Update on the Assessment, Diagnosis, and Treatment of Individuals with Social Anxiety Disorder
Abstract
Social anxiety disorder, also called social phobia, is a disorder characterized by extreme fear and/or avoidance of social or performance situations that involve evaluation or possible scrutiny by others. This disorder encompasses both isolated performance anxiety and generalized fears of many social encounters, leading to significant impairment and dysfunction in social, family, educational, and occupational functioning. It is often complicated by the presence of comorbid mood disorders, such as depression, and alcohol and substance use disorders. This article reviews the epidemiology, associated impairment, comorbidity, and treatment of social anxiety disorder, including pharmacotherapy and psychosocial therapies.
DEFINITION
Social anxiety disorder (SAD), also called social phobia, is characterized by marked and persistent fear of embarrassment or humiliation in situations involving performance or interaction with or scrutiny by others (see Table 1 for DSM-IV criteria). The situation(s) are either avoided or endured with marked distress. The affected individual will often experience marked anticipatory anxiety before a feared interactional or performance situation and may experience a panic attack during the exposure. This anticipatory anxiety, avoidance, or distress in the social situation has a negative effect on the individual's social, academic, or occupational function and interpersonal relationships and/or causes marked distress.
 |
Table 1. DSM-IV Diagnostic Criteria for Social Anxiety Disorder
The true extent of the impairment and dysfunction associated with SAD may have been unrecognized in earlier years because the diagnostic term “social phobia” tended to conflate individuals with both subtypes of the disorder: those with nongeneralized or performance anxiety and those with the more impairing generalized subtype, in which the severity of symptoms and extent of impairment in function and quality of life are amplified (1, 2).
GENERALIZED SOCIAL ANXIETY DISORDER
Generalized social anxiety disorder (GSAD) accounts for two-thirds of individuals with SAD (2) and is characterized by fear and avoidance of numerous interactional as well as performance social situations. Compared with the nongeneralized subtype, GSAD is more pervasive and is associated with greater distress and dysfunction in affected individuals, increased alcohol and drug abuse, depression, suicide attempts, poor marital functioning, vocational impairment, financial dependence, increased utilization of health care resources, and decreased educational attainment (3–8).
NONGENERALIZED SOCIAL ANXIETY DISORDER
Nongeneralized SAD or “performance anxiety” refers to marked anticipatory anxiety, distress, and avoidance associated with public speaking or other performance-type situations. Although it is less pervasive and generally considered less disabling than the generalized subtype (4), nongeneralized SAD may result in significant impairment and underachievement at school and work as well (9). Most individuals with GSAD also experience performance anxiety.
PREVALENCE
A number of both national and international epidemiologic studies suggest that SAD is a common psychiatric disorder (10). Recently, the National Comorbidity Survey (NCS) Replication documented that SAD has a lifetime prevalence of 12.1%, making it the fourth most common psychiatric condition in the United States behind major depressive disorder, alcohol abuse, and specific phobias (11). A more stringent reanalysis of data from the Epidemiological Catchment Area Study and the NCS included the requirement that the disorder be clinically significant, defined as requiring treatment or causing impairment. Even with this more rigorous assessment, the disorder remained relatively common with a reported 12-month prevalence of 3.7% (12).
COMORBIDITY
SAD frequently presents comorbidly with other psychiatric disorders including other anxiety disorders, major depressive disorder, bipolar disorder, and alcohol and substance use. The NCS documented that 81% of individuals with SAD report at least one other lifetime DSM-III-R psychiatric diagnosis (5). Not surprisingly given its overall greater severity, GSAD was more commonly associated with comorbid psychiatric illnesses than was the nongeneralized subtype (1, 2).
The onset of SAD frequently precedes and may in fact be a risk factor for the development of other comorbid disorders such as major depressive disorder (5, 6, 13, 14). In the NCS, secondary major depressive disorder was present in 37% of those with SAD (15); the lifetime rate of comorbid major depressive disorder was reported to be near 60% in clinical samples (16). Similarly, 22% of patients from a large study of bipolar disorder had SAD (17).
