The Neurobiological and Therapeutic Intersection of Pain and Affective Disorders
Abstract
The majority of patients suffering from chronic pain have comorbid mood or anxiety disorders. This is not surprising as there is an affective component to all pain, and the chronic stress and social disruption caused by unrelenting pain erodes the individual’s ability to maintain a sense of identity, social context, and physical and emotional stability. Conversely, patients with chronic affective disorders develop an alarmingly high incidence of chronic pain. Regardless of primacy, when each disorder is present, both should be identified and treated. Anatomical, neurochemical, and neurophysiological commonalities between chronic pain and chronic affective disorders lead to striking overlap of medication treatments used with each group of disorders. Moreover, in treating both types of disorders, sophisticated polypharmacology is often the rule not the exception. With so much biopsychosocial complexity and overlap in mechanisms and treatments, the well-trained psychiatrist is ideally positioned to provide care for patients with chronic pain disorders.
Clinical context
Since their respective beginnings, psychiatry and pain medicine have been closely linked. For instance Sigmund Freud was the first physician to publish on the local anesthetic properties of cocaine, whereas anesthesiologist Henry K. Beecher provided early evidence to support the placebo effect, one of the most profound psychological constructs in medicine. Both fields have embraced the biopsychosocial approach to medical education and care, pioneered by George Engel (1).
Chronic pain disorders and psychiatric disorders share a commonality from both descriptive phenomenology and underlying biology. Chronic pain is not merely acute pain that lasts for some predetermined time, just as major depression and generalized anxiety do not represent protracted sadness or fear. Chronic pain is described in terms of being excessive, relentless, and unbearable. It is often shown on the face and in the body habitus of its sufferer. Too often it disrupts psychological and physical functioning, potentially leading to self-destructive thoughts and even suicide. Such descriptors sound familiar to psychiatrists because one could easily substitute the words “depression” or “anxiety” for “chronic pain.” Whereas many patients with chronic pain exhibit mood and anxiety disturbances, the converse is also true (2). Biologically, pain, mood, and anxiety disorders overlap in the limbic, hypothalamic, prefrontal cortex, locus ceruleus, and spinal centers (3). Loss of neurochemical homeostasis is a hallmark of these disorders that results in or is the result of maladaptive neuroplasticity (4, 5). It remains unclear whether the observation of chronic pain and chronic affective disorders frequently occurring simultaneously reflects comorbidity of distinct disease states or expression of the same underlying biological disease. Most likely this phenomenon is the result of both.
There is a high incidence of chronic pain in the general population. Estimates suggest that about one in every four to six persons has chronic pain (6–9). There is increasing evidence that psychological disorders such as depression or anxiety often coexist and may be correlated with chronic pain (Table 1). One study found that patients in chronic pain meet the criteria for an anxiety disorder at almost twice the rate of the general population (35% versus 18%) (10). The number of patients with chronic pain and comorbid depression is even more impressive. Almost two thirds of patients with persistent pain have a lifetime history of major depression. In untreated and treatment-resistant depression the number of patients who exhibit chronic pain is between 30% and 50% (11–13).
Pain is a complex physical and emotional experience that is unpleasant and may represent actual or potential tissue damage (14). Price (15) pointed out that acute pain engenders the emotional responses of fear and anger, whereas chronic pain is more likely to elicit despair and hopelessness. In acute pain the brain and spinal cord collaborate to perceive the sensation, react to minimize the potential damage, and dampen the actual experience. With chronic pain, these built-in mechanisms fail through a process of excessive neuronal activation in the spinal cord and brain, in part due to a phenomenon known as wind-up. This results in an amplified perception of pain relative to evoked peripheral stimuli and impaired down-regulation of pain via normal descending modulatory mechanisms in the CNS. The concurrent emotional response is also out of proportion, and the pain and emotional systems form an unfortunate positive feedback loop that escalates and perpetuates the pain disorder.
The neuroanatomy involved in chronic pain demonstrates substantial overlap in systems subserving pain and emotion. Pain processing in the brain occurs via the spinothalamic tracts and occurs in multiple deep brain and cerebral areas. Thalamic relays to hypothalamic and limbic systems pass further upstream to the prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex, insula, posterior parietal complex, somatosensory cortex, and supplementary motor cortex. This results in a broad response to pain including emotional, neuroendocrine, arousal, autonomic, and somatosensory responses (16).
