazepines (which are an independent risk factor for delirium for patients in the ICU) (102), considering opioid rotation, and evaluating the need for neuroimaging. Nonpharmacologic interventions found to be effective in decreasing the rate of delirium (103) include frequent reorientation, performing cognitively stimulating activities, limiting noise and nighttime medications as well as establishing sleep routines and using visual and hearing aids. As for pharmacologic treatment, no medication has been approved by the U.S. Food and Drug Administration for the treatment of delirium, and there are no published double-blind randomized placebo-controlled trials. Small comparison trials in various medical patient populations have revealed approximately equal efficacy of haloperidol, olanzapine, and risperidone (104–106), with quetiapine being effective in several small studies without a comparison arm (107, 108).
Fatigue
Pharmacologic treatment strategies include hematopoietic agents for anemia (which have the largest body of evidence for use), activating antidepressants such as bupropion, stimulants, l-carnitine, corticosteroids, and modafinil (80). Effective nonpharmacologic interventions include exercise and psychosocial support/education/stress management; there is promising but limited evidence supporting the efficacy of yoga, mindfulness-based stress reduction, nutritional, sleep, and other restorative therapies (80).
Character disorders
Management strategies include acknowledging and empathizing with the real stresses in the patient's situation and repeatedly appealing to the patient's sense of worth with comments such as “you deserve the best medical care we can give, and that is why we are recommending this course of action” (109). Mental health consultants can help medical clinicians tailor their approach to the patient's particular personality style (110), set appropriate limits with the patient, guide team meetings to reduce conflict among staff, and provide education and emotional support to the team (111). Although clearly the treatment of choice for personality disorders, psychotherapy can often take years to be effective and thus is often not feasible for patients with advanced disease.
Schizophrenia
Schizophrenic patients may have poor insight into illness severity and poor recall of medical recommendations, and in these patients disease is often diagnosed at a late stage because of lack of access to or poor compliance with primary care. In addition, psychotic patients may not verbalize pain/discomfort and may tolerate malodorous or fungating lesions without complaint (112).
Optimal treatment strategies include streamlining oncologic care to include the smallest number of medical providers, with a social worker or psychiatrist as liaison between the medical staff, family, and patient. Early psychiatric evaluation, preferably before surgery, is crucial, as is communication with family and/or group home staff. In patients with breast cancer, prolactin elevations do not mediate increased recurrence risk, so treatment with first-generation antipsychotics or risperidone can proceed as previously (113). Clozapine use can be a concern with the use of chemotherapeutic agents that cause myelosuppression, although there have been six published cases of patients receiving clozapine and chemotherapy without agranulocytosis, despite expected neutropenia (114). Potential alternatives to discontinuing clozapine treatment are adding lithium or granulopoiesis-stimulating factors and discontinuing concomitant medications (carbamazepine, valproate, or risperidone) (115).
Although some schizophrenic patients may lack decision-making capacity regarding cancer treatments or end-of-life care, many are interested in advance care planning, are able to consider and communicate their preferences, and share the same end of life concerns as patients without mental illness (116).
Substance use disorders
Traditional substance abuse treatment modalities may be difficult in the setting of advanced cancer and opiate requirements. Table 4 summarizes guidelines for management in psycho-oncology (117).
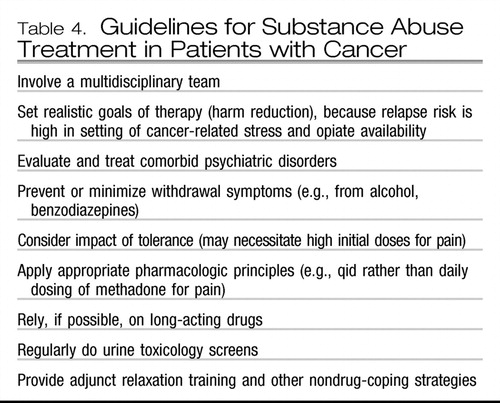 |
Table 4. Guidelines for Substance Abuse Treatment in Patients with Cancer
Chemotherapy-induced cognitive dysfunction
Treatment strategies include cognitive rehabilitation and treatment of comorbidities such as anemia and depression. Pharmacologic options include ginkgo biloba, methylphenidate, and cholinesterase inhibitors, although the rationale for their use at this point is hypothetical and borrowed from other diseases with similar symptom constellations (45).
WBRT-induced cognitive dysfunction and fatigue
One proposed treatment is donepezil, which in a phase II study of patients undergoing radiation for brain tumors improved cognitive functioning, mood, and quality of life (118) and which in our experience at Dana-Farber Cancer Institute is also highly effective for reducing fatigue in patients with brain metastases (119). There are limited data supporting the use of methylphenidate, vitamin E, and memantine but further studies are planned.
Paraneoplastic limbic encephalitis
Treatment is directed to the underlying malignancy and may also include immune therapy with steroids, immunoglobulins, or plasma exchange, which results in symptomatic stabilization in only 25% of patients (48).
Psychosocial therapy modalities
As noted above, adjustment disorders are among the most frequent diagnoses in an oncology population. Interventions for depression and anxiety are also mainstays of therapy with the aim of fostering coping and adjustment. The fact that most positive trials of psychosocial therapies were not limited to those with clinical diagnoses suggests that they may be quite effective in adjustment disorders.