Comorbid anxiety disorders including panic disorder, posttraumatic stress disorder, generalized anxiety disorder, and obsessive-compulsive disorder are also relatively common among individuals with SAD. For instance, in the NCS, a lifetime history of posttraumatic stress disorder was present in 16% of individuals with SAD, panic disorder was present in 11%, and generalized anxiety disorder was present in 13% (5). Individuals with SAD also have an increased risk for alcohol abuse and dependence and other substance abuse disorders. Socially anxious individuals may use alcohol in an attempt to decrease anticipatory anxiety and reduce avoidance of feared social and/or performance situations. In the NCS, the lifetime prevalence rate of alcohol dependence was 24% among those with social phobia (5); thus, individuals with SAD have a two- to threefold increased risk of developing alcohol abuse or dependence compared with the general population. As with other comorbidities, SAD onset typically precedes that of alcohol abuse; in a prospective study of individuals with social phobia or subclinical social fears, the risk of the developing alcohol abuse or dependence was more than twice that of the general population without such fears (18). The rate of alcohol abuse among those with social phobia in the clinical setting approaches 40% (19).
TREATMENT
The aim of treatment of SAD is to ultimately eliminate the patient's anticipatory and phobic anxiety around social interaction and performance situations, eradicating attendant avoidance behavior, and improving overall quality of life and function.
PHARMACOTHERAPY
A variety of pharmacologic agents have demonstrated efficacy for the treatment of SAD (Table 2).
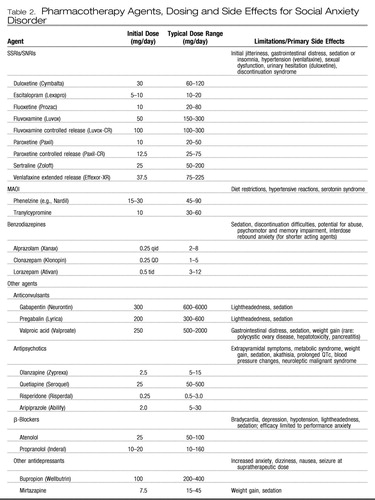 |
Table 2. Pharmacotherapy Agents, Dosing and Side Effects for Social Anxiety Disorder
Serotonin selective and serotonin-norepinephrine reuptake inhibitors.
The serotonin selective reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) have become first-line pharmacotherapy for the treatment of SAD because of their demonstrated efficacy for SAD and for major depressive disorder as well as other anxiety disorders that may often present comorbidly. In addition, they have a more favorable tolerability and safety profile than the monoamine oxidase inhibitors (MAOIs), which were the former “gold standard” agents for SAD and are not associated with the potential for abuse and dependence or ineffectiveness for comorbid major depressive disorder that complicates treatment with benzodiazepines.
The SSRIs paroxetine, sertraline, and fluvoxamine (controlled release) and the SNRI venlafaxine (extended release) have received Food and Drug Administration approval for the SAD indication. Other agents from this class also have demonstrated efficacy in randomized placebo-controlled studies and are likely effective as well, although differences in side effect profiles may be clinically relevant in some cases (20). Administration of SSRIs and SNRIs may be associated with a variety of side effects including sexual dysfunction, weight gain, increased anxiety, sedation, dizziness, headache, and gastrointestinal distress, as well as hypertension with venlafaxine and urinary retention with duloxetine. The onset of therapeutic effects usually takes at least 2–3 weeks, with greater benefits accruing over weeks or months as patients begin to expose themselves to previously feared situations. Roughly one-half to two-thirds of patients respond in acute treatment trials, with about one-half of these experiencing remission. Although the benefits of acute treatment are maintained in long-term follow-up (21), a substantial number of patients remain at least somewhat symptomatic over time (22). Given that most large randomized controlled trials (RCTs) often exclude patients with significant psychiatric or medical comorbidity as well as other complicating factors, it is likely that response and remission rates in clinical practice are even lower, underscoring the need to develop more effective treatment paradigms.