Psychiatrists have long known that the limbic system is only a part of the overall emotional response system. The emotional response to pain involves the ascending reticular activating system, which sends fibers to the frontal cortex, limbic system, hypothalamus, cerebellum, and dorsal horn of the spinal cord (17). Anatomically the pain-processing areas of the brain include the same areas seen in mood and anxiety disorders (18) (Figures 1, A and B).
What is the difference between acute and chronic pain? There is no one answer to this important question. However, answers may be found through comparing the differential physiological and cellular molecular biological characteristics of acute versus chronic pain.
In acute pain, a signal generated at peripheral nociceptors (specialized nerve receptor endings embedded throughout peripheral tissues) is sent to the CNS via nerves that end at the spinal cord. Synaptic processes pass the signal to second-order neurons in the dorsal horn of the spinal cord, which pass the signal to third-order neurons in the dorsal horn. These cross to the contralateral side of the spinal cord and travel via nerve tracts to the brain, where the signal diffuses to multiple areas evoking a varied response. The brain sends a counter signal to the dorsal horn and suppresses the incoming pain signal (19).
In chronic pain, these processes break down peripherally and centrally, resulting in problems ranging from increased peripheral activation, release of increased neurotransmitters, activation of normally inactive receptors leading to amplified signaling (termed wind-up), and abnormal neuroplastic reorganization. The CNS becomes sensitized as amplified signals are sent to the same brain centers as in the acute state, but subsequent regulation from the brain is frustrated by maladaptive sensory processes, such as those that wind up at the dorsal horn. The end result is an experience of pain unpleasantness that is more robust and enduring than the peripheral stimulus, which sustains itself independently of ongoing peripheral input. Unlike the normal process in which pain is a symptom of a normally activated alarm that registers harm, potential harm, or ongoing healing in the body, this pain is evidence of an injured alarm system. Therefore, chronic pain is usually not just a symptom but is the manifestation of a chronic disease process resulting from runaway neuroplasticity (20).
The neuroplasticity and loss of neural regulatory control seen in pain, mood, and anxiety have striking overlap. γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter that regulates sleep, pain, anxiety, and seizures. Chronic pain results in loss of GABAergic control, leading to disproportionate excitation of the CNS mediated by unleashed glutamate (21). Substance P is excessively released at presynaptic terminals due to inflammation, pain, emesis, depression, and anxiety (22). Activation of N-methyl-d-aspartic acid (NMDA) receptors is currently considered to be a central event in the neuroplasticity of pain (23) and affective disorders (24). In either case, normally dormant postsynaptic NMDA receptor complexes become activated by excessive peripheral stimuli (release of high levels of Substance P and glutamate in pain and high circulating serum cortisol levels in affective disorders). As glutamate attaches to internal sites, excessive calcium flows through the activated NMDA receptor complexes into the postsynaptic nerve cell, leading to threshold changes at the cell membrane and chemical alteration within the cell (25). Malfunction of serotonin and norepinephrine centers in the brainstem may result in depression and anxiety in patients with pain (26) and pain in depressed and anxious patients (2). All of these changes result in decreased brain-derived neurotrophic factor, which, in the face of excitatory CNS tone, is postulated to result in nerve cell death and loss of dendritic arborization (24).
Treatment and evidence
Assessing and treating mood disorders and pain simultaneously is the best strategy for improved outcomes and often involves an approach of rational polypharmacology (27). It is notable that the pharmacopeia for the disciplines of pain medicine and psychiatry have major overlap. Figure 2 reveals that most major drug groups used in psychiatry are also used as analgesics. Whereas some analgesics used for treating chronic pain work on peripheral pain targets, most have mechanisms of action in the CNS. The major classes of medication to treat chronic pain include opioids, antiinflammatory agents, antidepressants, antiepileptic drugs, and antiarrhythmic agents. The use of many antidepressant, antiepileptic, and antiarrhythmic drugs for neuropathic pain may be viewed as use of a single drug class, as agents in each of these drug groups possess membrane stabilization properties such as those seen in local anesthetics. The use of tricyclic antidepressants (TCAs) as part of this group may be perplexing to some until their sodium channel blockade properties are considered. This sodium channel blockade is identical to the properties of local anesthetics and almost certainly contributes to the neuropathic analgesic effects of TCAs as well as to their potential cardiotoxicity. Drugs such as venlafaxine and duloxetine appear to reduce neuropathic pain independent of their antidepressant effects. However, unlike all of the other drugs mentioned above, these do not appear to be membrane stabilizers and their exact mechanism of neuropathic analgesia is not clear.