Themes
Existential concerns, such as the struggle to maintain identity, to find meaning in life, and to maintain hope, as well as to answer questions of transcendence and spirituality, are more prominent in psycho-oncologic care (120). Authors such as Block, and Chochinov et al. have described therapeutic approaches to these concerns (30, 95, 121).
Developmental stage, coping style, and the impact of the cancer on self-esteem and object relations should all be assessed when one is beginning psychotherapy in this setting. An empathic style, with acknowledgment of suffering and grief, is favored (30, 68, 95). Narrative therapies help alleviate demoralization, with the dignity-conserving therapy of Chochinov et al. (121) exemplifying an end-of-life-specific modality. Other manualized interventions include “The Healing Journey” and “Re-Creating Your Life” (122).
Group therapy
Group treatment is a widely used and evidence-supported modality in cancer. Groups vary from educational to supportive to cognitive behavior. Most are short-term. They are effective for both patients and family members (123). Supportive-expressive group therapy has the longest history in this setting and involves expressing emotions, detoxifying dying, redefining priorities, and improving supportive/treatment relationships. There appears to be a lasting effect of group treatment on quality of life (122, 123). Meaning-centered therapy, which draws on Frankl's logotherapy as well as the role of spirituality in making meaning, is another treatment developed for those facing life-threatening illness (30, 68, 95, 121, 124).
Early studies of efficacy of group therapy appeared to show a survival advantage (125, 126); however, survival rates in the studies were anomalous compared with Surveillance Epidemiology and End Results (SEER) data. Quality-of-life effects are more clearly positive, which has been confirmed in further studies; benefits include decreased distress, better coping, and prevention of new episodes of depression (53). Satisfaction with groups is high (127).
Couples therapy
Cancer challenges marital bonds by altering the affected partner's physical and sexual function and often results in significant shifts in roles, responsibilities, and communication styles. Brief interventions in early-stage disease may reduce emotional distress and depression, increase sexual satisfaction, and improve family communication (128). Couples therapy during the terminal phase may reduce distress, provide an opportunity for relational growth, and ease the bereavement process for the surviving spouse (often preparing him or her for bereavement follow-up therapy) (129, 130).
OTHER SIGNIFICANT TOPICS
Goals of care
Often, psychiatric symptoms in advanced illness are exacerbated by unclear goals of care. A psychiatric consultation may be requested when the patient manifests symptoms, behaviors, or beliefs that are a result of mixed messages, incomplete information, family or treatment team anxieties, or a complicated social, familial, legal, or ethical picture (131). The implicit request may be “the patient won't do what is best for him/her, so please convince him/her or find incapacity to decide” or “please mediate this conflict between family members, between family and oncologists, between patient and specialists, or among members of the treatment team.”
Although it is gratifying to the psychiatrist to be perceived as being able to make these Solomonic decisions, often these conflicts resolve with better communication or an ethics consultation. In addition, psychiatrists (even those specializing in psycho-oncology) may not be fully able to assess or discuss the vital oncologic issues of prognosis and effectiveness of treatment. These are issues sometimes avoided by treaters in the hope that the psychiatrist will be able to deliver the bad news, avert the predicted emotional catastrophe, and convince the patient of the rightness of the recommended course. However, psychiatrists are rarely qualified to be judges, oncologists, detectives, or theologians.
There are appropriate roles for psychiatrists in evaluating and facilitating the adjustment to new care goals (132). Oncologic and palliative care treatment teams require input about the prognosis of psychiatric conditions and their effect on decision making. Clarity of goals and psychological adjustment to changing goals can be advanced by increasing patients' understanding of the relation of their current illness to past fears, traumas, or hopes. Meaning, legacy, and transcendence are issues that psychiatrists can help patients address. And if discussion of goals of care is impeded by anxiety or other psychiatric conditions, treatment for these is indicated.
Survivorship
Because of improved diagnosis and treatment, 59% of patients with cancer in the United States will live for 5 or more years, and 10 million Americans are living with a medical history including cancer (50). Lingering treatment-related concerns include infertility, early menopause, pain, and complications due to a compromised immune system and the development of new cancers—all issues that may potentiate mental health problems. Quality of life improves over time for many cancer survivors, with the exception of prostate cancer survivors (133). A significant proportion of hematopoietic stem cell transplant survivors experience persistent anxiety and depressive symptoms (134). PTSD symptoms may also develop in the aftermath of cancer treatment in 2% to 39% of survivors, with parents of pediatric survivors consistently reporting the highest PTSD rates (suggesting that the experience of parenting a child with life-threatening illness may be inherently more stressful than the experience of actual cancer or survivorship) (135). Young adults with cancer may have a higher risk for PTSD, perhaps because of a lack of psychological preparedness for the possibility of imminent death. Maintaining employment or returning to work is complicated for many cancer survivors. Data from the national health interview survey in the United States showed that one in six correspondents with a history of cancer reported being unable to work (136). In several studies, a nonsupportive work environment, manual labor, head and neck cancer, and fatigue level were associated with failure to return to work (137, 138). Impaired neuropsychological functioning (which is especially common in stem cell transplantation survivors) (43) may be correlated with lower vocational functioning, although more research is needed to test this hypothesis (139). Improved occupational rehabilitation procedures for cancer survivors are needed (140).