β-Blockers.
β-Blockers, such as propranolol (10–80 mg/day) and atenolol (50–150 mg/day) are effective for performance anxiety regarding public speaking or other performance situations (23, 24). They are typically administered on an “as needed” basis 1–2 hours before a performance situation, although some patients facing frequent performance challenges take them on a more routine basis. β-Blockers seem to reduce anxiety in performance situations by blunting the symptoms of physiological arousal such as tachycardia and tremor that are often an individual's focus when performing and drive an escalating cycle of fear and further anxiety. However, given their relatively short duration of action and lack of effect on the emotional and cognitive (relative to physiological) symptoms of social anxiety, these agents have not been considered to be first-line agents for GSAD (25). Side effects of β-blockers include lightheadedness, bradycardia, sedation, and nausea. Of interest, pindolol, a β-blocker with serotonin type 1A (5-HT1A) autoreceptor antagonist properties, which may accelerate or augment responses to antidepressants for major depressive disorder (26) and panic disorder (27), was ineffective in a placebo-controlled randomized augmentation trial in social phobics.
Monoamine oxidase inhibitors.
Until supplanted by the better tolerated and safer SSRIs and SNRIs, the monoamine oxidase inhibitors (MAOIs), including phenelzine and tranylcypromine, were the gold standard pharmacotherapy for SAD (25, 28). Early observations of the efficacy of MAOIs in atypical major depressive disorder, a syndrome characterized by marked sensitivity to rejection evocative of the focus of anxiety in those with social phobia (29), led to the use of the these agents in SAD and subsequent demonstration of efficacy in RCTs (25). However, the association of MAOI administration with troubling side effects including orthostatic hypotension, paresthesias, weight gain, and sexual dysfunction, as well as the need for proscribed dietary intake of tyramine-containing foods and sympathomimetic medication because of risk of potentially fatal hypertensive and serotonergic syndromes, has limited the widespread use of these agents and they are now generally reserved for use in refractory cases.
Benzodiazepines.
Benzodiazepines appear to be effective in SAD, with studies in nondepressed individuals treated with clonazepam and alprazolam suggesting efficacy beginning within 2 weeks (3, 30, 31). In addition to rapid onset of effect relative to other classes of effective agents, benzodiazepines have a favorable side effect profile and the flexibility to be used on an as-needed basis for situational anxiety. In addition, they can be used to augment the efficacy of antidepressants for generalized SAD. Data from a double-blind, randomized, placebo-controlled study demonstrated that the addition of clonazepam to paroxetine improved outcome compared with the SSRI alone (32). Adverse effects associated with benzodiazepine administration include sedation, ataxia, and cognitive and psychomotor impairment; further, physiological dependence may develop with regular use. In addition, it is important to recognize that benzodiazepines as monotherapy are generally not effective for the depressive disorders that commonly present comorbidly with SAD and in fact may lead to worsened mood. The abuse liability of benzodiazepines is generally limited to those with a predisposing diathesis or history of alcohol or substance abuse, although given the relatively high rates of concurrent alcohol or substance abuse in SAD, their abuse potential should be taken into account when a treatment plan for comorbidly affected individuals is developed and alternative therapeutic interventions should be used when possible.
Other medications.
Although not subjected to extensive testing for this indication, the tricyclic antidepressants seem to lack efficacy for SAD (33), whereas there is evidence from a small open trial suggesting potential efficacy for bupropion (34) and a small controlled study with mirtazapine that showed positive results (35). The azapirone buspirone, a 5-HT1A partial agonist, has not demonstrated efficacy for SAD as a monotherapy, although it may be useful for augmentation in patients incompletely responsive to SSRI therapy (36). Although small studies suggest the potential efficacy of atypical antipsychotic drugs including olanzapine (37), risperidone (38), aripiprazole (39), and quetiapine (40), they have not been tested in large RCTs in SAD, and given concerns about associated metabolic syndrome, weight gain, and extrapyramidal effects, their use is best reserved for patients remaining symptomatic despite standard interventions.