In patients with comorbid pain and affective disorder, it is often impossible to determine which is primary and which is secondary. The overlap of the pharmacopeia for pain and psychiatric disorders is the result of the many commonalities of the underlying neurochemistry (Figure 2). Some pharmacological effects that are beneficial for analgesia may be viewed as side effects of the medications when they are used for psychiatric purposes, such as TCA-related sodium channel blockade or sedation induced by mirtazapine. Others are direct therapeutic benefits, but on alternate pathways than those seen for normal treatment, such as serotonergic and noradrenergic effects of serotonin-norepinephrine reuptake inhibitors (SNRIs) or TCAs on the dorsal horn of the spinal cord to regulate pain. Still other effects reflect a shared response for pain and affective disorders in the brain itself, for example, the calcium channel-blocking effects of lamotrigine. For these reasons and others, treating the affective component of patients with chronic pain usually reduces pain and treating the painful component of patients with affective disorders often reduces mood and anxiety disturbances.
Table 2 highlights medications used for pain with strong psychotropic effects. Opioids are included in this group, with their major psychotropic actions being undesirable. The medications are reviewed by their class, mechanisms of action, site of action, therapeutic effects, and side effects. Although not an exhaustive list, this list can be used as a reference when one is considering monotherapy and polypharmacology for treating comorbid pain and affective disorders. Although it provides a compendium of drugs, this table does not cover all medications available for either pain or psychiatric treatment. The art of treating these pain and psychiatric disorders is in the selection and combination of agents that maximize efficacy, while minimizing side effects and drug interactions.
Questions and controversies
Most major psychiatric drug groups are also used for analgesia (Figure 1). This confluence of therapeutic effect is unlikely to be a coincidence; the algesic effect of major affective disorders and the analgesic effects of treating these conditions would predict this overlap. New medications, such as pregabalin and duloxetine, hold promise as single agents to treat disorders that comprise anxiety, depression, and pain as comorbidities (28).
For a drug to be directly analgesic, the analgesia must be independent of the drug’s other psychiatric effects. The classic examples are the TCAs that, with their sodium channel blockade properties, are demonstrably analgesic, whereas selective serotonin reuptake inhibitors (SSRIs) have not convincingly been shown to be better than placebo for neuropathic pain. Nonetheless, many patients whose depression improves from SSRI therapy also report a decrease in pain. What is it about the SNRIs duloxetine and venlafaxine that make them analgesic independent of their antidepressant action? Perhaps this efficacy has to do with the SNRI norepinephrine blockade that usually occurs only at higher doses than serotonin blockade, and may be predictable by the mechanism of action of SNRIs, despite their lacking the sodium channel-blocking effects of tricyclic antidepressants. On the other hand, there have been colossal failures in both pain medicine and psychiatry when clinical studies have attempted to predict efficacy based on mechanism of action. For instance, recently tested Substance P inhibitors failed as antidepressants and analgesics.
In treating a patient with an affective disorder and pain, there is a natural tendency to want to treat with monotherapy. This often does not work, because dosing for the affective disorder and pain often requires different titrations. When dealing with patients with comorbid conditions, practitioners need to recognize that patients are in an amplified state. Affective symptoms, as well as side effects, are going to be even less well tolerated. For instance, in treating a patient who has postherpetic neuralgia and depression, a low-dose TCA may be effective for pain relief, but treatment of the depression may require a much higher dose that may be accompanied by unacceptable side effects. Use of a TCA at low dose or an anticonvulsant for pain and separate antidepressant dosing of an SSRI for mood may result in greater efficacy and fewer adverse effects.
Although the analgesics with the broadest efficacy are opioids, their use for treating chronic pain remains controversial. Opiophobia remains a reality for many physicians, although this is slowly yielding to a more balanced use of drugs in this therapeutic class. Opioids are useful in long- and short-acting forms, are delivered via every route of administration, treat nociceptive and neuropathic pain, have few drug interactions, and are relatively safe if treatment is properly supervised. They are, however, highly abused and diverted for profit, leading to their heightened state of regulation. Such need for pharmacovigilance, as well as their stigma as abusable drugs is too often seen as a reason to avoid using opioids. Instead less regulated or scrutinized drugs are frequently substituted, despite less efficacy or greater risk.