Bereavement
The loss of a loved one has been described as one of the most difficult experiences an individual may face. Widowed individuals generally go through identified stages of grief, with the vast majority coming to terms with the loss within approximately 1 year (141). The grief associated with the loss of a child may be more intense and longer-lasting, with a recent study finding that parents with unresolved grief have an increased risk of long-term mental and physical morbidity, health services use, and increased sick leave (142). Grief experiences have been divided into nonpathologic and pathologic, with symptoms of the latter (complicated grief) including intense yearning, difficulty accepting the death, numbness, emptiness, and sense of a bleak future (143). Complicated grief has been further divided into anticipatory grief reactions and bereavement reactions, with younger caregivers (age <60 years) possibly having a higher risk for the former (144). Symptoms of major depression that distinguish it from bereavement include guilt extending beyond actions taken by the survivor at the time of death, active suicidal thoughts, morbid preoccupation with worthlessness, marked psychomotor retardation, and hallucinations other than hearing the voice or seeing the image of the deceased person (DSM-IV-TR). Aggressive care (including ICU admissions, feeding tubes, chemotherapy, mechanical ventilation, and resuscitation) at the end of life may increase the risk of major depression in bereaved caregivers (145).
FUTURE DIRECTIONS
In the next 15 years, cancer prevalence is expected to double in developed countries because of improved survival rates, and cancer incidence will also increase with an aging population. In 2008, the Institute of Medicine affirmed that psychosocial care is possible in any oncology practice (146). However, funding for psychosocial oncology services remains quite limited, even though psychosocial services have been shown to provide cost offsets of up to 22% to the medical system (147). Integrated on-site biopsychosocial screening (rather than the compartmentalized approach of medical and psychosocial models) may help treatment planning and allow early detection and management of emotional distress (148). Funding streams must support this integrated work. Trainees from psychiatry are needed in psycho-oncology to expand services into community hospitals and smaller cancer centers. Productive areas for research include clarifying the impact of coping style and social support on cancer mortality, assessing which specific psychotherapies benefit which patients with cancer, and determining effects of chemotherapy, cytokines, and immunotherapies on the CNS as well as the interaction among these effects, character/coping styles, and primary mood disorders (2). Finally, with an ever-increasing prevalence of cancer survivors requiring treatment, an enhanced body of research knowledge on the psychological consequences of survivorship will benefit both psycho-oncologists and general psychiatrists.
1 Holland J, Sacks A: Principles of Psycho-Oncology, in Holland-Frei Cancer Medicine, 7th ed. Edited by Kufe DW, Frei E III, Holland JF, Weichselbaum RR, Pollock RE, Bast RC Jr, Hong WK, Hait WN, Hamilton, ON, Canada, B. C. Decker Inc., 2006Google Scholar
2 Holland JC: History of psycho-oncology: overcoming attitudinal and conceptual barriers. Psychosom Med 2002; 64: 206– 221Crossref, Google Scholar
3 McDonald MV, Passik SD, Dugan W, Rosenfeld B, Theobold DE, Edgerton S: Nurses' recognition of depression in their patients with cancer. Oncol Nurs Forum 1999; 26: 593– 599Google Scholar
4 Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobold DE, Edgerton S: Oncologists' recognition of depression in their patients with cancer. J Clin Oncol 1998; 16: 1594– 1600Crossref, Google Scholar
5 Kadan-Lottick NS, Vanderwerker LC, Block SD, Zhang B, Prigerson HG: Psychiatric disorders and mental health service use in patients with advanced cancer: a report from the coping with cancer study. Cancer 2005; 104: 2872– 2881Crossref, Google Scholar
6 Derogatis LR, Morrow GR, Fetting J, Penman D, Piasetsky S, Schmale AM, Henrichs M, Carnicke CL Jr: The prevalence of psychiatric disorders among cancer patients. JAMA 1983; 249: 751– 757Crossref, Google Scholar
7 Miovic M, Block S: Psychiatric disorders in advanced cancer. Cancer 2007; 110: 1665– 1676Crossref, Google Scholar
8 Wilson KG, Chochinov HM, Skirko MG, Allard P, Chary S, Gagnon PR, Macmillan K, De Luca M, O'Shea F, Kuhl D, Fainsinger RL, Clinch JJ: Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage 2007; 33: 118– 129Crossref, Google Scholar
9 Gurevich M, Devins GM, Rodin GM: Stress response syndromes and cancer: conceptual and assessment issues. Psychosomatics 2002; 43: 259– 281Crossref, Google Scholar