The anticonvulsants gabapentin, an α2δ calcium channel antagonist, and a related compound pregabalin demonstrated efficacy for social phobia in RCTs (41, 42). An open trial of valproic acid suggested potential efficacy for SAD (43), whereas results in small studies with levetiracetam have been mixed (44, 45) and results of a larger RCT with that agent were negative (Stein MB, Ravindran L, Simon NM, Khan A, Liebowitz M, Brawman-Mintzer O, Lydiard RB, Pollack MH: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Assess the Efficacy and Safety of Levetiracetam for Treatment of Generalized Social Anxiety Disorder. Submitted for publication, 2008.).
COGNITIVE BEHAVIORAL THERAPY
Cognitive behavioral therapy (CBT) is typically a time-limited psychosocial intervention, administered either in individual or group settings, that has demonstrated clear efficacy for the treatment of SAD (46, 47). Typical components of CBT include psychoeducation, somatic management techniques such as muscle relaxation, in vivo and imaginal exposure, video feedback, cognitive restructuring, and social skills training (46, 48). CBT has demonstrated efficacy comparable to that of pharmacotherapy, with a slightly slower onset of therapeutic effect but greater persistence of benefit after treatment discontinuation (31, 49–52).
Although combined pharmacotherapy and CBT may be presumed to be more effective than either intervention administered individually, evidence from one large RCT with fluoxetine and CBT did not demonstrate a significant advantage for the combined intervention over each effective monotherapy (53). This finding suggests that it would be reasonable to initiate treatment with either intervention alone and consider adding the alternative intervention for individuals who do not show a satisfactory response to the single intervention, although there are few systematic data addressing this hypothesis.
Recently, another paradigm for combining pharmacological and CBT interventions has emerged, with the aim of using pharmacotherapy to enhance the effects of exposure-based treatment. This approach is based on translational research derived from preclinical work on the neural circuitry underlying fear extinction, demonstrating the importance of the N-methyl-d-aspartate (NMDA) receptor within the amygdala for fear extinction and the effect of NMDA partial agonists administered systemically or in the amygdala to facilitate extinction (54, 55). Subsequent work in humans demonstrated that administration of the antibiotic d-cycloserine (DCS), an NMDA receptor partial agonist, before a CBT session, enhanced its efficacy for acrophobia (56, 57). More recently, DCS demonstrated efficacy for the enhancement of CBT in the treatment of SAD as well (58). If these early findings are confirmed, DCS and other agents active at the NMDA receptor and glutamatergic system may be routinely administered to enhance the effectiveness of exposure-based treatment of SAD and other phobic disorders.
CONCLUSION
Over the last two to three decades, advances in our understanding of SAD have been spurred by the growing recognition of its prevalence, early onset, chronicity, and morbid impact. Although currently available pharmacotherapies and CBT are clearly effective for the treatment of SAD, many of those treated remain symptomatic or fail to respond at all, and there is a significant unmet need to discover ways to optimize the use of currently available interventions and to develop novel therapies. Translational research derived from growing understanding of the underlying neurobiology of fear-based disorder offers the promise of improving outcomes for the treatment of SAD.