Recommendations
Although pain medicine and psychiatry share common historical routes, underlying pathology, and pharmacological approaches, there are relatively few psychiatrists who are also pain specialists. Both specialties are steeped in neuroscience and embrace the biopsychosocial model of care. The long-term effect of pain is often deterioration of mood and unrelenting affective disorders that frequently amplify the pain. When the conditions present simultaneously, treating both should be considered the best practice. Effective care of either condition requires understanding of the underlying relevant pharmacology and primary and secondary psychological and social impact. Psychiatrists are uniquely qualified to accomplish this task.
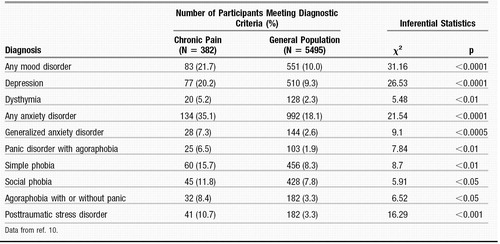 |
Table 1. Prevalence of Past-Year DSM-III-R Diagnosis in Patients with Chronic Pain
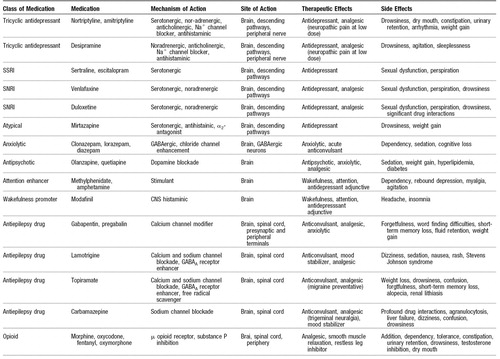 |
Table 2. Medications for Treating Pain and Affective Disorders
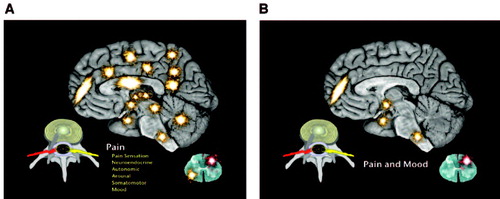
Figure 1. A) Pathways Involved in Chronic Pain Showing the Experience of Pain as More Than Induction of Brain-Based Sensation, but Also Including Neuroendocrine, Autonomic, Arousal, Sensorimotor and Mood Centers and Pathways. B) Overlap of Pain and Mood Centers in the Limbic System, Hypothalamus, Prefrontal Cortex, Locus Ceruleus of the Brain and Dorsal Horn of the Spinal Cord.
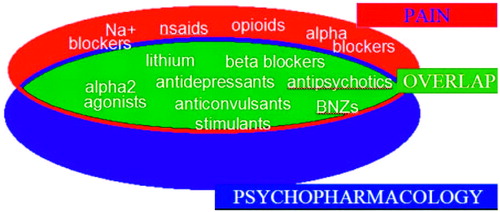
Figure 2. Overlap of Major Drug Groups Used in Pain Medicine and Psychiatry
Note: BNZs, benzodiazepines; nsaids, nonsteroidal antiinflammatory drugs.
CME Disclosure Michael Moskowitz, M.D., M.P.H.; Assistant Professor of Anesthesiology and Pain Medicine, School of Medicine, University of California, Davis, Sacramento, CA; Psychiatrist and Pain Medicine Specialist, Bay Area Pain Medical Associates, Mill Valley, CA. No significant financial conflict of interest or affiliation to report. Scott Fishman, M.D.; Professor, University of California Davis, School of Medicine. Consultant, Speakers Bureau, Grants/Research Support: Elan Co, Endo Pharmaceuticals, Janssen Medical Affairs, lic, Merck, Pfizer, Purdue Pharma, Lilly. Consultant: Cephalon.