10 Spiegel D, Giese-Davis J: Depression and cancer: mechanisms and disease progression. Biol Psychiatry 2003; 54: 269– 282Crossref, Google Scholar
11 Kissane DW, Wein S, Love A, Lee XQ, Kee PL, Clarke DM: The Demoralization Scale: a report of its development and preliminary validation. J Palliat Care 2004; 20: 269– 276Crossref, Google Scholar
12 Weiner MF, Lovitt R: Conservation-withdrawal versus depression. Gen Hosp Psychiatry 1979; 1: 347– 349Crossref, Google Scholar
13 Lichtermann D, Ekelund J, Pukkala E, Tanskanan A, Lönnqvist J: Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 2001; 58: 573– 578Crossref, Google Scholar
14 Ciaramella A, Poli P: Assessment of depression among cancer patients: the role of pain, cancer type and treatment. Psychooncology 2001; 10: 156– 165Crossref, Google Scholar
15 Evans DL, Staab JP, Petitto JM, Morrison MF, Szuba MP, Ward HE, Wingate B, Luber MP, O'Reardon JP: Depression in the medical setting: biopsychological interactions and treatment considerations. J Clin Psychiatry 1999; 60( suppl 4): 40– 55Google Scholar
16 Lichtenthal W, Nilsson M, Zhang B, Trice ED, Kissane DW, Breitbart W, Prigerson HG: Do rates of mental disorders and existential distress among advanced stage cancer patients increase as death approaches?, Psychooncology 2009: 18: 50– 61Crossref, Google Scholar
17 Lawlor PG, Gagnon B, Mancini IL, Pereira JL, Hanson J, Suarez-Almazor ME, Bruera ED: Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med 2000; 160: 786– 794Crossref, Google Scholar
18 Spiller JA, Keen JC: Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med 2006; 20: 17– 23Crossref, Google Scholar
19 Ross CA, Peyser CE, Shapiro I, Folstein MF: Delirium: phenomenologic and etiologic subtypes. Int Psychogeriatr 1991; 3: 135– 147Crossref, Google Scholar
20 Breitbart W, Alici Y: Agitation and delirium at the end of life: ‘We couldn't manage him.’ JAMA 2008; 300: 2898– 2910Crossref, Google Scholar
21 Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR: Cancer-related fatigue: the scale of the problem. Oncologist 2007; 12( suppl 1): 4– 10Crossref, Google Scholar
22 Pandiani JA, Boyd MM, Banks SM, Johnson AT: Elevated cancer incidence among adults with serious mental illness. Psychiatr Serv 2006; 57: 1032– 1034Crossref, Google Scholar
23 Bruera E, Moyano J, Seifert L, Fainsinger RL, Hanson J, Suarez-Almazor M: The frequency of alcoholism among patients with pain due to terminal cancer. J Pain Symptom Manage 1995; 10: 599– 603Crossref, Google Scholar
24 Parsons HA, Delgado-Guay MO, El Osta B, Chacko R, Poulter V, Palmer JL, Bruera E: Alcoholism screening in patients with advanced cancer: impact on symptom burden and opioid use. J Palliat Med 2008; 11: 964– 968Crossref, Google Scholar
25 Mehanna HM, De Boer MF, Morton RP: The association of psycho-social factors and survival in head and neck cancer. Clin Otolaryngol 2008; 33: 83– 89Crossref, Google Scholar
26 Kugaya A, Akechi T, Okuyama T, Nakano T, Mikami I, Okamura H, Uchitomi Y: Prevalence, predictive factors, and screening for psychologic distress in patients with newly diagnosed head and neck cancer. Cancer 2000; 88: 2817– 2823Crossref, Google Scholar
27 Schneider KL, Shenassa E: Correlates of suicide ideation in a population-based sample of cancer patients. J Psychosoc Oncol 2008; 26: 49– 62Crossref, Google Scholar
28 Chochinov HM, Wilson KG, Enns M, Lander S: Depression, hopelessness, and suicidal ideation in the terminally ill. Psychosomatics 1998; 39: 366– 370Crossref, Google Scholar
29 Rasic DT, Belik S-L, Bolton JM, Chochinov HM, Sareen J: Cancer, mental disorders, suicidal ideation and attempts in a large community sample. Psychooncology 2008; 17: 660– 667Crossref, Google Scholar
30 Block SD: Assessing and managing depression in the terminally ill patient. ACP-ASIM End-of-Life Care Consensus Panel. American College of Physicians-American Society of Internal Medicine. Ann Intern Med 2000; 132: 209– 218Crossref, Google Scholar
31 Breitbart W, Rosenfeld B, Pessin H, Breitbart W: Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA 2000; 284: 2907– 2911Crossref, Google Scholar
32 Gutheil TG, Schetky D: A date with death: management of time-based and contingent suicidal intent. Am J Psychiatry 1998; 155: 1502– 1507Crossref, Google Scholar
33 Peteet JR, Maytal G, Rokni H: Unimaginable loss: contingent suicidal ideation in family members. Psychosomatics, 2009Google Scholar
34 Miller M, Mogun H, Azrael D, Solomon DH: Cancer and the risk of suicide in older Americans. J Clin Oncol 2008; 26: 4720– 4724Crossref, Google Scholar
35 Misono S, Weiss NS, Fann JR, Redman M, Yueh B: Incidence of suicide in persons with cancer. J Clin Oncol 2008; 26: 4731– 4738Crossref, Google Scholar
36 Rodin G, Zimmermann C, Rydall A, Jones J, Shepherd FA, Moore M, Fruh M, Donner A, Gagliese L: The desire for hastened death in patients with metastatic cancer. J Pain Symptom Manage 2007; 33: 661– 675Crossref, Google Scholar