1 Wittchen HU, Ustün TB, Kessler RC: Diagnosing mental disorders in the community. A difference that matters? Psychol Med 1999; 29:1021–1027Crossref, Google Scholar
2 Kessler RC Stein Mb, Berglund P: Social phobia subtypes in the National Comorbidity Survey. Am J Psychiatry 1998; 155:613–619Crossref, Google Scholar
3 Davidson JR, Potts N, Richichi E, Krishnan R, Ford SM, Smith R, Wilson WH: Treatment of social phobia with clonazepam and placebo. J Clin Psychopharmacol 1993; 13:423–428Crossref, Google Scholar
4 Stein MB, Kean YM: Disability and quality of life in social phobia: epidemiologic findings. Am J Psychiatry 2000; 157:1606–1613Crossref, Google Scholar
5 Magee WJ, Eaton WW, Wittchen HU, McGonagle KA, Kessler RC: Agoraphobia, simple phobia, and social phobia in the National Comorbidity Survey. Arch Gen Psychiatry 1996; 53:159–168Crossref, Google Scholar
6 Schneier FR, Johnson J, Hornig CD, Liebowitz MR, Weissman MM: Social phobia. Comorbidity and morbidity in an epidemiologic sample. Arch Gen Psychiatry 1992; 49:282–288Crossref, Google Scholar
7 Weiller EBP, Lepine JP, Lecrubier Y: Social phobia in general health care: an unrecognised undertreated disabling disorder. Int Clin Psychopharmacol 1996; 11(suppl. 3):25–28Crossref, Google Scholar
8 Katzelnick DJ, Kobak KA, DeLeire T, Henk HJ, Greist JH, Davidson JR, Schneier FR, Stein MB, Helstad CP: Impact of generalized social anxiety disorder in managed care. Am J Psychiatry 2001; 158:1999–2007Crossref, Google Scholar
9 Stein MB, Walker JR, Forde DR: Public speaking fears in a community sample: prevalence, impact on functioning, and diagnostic classification. Arch Gen Psychiatry 1996; 53:169–174Crossref, Google Scholar
10 Furmark T: Social phobia: overview of community surveys. Acta Psychiatr Scand 2002; 105:84–93Crossref, Google Scholar
11 Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE: Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602Crossref, Google Scholar
12 Narrow WE, Rae DS, Robins LN, Regier DA: Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys' estimates. Arch Gen Psychiatry 2002; 59:115–123Crossref, Google Scholar
13 Kessler RC, Stang P, Wittchen HU, Stein M, Walters EE: Lifetime co-morbidities between social phobia and mood disorders in the US National Comorbidity Survey. Psychol Med 1999; 29:555–567Crossref, Google Scholar
14 Stein MB, Gorman JM: Unmasking social anxiety disorder. J Psychiatry Neurosci 2001; 26:185–189Google Scholar
15 Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ: The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. Am J Orthopsychiatry 1996; 66:17–31Crossref, Google Scholar
16 Merikangas KR, Angst J: Comorbidity and social phobia: evidence from clinical, epidemiologic, and genetic studies. Eur Arch Psychiatry Clin Neurosci 1995; 244:297–303Crossref, Google Scholar
17 Simon NM, Otto MW, Wisniewski SR, Fossey M, Sagduyu K, Frank E, Sachs GS, Nierenberg AA, Thase ME, Pollack MH: Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry 2004; 161:2222–2229Crossref, Google Scholar
18 Crum RM, Pratt LA: Risk of heavy drinking and alcohol use disorders in social phobia: a prospective analysis. Am J Psychiatry 2001; 158:1693–1700Crossref, Google Scholar
19 Otto MW, Pollack MH, Sachs GS, O'Neil CA, Rosenbaum JF: Alcohol dependence in panic disorder patients. J Psychiatr Res 1992; 26:29–38Crossref, Google Scholar
20 Fava M: Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21Google Scholar
21 Walker JR, Van Ameringen MA, Swinson R, Bowen RC, Chokka PR, Goldner E, Johnston DC, Lavallie YJ, Nandy S, Pecknold JC, Hadrava V, Lane RM: Prevention of relapse in generalized social phobia: results of a 24-week study in responders to 20 weeks of sertraline treatment. J Clin Psychopharmacol 2000; 20:636–644Crossref, Google Scholar