1 Engel GL: The clinical application of the biopsychosocial model Am J Psychiatry 1980; 137:535–544Crossref, Google Scholar
2 Stahl SM: Does depression hurt? J Clin Psychiatry 2002; 63:273–274Crossref, Google Scholar
3 Price D: Psychological Mechanisms of Pain and Analgesia. Seattle, IASP Press, 1999, pp 9-71-153Google Scholar
4 Melzack R: Toward a new concept of pain for the new millennium, in Interventional Pain Management, 2nd ed. Edited by Waldman SD. Philadelphia, WB Saunders, Philadelphia, 2001, pp 1–20Google Scholar
5 Stahl SM: The psychopharmacology of painful physical symptoms in depression. J Clin Psychiatry 2002; 63:382–383Crossref, Google Scholar
6 Mantyselka P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamaki H, Halonen P, Takala J: Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain 2001; 89:175–180Crossref, Google Scholar
7 Fishman SM: The War on Pain. New York: Harper Collins, 2001Google Scholar
8 Harstall C, Ospina M: Pain. Clin Updates 2003; XI(2).Google Scholar
9 ABC News/USA Today/Stanford Medical Center poll. Available at: http://abcnews.go.com/images/Politics/979a1TheFightAgainstPain.pdf. Accessed April 4, 2006Google Scholar
10 McWilliams LA, Cox BJ, Enns MW: Mood and anxiety associated with chronic pain: an examination in a nationally representative sample. Pain 2003; 106:127–133Crossref, Google Scholar
11 Katon W, Von Korff M, Lin E, Lipscomb P, Russo J, Wagner E, Polk E: Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990; 12:355–362Crossref, Google Scholar
12 Kroenke K, Mangelsdorff AD: Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Am J Med 1989; 86:262–266Crossref, Google Scholar
13 Kroenke K, Spitzer RL, Williams JB, Linzer M, Hahn SR, deGruy FV, Brody D: Physical symptoms in primary care: predictors of psychiatric disorders and functional impairment. Arch Fam Med 1994; 3:774–779Crossref, Google Scholar
14 Mersky H, Bogduk N: Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed. Seattle, IASP Press, 1994, p 210Google Scholar
15 Price D: Psychological Mechanisms of Pain and Analgesia. Seattle, IASP Press, 1999, pp 9–10Google Scholar
16 Price D: Psychological Mechanisms of Pain and Analgesia. Seattle, IASP Press, 1999, pp 71–153Google Scholar
17 Siegal GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD (eds): Basic neurochemistry: Molecular, cellular and medical aspects, 6th ed. Philadelphia, Lippincott, Williams & Wilkins, 1999, pp 308–309Google Scholar
18 Yaksh T: Anatomy of the pain-processing system, in International Pain Management, 2nd ed. Edited by Waldman S. Philadelphia, WB Saunders, 2001, pp 11–20Google Scholar
19 Price D: Psychological Mechanisms of Pain and Analgesia. Seattle, IASP Press, 1999, pp 48–135Google Scholar
20 Borsook D (ed): Molecular Neurobiology of Pain. Seattle, IASP Press, 1997, pp 221–304Google Scholar
21 Roberts E: Adventures with GABA: Fifty Years On, in GABA in the nervous system. Edited by Martin D, Oslen R. Philadelphia, Lippincott, Williams & Wilkins, Philadelphia, 2000, pp 1–24Google Scholar
22 Mantyh PW: Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry 2002; 63(Suppl 11):6–10Google Scholar
23 Wiesenfeld-Hallin Z, Alster P, Grass S, Hoffman O, de Araújo Lucus G, Plesan A, Xu X-J: Opioid sensitivity in antinociception: role of anti-opioid systems with emphasis on cholecystokinin and NMDA receptors, in Opioid Sensitivity of Chronic Non-Cancer Pain. Edited by Kalso E, McQuay HJ, Wiesenfeld-Hallin Z. Seattle, IASP Press, 1999, pp 237–252Google Scholar
24 Mathew SJ, Coplan JD, Schoepp DD, Smith EL, Rosenblum LA, Gorman JM: Glutamate-hypothalamic-pituitary adrenal axis interactions: implications for mood and anxiety disorders. CNS Spectr 2001; 6:555–556, 561–564Crossref, Google Scholar
25 Cooper J, Bloom F, Roth R: The Biochemical Basis of Neuropharmacology. Oxford, Oxford University Press, 2003, pp 105–150Google Scholar
26 Yaksh T: Alpha-2-agonists as analgesics, in Novel Aspects of Pain Management: Opioids and Beyond. Edited by Sawynok J, Cowan A. New York, Wiley-Liss, 1999, pp 179–202Google Scholar
27 Moskowitz MH: Pharmacotherapy of neuropathic low back pain. Curr Pain Headache Rep 2003; 7:78–87Crossref, Google Scholar
28 Stahl SM: Anticonvulsants and Anxiolytics. Part 2. J Clin Psychiatry 2004; 65:460–461Crossref, Google Scholar