37 Ganzini L, Goy ER, Dobscha SK: Prevalence of depression and anxiety in patients requesting physicians' aid in dying: cross sectional survey. BMJ 2008; 337: a1682Crossref, Google Scholar
38 Eichler AF, Loeffler JS: Multidisciplinary management of brain metastases. Oncologist 2007; 12: 884– 898Crossref, Google Scholar
39 Chow E, Fan G, Hadi S, Wong J, Kirou-Mauro A, Filipczak L: Symptom clusters in cancer patients with brain metastases. Clin Oncol (R Coll Radiol) 2008; 20: 76– 82Crossref, Google Scholar
40 DeAngelis LM, Boutros D: Leptomeningeal metastasis. Cancer Invest 2005; 23: 145– 154Crossref, Google Scholar
41 Alici-Evcimen Y, Breitbart WS: Ifosfamide neuropsychiatric toxicity in patients with cancer. Psychooncology 2007; 16: 956– 960Crossref, Google Scholar
42 Paraska K, Bender CM: Cognitive dysfunction following adjuvant chemotherapy for breast cancer: two case studies. Oncol Nurs Forum 2003; 30: 473– 478Crossref, Google Scholar
43 Poppelreuter M, Weis J, Mumm A, Orth HB, Bartsch HH: Rehabilitation of therapy-related cognitive deficits in patients after hematopoietic stem cell transplantation. Bone Marrow Transplant 2008; 41: 79– 90Crossref, Google Scholar
44 Malmstrom H, Karlsson EA: Cognitive functions in patients with ovarian cancer receiving chemotherapy. Proc Am Soc Clin Oncol 2003; 22: 462Google Scholar
45 Staat K, Segatore M: The phenomenon of chemo brain. Clin J Oncol Nurs 2005; 9: 713– 721Crossref, Google Scholar
46 Li J, Bentzen SM, Renschler M, Mehta MP: Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol 2007; 25: 1260– 1266Crossref, Google Scholar
47 Khuntia D, Brown P, Li J, Mehta MP: Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol 2006; 24: 1295– 1304Crossref, Google Scholar
48 Voltz R: Neuropsychological symptoms in paraneoplastic disorders. J Neurol 2007; 254( Suppl 2): II84– II86Crossref, Google Scholar
49 Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J: Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 2000; 123( Pt 7): 1481– 1494Crossref, Google Scholar
50 American Cancer Society: Cancer Facts and Figures: 2003–2004. Atlanta, American Cancer Society, 2005Google Scholar
51 Falagas ME, Zarkadoulia EA, Ioannidou EN, Peppas G, Christodoulou C, Rafailidis PI: The effect of psychosocial factors on breast cancer outcome: a systematic review. Breast Cancer Res 2007; 9: R44Crossref, Google Scholar
52 Phillips K-A, Osborne RH, Giles GG, Dite GS, Apicella C, Hopper JL, Milne RL: Psychosocial factors and survival of young women with breast cancer: a population-based prospective cohort study. J Clin Oncol 2008; 26: 4666– 4671Crossref, Google Scholar
53 Kissane D: Beyond the psychotherapy and survival debate: the challenge of social disparity, depression and treatment adherence in psychosocial cancer care. Psychooncology 2009; 18: 1– 5Crossref, Google Scholar
54 Ross L, Frederiksen K, Boesen S, Karlsen R, Rasmussen M, Sorensen L, Jørgensen T, Claesson M, Johansen C: No effect on survival of home psychosocial intervention in a randomized study of Danish colorectal cancer patients. Psychooncology ( in press)Google Scholar
55 Paton CC, Perez DJ: Psychosocial variables as prognostic factors in metastatic cancer: a brief review. N Z Med J 2006; 119: U2054Google Scholar
56 Petticrew M, Bell R, Hunter D: Influence of psychological coping on survival and recurrence in people with cancer: systematic review. BMJ 2002; 325: 1066Crossref, Google Scholar
57 Gallo JJ, Bogner HR, Morales KH, Post EP, Lin JY, Bruce ML: The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med 2007; 146: 689– 698Crossref, Google Scholar
58 Strasser-Weippl K, Ludwig H: Psychosocial QOL is an independent predictor of overall survival in newly diagnosed patients with multiple myeloma. Eur J Haematol 2008; 81: 374– 379Google Scholar
59 Vos MS, de Haes JCJM: Denial in cancer patients, an explorative review. Psychooncology 2007; 16: 12– 25Crossref, Google Scholar
60 Rodin G, Zimmermann C: Psychoanalytic reflections on mortality: a reconsideration. J Am Acad Psychoanal Dyn Psychiatry 2008; 36: 181– 196Crossref, Google Scholar
61 Weisman AD, Worden JW: Coping and Vulnerability: A Research Report. Boston, Massachusetts General Hospital, 1977Google Scholar
62 Weisman AD: Coping with cancer. New York: McGraw-Hill, 1979.Google Scholar
63 Weisman AD: Vulnerability and the psychological disturbances of cancer patients. Psychosomatics 1989; 30: 80– 85Crossref, Google Scholar
64 Weisman AD: A model for psychosocial phasing in cancer. Gen Hosp Psychiatry 1979; 1: 187– 195Crossref, Google Scholar
65 Josephson AM, Peteet JR: Talking with patients about spirituality and worldview: practical interviewing techniques and strategies. Psychiatr Clin North Am 2007; 30: 181– 197Crossref, Google Scholar
66 McClung E, Grossoehme DH, Jacobson AF: Collaborating with chaplains to meet spiritual needs. Medsurg Nurs 2006; 15: 147– 156Google Scholar