22 Allgulander C, Nilsson B: A prospective study of 86 new patients with social anxiety disorder. Acta Psychiatr Scand 2001; 103:447–452Crossref, Google Scholar
23 Brantigan CO, Brantigan TA, Joseph N: Effect of beta blockade and beta stimulation on stage fright. Am J Med 1982; 72:88–94Crossref, Google Scholar
24 Gossard D, Dennis C, DeBusk RF: Use of beta-blocking agents to reduce the stress of presentation at an international cardiology meeting: results of a survey. Am J Cardiol 1984; 54:240–241Crossref, Google Scholar
25 Liebowitz MR, Schneier F, Campeas R, Hollander E, Hatterer J, Fyer A, Gorman J, Papp L, Davies S, Gully R: Phenelzine vs atenolol in social phobia: a placebo-controlled comparison. Arch Gen Psychiatry 1992; 49:290–300Crossref, Google Scholar
26 Martinez D, Broft A, Laruelle M: Pindolol augmentation of antidepressant treatment: recent contributions from brain imaging studies. Biol Psychiatry 2000; 48:844–853Crossref, Google Scholar
27 Hirschmann S, Dannon PN, Iancu I, Dolberg OT, Zohar J, Grunhaus L: Pindolol augmentation in patients with treatment-resistant panic disorder: a double-blind, placebo-controlled trial. J Clin Psychopharmacol 2000; 20:556–559Crossref, Google Scholar
28 Versiani M, Mundim FD, Nardi AE, Liebowitz MR: Tranylcypromine in social phobia. J Clin Psychopharmacol 1988; 8:279–283Crossref, Google Scholar
29 Welkowitz LA L, Liebowitz MR: Handbook of Anxiety. Elsevier, 1990Google Scholar
30 Gelernter CS, Uhde TW, Cimbolic P, Arnkoff DB, Vittone BJ, Tancer ME, Bartko JJ: Cognitive-behavioral and pharmacological treatments of social phobia: a controlled study. Arch Gen Psychiatry 1991; 48:938–945Crossref, Google Scholar
31 Otto MW, Pollack MH, Gould RA, Worthington JJ 3rd, McArdle ET, Rosenbaum JF: A comparison of the efficacy of clonazepam and cognitive-behavioral group therapy for the treatment of social phobia. J Anxiety Disord 2000; 14:345–358Crossref, Google Scholar
32 Seedat S, Stein MB: Double-blind, placebo-controlled assessment of combined clonazepam with paroxetine compared with paroxetine monotherapy for generalized social anxiety disorder. J Clin Psychiatry 2004; 65:244–248Google Scholar
33 Emmanuel NP, Johnson M, Villareal G: Imipramine in the treatment of social phobia: a double-blind study. Presented at the 36 meeting of the American College of Neuropsychopharmacology, Waikoloa, Hawaii, 1997Google Scholar
34 Emmanuel NP, Brawman-Mintzer O, Morton WA, Book SW, Johnson MR, Lorberbaum JP, Ballenger JC, Lydiard RB: Bupropion-SR in treatment of social phobia. Depress Anxiety 2000; 12:111–113Crossref, Google Scholar
35 Muehlbacher M, Nickel MK, Nickel C, Kettler C, Lahmann C, Pedrosa Gil F, Leiberich PK, Rother N, Bachler E, Fartacek R, Kaplan P, Tritt K, Mitterlehner F, Anvar J, Rother WK, Loew TH, Egger C: Mirtazapine treatment of social phobia in women: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 2005; 25:580–583Crossref, Google Scholar
36 Van Ameringen M, Mancini C, Wilson C: Buspirone augmentation of selective serotonin reuptake inhibitors (SSRIs) in social phobia. J Affect Disord 1996; 39:115–121Crossref, Google Scholar
37 Barnett SD, Kramer ML, Casat CD, Connor KM, Davidson JR: Efficacy of olanzapine in social anxiety disorder: a pilot study. J Psychopharmacol 2002; 16:365–368Crossref, Google Scholar
38 Simon NM, Hoge EA, Fischmann D, Worthington JJ, Christian KM, Kinrys G, Pollack MH: An open-label trial of risperidone augmentation for refractory anxiety disorders. J Clin Psychiatry 2006; 67:381–385Crossref, Google Scholar
39 Worthington JJ 3rd, Kinrys G, Wygant LE, Pollack MH: Aripiprazole as an augmentor of selective serotonin reuptake inhibitors in depression and anxiety disorder patients. Int Clin Psychopharmacol 2005; 20:9–11Crossref, Google Scholar