67 Rauch PK, Muriel AC, Cassem NH: Parents with cancer: who's looking after the children? J Clin Oncol 2003; 21: 117s– 121sCrossref, Google Scholar
68 Lloyd-Williams M, Reeve J, Kissane D: Distress in palliative care patients: developing patient-centred approaches to clinical management. Eur J Cancer 2008; 44: 1133– 1138Crossref, Google Scholar
69 Mitchell SL, Kiely DK, Hamel MB: Dying with advanced dementia in the nursing home. Arch Intern Med 2004; 164: 321– 326Crossref, Google Scholar
70 Muskin PR: The request to die: role for a psychodynamic perspective on physician-assisted suicide. JAMA 1998; 279: 323– 328Crossref, Google Scholar
71 Ljubisavljevic V, Kelly B: Risk factors for development of delirium among oncology patients. Gen Hosp Psychiatry 2003; 25: 345– 352Crossref, Google Scholar
72 Lawlor PG, Nekolaichuk C, Gagnon B, Mancini IL, Pereira JL, Bruera ED: Clinical utility, factor analysis, and further validation of the memorial delirium assessment scale in patients with advanced cancer: assessing delirium in advanced cancer. Cancer 2000; 88: 2859– 2867Crossref, Google Scholar
73 Adamis D, Morrison C, Treloar A, Macdonald AJ, Martin FC: The performance of the Clock Drawing Test in elderly medical inpatients: does it have utility in the identification of delirium? J Geriatr Psychiatry Neurol 2005; 18: 129– 133Crossref, Google Scholar
74 Breitbart W, Gibson C, Tremblay A: The delirium experience: delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics 2002; 43: 183– 194Crossref, Google Scholar
75 Jones C, Griffiths RD, Humphris G, et al.: Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med 2001; 29: 573– 580Crossref, Google Scholar
76 Stagno D, Gibson C, Breitbart W: The delirium subtypes: a review of prevalence, phenomenology, pathophysiology, and treatment response. Palliat Support Care 2004; 2: 171– 179Crossref, Google Scholar
77 Buss MK, Vanderwerker LC, Inouye SK, Zhang B, Block SD, Prigerson HG: Associations between caregiver-perceived delirium in patients with cancer and generalized anxiety in their caregivers. J Palliat Med 2007; 10: 1083– 1092Crossref, Google Scholar
78 Hines SC, Glover JJ, Babrow AS, Holley JL, Badzek LA, Moss AH: Improving advance care planning by accommodating family preferences. J Palliat Med 2001; 4: 481– 489Crossref, Google Scholar
79 Passik SD, Portenoy RK, Ricketts PL: Substance abuse issues in cancer patients. Part 1: Prevalence and diagnosis. Oncology (Williston Park) 1998; 12: 517– 521, 524Google Scholar
80 Carroll JK, Kohli S, Mustian KM, Roscoe JA, Morrow GR: Pharmacologic treatment of cancer-related fatigue. Oncologist 2007; 12 ( suppl 1): 43– 51Crossref, Google Scholar
81 Minisini A, Atalay G, Bottomley A, Puglisi F, Piccart M, Biganzoli L: What is the effect of systemic anticancer treatment on cognitive function? Lancet Oncol 2004; 5: 273– 282Crossref, Google Scholar
82 Lorenz KA, Lynn J, Dy SM, Shugarman LR, Wilkinson A, Mularski RA, Morton SC, Hughes RG, Hilton LK, Maglione M, Rhodes SL, Rolon C, Sun VC, Shekelle PG: Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med 2008; 148: 147– 159Crossref, Google Scholar
83 O'Mahony S, Goulet J, Kornblith A, Abbatiello G, Clarke B, Kless-Siegel S, Breitbart W, Payne R: Desire for hastened death, cancer pain and depression: report of a longitudinal observational study. J Pain Symptom Manage 2005; 29: 446– 457Crossref, Google Scholar
84 Strong V, Waters R, Hibberd C, Murray G, Wall L, Walker J, McHugh G, Walker A, Sharpe M: Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet 2008; 372: 40– 48Crossref, Google Scholar
85 Carr D, Goudas L, Lawrence D, Pirl W, Lau J, DeVine D, Kupelnick B, Miller K: Management of cancer symptoms: pain, depression, and fatigue. Evid Rep Technol Assess (Summ) 2002; 7: 1– 5Google Scholar
86 Kumar R: Approved and investigational uses of modafinil: an evidence-based review. Drugs 2008; 68: 1803– 1839Crossref, Google Scholar
87 Rao A, Cohen HJ: Symptom management in the elderly cancer patient: fatigue, pain, and depression. J Natl Cancer Inst Monogr 2004; 32: 150– 157Crossref, Google Scholar
88 Rasmussen KG, Perry CL, Suter B, et al.: ECT in patients with intracranial masses. J Neuropsychiatry Clin Neurosci 2007; 19: 191– 193Crossref, Google Scholar
89 Akechi T, Okuyama T, Onishi J, Morita T, Furukawa TA: Psychotherapy for depression among incurable cancer patients. Cochrane Database Syst Rev 2008; CD005537Google Scholar
90 Jacobsen PB: Promoting evidence-based psychosocial care for cancer patients. Psychooncology 2009; 18: 6– 13Crossref, Google Scholar
91 Jacobsen PB, Jim HS: Psychosocial interventions for anxiety and depression in adult cancer patients: achievements and challenges. CA Cancer J Clin 2008; 58: 214– 230Crossref, Google Scholar
92 Luebbert K, Dahme B, Hasenbring M: The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytical review. Psychooncology 2001; 10: 490– 502Crossref, Google Scholar
93 Rajasekaran M, Edmonds PM, Higginson IL: Systematic review of hypnotherapy for treating symptoms in terminally ill adult cancer patients. Palliat Med 2005; 19: 418– 426Crossref, Google Scholar
94 Qaseem A, Snow V, Shekelle P, Casey DE Jr, Cross JT Jr, Owens DK, Dallas P, Dolan NC, Forciea MA, Halasyamani L, Hopkins RH Jr: Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2008; 148: 141– 146Crossref, Google Scholar
95 Block SD: Psychological issues in end-of-life care. J Palliat Med 2006; 9: 751– 772Crossref, Google Scholar
96 Flory N, Lang E: Practical hypnotic interventions during invasive cancer diagnosis and treatment. Hematol Oncol Clin North Am 2008; 22: 709– 725, ixCrossref, Google Scholar
97 Jackson KC, Lipman AG: Drug therapy for anxiety in palliative care. Cochrane Database Syst Rev 2004; CD004596Google Scholar
98 Holland JC, Morrow GR, Schmale A, Derogatis L, Stefanek M, Berenson S, Carpenter PJ, Breitbart W, Feldstein M: A randomized clinical trial of alprazolam versus progressive muscle relaxation in cancer patients with anxiety and depressive symptoms. J Clin Oncol 1991; 9: 1004– 1011Crossref, Google Scholar
99 Henoch I, Bergman B, Danielson E: Dyspnea experience and management strategies in patients with lung cancer. Psychooncology 2008; 17: 709– 715Crossref, Google Scholar
100 Bausewein C, Booth S, Gysels M, Higginson I: Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev 2008; CD005623Google Scholar
101 Hirst A, Sloan R: Benzodiazepines and related drugs for insomnia in palliative care. Cochrane Database Syst Rev 2002; CD003346Google Scholar
102 Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW: Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 2006; 104: 21– 26Crossref, Google Scholar
103 Inouye SK, Bogardus STJ, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LMJ: A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340: 669– 676Crossref, Google Scholar
104 Han C-S, Kim Y-K: A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics 2004; 45: 297– 301Crossref, Google Scholar
105 Sipahimalani A, Masand PS: Olanzapine in the treatment of delirium. Psychosomatics 1998; 39: 422– 430Crossref, Google Scholar
106 Skrobik YK, Bergeron N, Dumont M, Gottfried SB: Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med 2004; 30: 444– 449Crossref, Google Scholar
107 Maneeton B, Maneeton N, Srisurapanont M: An open-label study of quetiapine for delirium. J Med Assoc Thai 2007; 90: 2158– 2163Google Scholar
108 Sasaki Y, Matsuyama T, Inoue S, Sunami T, Inoue T, Denda K, Koyama T: A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry 2003; 64: 1316– 1321Crossref, Google Scholar
109 Groves JE, Beresin EV: Difficult patients, difficult families. New Horizons 1998; 6: 331– 343Google Scholar
110 Kahana RJ, Bibring GL: Personality types in medical management, in Psychiatric and Medical Practice in a General Hospital. Edited by Zinberg NE. New York, International Universities Press, 1964, pp 108– 123Google Scholar
111 Hay JL, Passik SD: The cancer patient with borderline personality disorder: suggestions for symptom-focused management in the medical setting. Psychooncology 2000; 9: 91– 100Crossref, Google Scholar
112 Talbott JA, Linn L: Reactions of schizophrenics to life-threatening disease. Psychiatr Q 1978; 50: 218– 227Crossref, Google Scholar
113 Massie MJ: In Handbook of Psycho-oncology. Edited by Holland JC. New York, Oxford University Press, 1989, pp 320– 323Google Scholar
114 Rosenstock J: Clozapine therapy during cancer treatment. Am J Psychiatry 2004; 161: 175Crossref, Google Scholar
115 Esposito D, Rouillon F, Limosin F: Continuing clozapine treatment despite neutropenia. Eur J Clin Pharmacol 2005; 60: 759– 764Crossref, Google Scholar
116 Foti ME, Bartels SJ, Merriman MP, Fletcher KE, Van Citters AD: Medical advance care planning for persons with serious mental illness. Psychiatr Serv 2005; 56: 576– 584Crossref, Google Scholar
117 Passik SD, Portenoy RK, Ricketts PL: Substance abuse in cancer patients. Part 2: Evaluation and treatment. Oncology (Williston Park) 1998; 12: 729–734; discussion 736, 741–722.Google Scholar
118 Shaw EG, Rosdhal R, D'Agostino RBJ, Lovato J, Naughton MJ, Robbins ME, Rapp SR: Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol 2006; 24: 1415– 1420Crossref, Google Scholar
119 Miovic MK: personal communication. 2008Google Scholar
120 Henoch I, Danielson E: Existential concerns among patients with cancer and interventions to meet them: an integrative literature review. Psychooncology 2008; 18: 225– 236Crossref, Google Scholar
121 Chochinov HM, Hack T, Hassard T, Kristjanson LJ, McClement S, Harlos M: Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol 2005; 23: 5520– 5525Crossref, Google Scholar
122 LeMay K, Wilson KG: Treatment of existential distress in life threatening illness: a review of manualized interventions. Clin Psychol Rev 2008; 28: 472– 493Crossref, Google Scholar