40 Schutters SI, van Megen HJ, Westenberg HG: Efficacy of quetiapine in generalized social anxiety disorder: results from an open-label study. J Clin Psychiatry 2005; v66:540–542Crossref, Google Scholar
41 Pande AC, Davidson JR, Jefferson JW, Janney CA, Katzelnick DJ, Weisler RH, Greist JH, Sutherland SM: Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999; 19:341–348Crossref, Google Scholar
42 Pande AC, Feltner DE, Jefferson JW, Davidson JR, Pollack M, Stein MB, Lydiard RB, Futterer R, Robinson P, Slomkowski M, DuBoff E, Phelps M, Janney CA, Werth JL: Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol 2004; 24:141–149Crossref, Google Scholar
43 Kinrys G, Pollack MH, Simon NM, Worthington JJ, Nardi AE, Versiani M: Valproic acid for the treatment of social anxiety disorder. Int Clin Psychopharmacol 2003; 18:169–172Google Scholar
44 Simon NM, Worthington JJ, Doyle AC, Hoge EA, Kinrys G, Fischmann D, Link N, Pollack MH: An open-label study of levetiracetam for the treatment of social anxiety disorder. J Clin Psychiatry 2004; 65:1219–1222Crossref, Google Scholar
45 Zhang W, Connor KM, Davidson JR: Levetiracetam in social phobia: a placebo controlled pilot study. J Psychopharmacol 2005; 19:551–553Crossref, Google Scholar
46 Heimberg, RG, Jester, HR: Cognitive behavioral treatments: literature review, in Social Phobia: Diagnosis, Assessment and Treatment. Edited by Heimberg, RG, Liebowitz, MR, Hope, DA, Schneier, FR. New York, Guilford Press, 1995Google Scholar
47 Heimberg RG: Cognitive-behavioral therapy for social anxiety disorder: current status and future directions. Biol Psychiatry 2002; 51:101–108Crossref, Google Scholar
48 Gould RA, Pollack MH, Yap L: Cognitive-behavioral and pharmacological treatment for social phobia: a meta-analysis. Clin Psychol Sci Pract 1997; 4:291–306Crossref, Google Scholar
49 Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, Holt CS, Welkowitz LA, Juster HR, Campeas R, Bruch MA, Cloitre M, Fallon B, Klein DF: Cognitive behavioral group therapy vs phenelzine therapy for social phobia: 12-week outcome. Arch Gen Psychiatry 1998; 55:1133–1141Crossref, Google Scholar
50 Liebowitz MR, Heimberg RG, Schneier FR, Hope DA, Davies S, Holt CS, Goetz D, Juster HR, Lin SH, Bruch MA, Marshall RD, Klein DF: Cognitive-behavioral group therapy versus phenelzine in social phobia: long-term outcome. Depress Anxiety 1999; 10:89–98Crossref, Google Scholar
51 Heimberg RG: Specific issues in the cognitive-behavioral treatment of social phobia. J Clin Psychiatry 1993; 54(suppl):36–45Google Scholar
52 Taylor S: Meta-analysis of cognitive-behavioral treatments for social phobia. J Behav Ther Exp Psychiatry 1996; 27:1–9Crossref, Google Scholar
53 Foa EB, Franklin ME, Moser J: Context in the clinic: how well do cognitive-behavioral therapies and medications work in combination? Biol Psychiatry 2002; 52:987–997Crossref, Google Scholar
54 Davis M: Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: clinical implications for exposure therapy. Eur J Neurosci 2002; 16:395–398Crossref, Google Scholar
55 Ledgerwood L, Richardson R, Cranney J: Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci 2003; 117:341–349Crossref, Google Scholar
56 Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M: Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004; 61:1136–1144Crossref, Google Scholar
57 Davis M, Ressler K, Rothbaum BO, Richardson R: Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry 2006; 60:369–375Crossref, Google Scholar
58 Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW: Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry 2006; 63:298–304Crossref, Google Scholar