123 Weis J: Support groups for cancer patients. Support Care Cancer 2003; 11: 763– 768Crossref, Google Scholar
124 Breitbart W, Gibson C, Poppito SR, Berg A: Psychotherapeutic interventions at the end of life: a focus on meaning and spirituality. Can J Psychiatry 2004; 49: 366– 372Crossref, Google Scholar
125 Fawzy FI, Fawzy NW, Hyun CS, Elashoff R, Guthrie D, Fahey JL, Morton DL: Malignant melanoma. Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry 1993; 50: 681– 689Crossref, Google Scholar
126 Spiegel D, Bloom JR, Kraemer HC, et al.: Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 1989; 2: 888– 891Crossref, Google Scholar
127 Gottlieb BH, Wachala ED: Cancer support groups: a critical review of empirical studies. Psychooncology 2007; 16: 379– 400Crossref, Google Scholar
128 Scott JL, Halford WK, Ward BG: United we stand? The effects of a couple-coping intervention on adjustment to early stage breast or gynecological cancer. J Consult Clin Psychol 2004; 72: 1122– 1135Crossref, Google Scholar
129 McLean LM, Jones JM: A review of distress and its management in couples facing end-of-life cancer. Psychooncology 2007; 16: 603– 616Crossref, Google Scholar
130 Mohr DC, Moran PJ, Kohn C, Hart S, Armstrong K, Dias R, Bergsland E, Folkman S: Couples therapy at end of life. Psychooncology 2003; 12: 620– 627Crossref, Google Scholar
131 Weiner JS, Roth J: Avoiding iatrogenic harm to patient and family while discussing goals of care near the end of life. J Palliat Med 2006; 9: 451– 463Crossref, Google Scholar
132 Back AL, Anderson WG, Bunch L, Marr LA, Wallace JA, Yang HB, Arnold RM: Communication about cancer near the end of life. Cancer 2008; 113: 1897– 1910Crossref, Google Scholar
133 Bloom JR, Petersen DM, Kang SH: Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007; 16: 691– 706Crossref, Google Scholar
134 Mosher C, Redd W, Rini C, Burkhalter J, Duhamel K: Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psychooncology 2009; 18: 113– 127Crossref, Google Scholar
135 Alter CL, Pelcovitz D, Axelrod A, Goldenberg B, Harris H, Meyers B, Grobois B, Mandel F, Septimus A, Kaplan S: Identification of PTSD in cancer survivors. Psychosomatics 1996; 37: 137– 143Crossref, Google Scholar
136 Hewitt M, Rowland JH, Yancik R: Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci 2003; 58: 82– 91Crossref, Google Scholar
137 Spelten ER, Sprangers MAG, Verbeek JHAM: Factors reported to influence the return to work of cancer survivors: a literature review. Psychooncology 2002; 11: 124– 131Crossref, Google Scholar
138 Spelten ER, Verbeek JHAM, Uitterhoeve ALJ, Ansink AC, van der Lelie J, de Reijke TM, Kammeijer M, de Haes JCJM, Sprangers MAG: Cancer, fatigue and the return of patients to work—a prospective cohort study. Eur J Cancer 2003; 39: 1562– 1567Crossref, Google Scholar
139 Nieuwenhuijsen K, de Boer A, Spelten E, Sprangers M, Verbeek J: The role of neuropsychological functioning in cancer survivors' return to work one year after diagnosis. Psychooncology ( in press)Google Scholar
140 Verbeek J, Spelten E, Kammeijer M, Sprangers M: Return to work of cancer survivors: a prospective cohort study into the quality of rehabilitation by occupational physicians. Occup Environ Med 2003; 60: 352– 357Crossref, Google Scholar
141 Maciejewski PK, Zhang B, Block SD, Prigerson HG: An empirical examination of the stage theory of grief. JAMA 2007; 297: 716– 723Crossref, Google Scholar
142 Lannen PK, Wolfe J, Prigerson HG, Onelov E, Kreicbergs UC: Unresolved grief in a national sample of bereaved parents: impaired mental and physical health 4 to 9 years later. J Clin Oncol 2008; 26: 5870– 5876Crossref, Google Scholar
143 Prigerson HG, Frank E, Kasl SV, Reynolds CF 3rd, Anderson B, Zubenko GS, Houck PR, George CJ, Kupfer DJ: Complicated grief and bereavement-related depression as distinct disorders: preliminary empirical validation in elderly bereaved spouses. Am J Psychiatry 1995; 152: 22– 30Crossref, Google Scholar
144 Tomarken A, Holland J, Schachter S, Vanderwerker L, Zuckerman E, Nelson C, Coups E, Ramirez PM, Prigerson H: Factors of complicated grief pre-death in caregivers of cancer patients. Psychooncology 2008; 17: 105– 111Crossref, Google Scholar
145 Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, Mitchell SL, Jackson VA, Block SD, Maciejewski PK, Prigerson HG: Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008; 300: 1665– 1673Crossref, Google Scholar
146 Institute of Medicine Report: Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC, Institute of MedicineGoogle Scholar
147 Simpson JS, Carlson LE, Trew ME: Effect of group therapy for breast cancer on healthcare utilization. Cancer Pract 2001; 9: 19– 26Crossref, Google Scholar
148 Thomas BC, Bultz BD: The future in psychosocial oncology: screening for emotional distress—the sixth vital sign. Future Oncol 2008; 4: 779– 784Crossref, Google Scholar



