Obstructive Sleep Apnea
| Clinical Principles Obstructive sleep apnea is an increasingly common disorder that is strongly linked to obesity. Neurocognitive sequelae, such as daytime sleepiness and impaired executive function, are important factors in motor vehicle accidents and probably contribute to loss of work-related productivity. Emerging clinical research studies suggest an increasing role of obstructive sleep anpnea in cardiovascular disease, particularly systemic hypertension and congestive heart failure. Continuous positive airway pressure (CPAP) is very effective at reversing sleep-related disorderd breathing events and consequent daytime symptoms, although maintaining patient adherence can be challenging. The role of CPAP and other modes of therapy in the management of systemic disease that coexists with obstructive sleep apnea is promising but remains to be determined by rigorous interventional controlled trials. | Pathophysiologic Principles Obstructive sleep apnea is associated with a reduced-caliber upper airway, which, despite an increase in compensatory pharyngeal dilator muscle electromyographic output, is vulnerable to further narrowing or collapse. Acute and repetitive effects of apneas and hypopneas include oxygen desaturation, reductions in intrathoracic pressure, and central nervous system arousals, all of which may contribute to the cardiovascular diseases that frequently coexist with obstructive sleep apnea. Disruption of neural circulatory control in obstructive sleep apnea is demonstrated by heightened peripheral chemoreflex sensitivity and sympathetic overactivity during both sleep and wakefulness. Metabolic abnormalities in obstructive sleep apnea appear to be mediated in part by insulin resistance, which may be independent of body weight, and by the dysregulation of leptin. |
Along with an increasingly sophisticated scientific approach to obstructive sleep apnea has come controversy over its true definition. By convention, the apnea–hypopnea index has been used to characterize obstructive sleep apnea. This index measures the frequency of reductions in airflow associated with upper-airway collapse or narrowing that occurs with the state change from wakefulness to sleep. The term sleep-disordered breathing encompasses these phenomena. However, a rigid diagnostic standard has not been applied to the apnea–hypopnea index. As a result, its definition differs among sleep laboratories and in the medical literature. A consensus statement was developed over 5 years ago in an attempt to standardize these criteria in human research (1), and although many sleep laboratories have adopted these recommendations, diagnostic variability remains. According to this statement, an apnea involves upper-airway collapse and is defined as nearly complete cessation of airflow associated with oxygen desaturation or an arousal from sleep. Hypopneas, which are associated with partial collapse of the upper airway, should be considered as existing on a pathologic continuum with apneas and may be clinically more important because they may make up the majority of disordered breathing events in a given night (2).
The apnea–hypopnea index measures the frequency of disordered breathing events but does not quantify other processes that may be operative in the pathophysiology of obstructive sleep apnea, such as the degree of oxygen desaturation. Moreover, the total number of arousals, some of which may occur in the absence of frank breathing abnormalities, may be a superior marker of sleep fragmentation than is the apnea–hypopnea index and may better explain daytime sleepiness (3). Nevertheless, no other metric has proven to be superior to the apnea–hypopnea index in assessing the overall effect of obstructive sleep apnea, even though it may prove to be inappropriate for characterizing obstructive sleep apnea in specific subsets of patients. For example, it may be that the frequency of arousal (perhaps independent of oxygen desaturation) has the greatest effect on daytime function. Or, perhaps the severity or duration of hypoxemic episodes will be found to have the greatest effect in terms of cardiovascular sequelae.
The obstructive sleep apnea syndrome is defined as sleep-disordered breathing associated with daytime symptoms, most often excessive sleepiness. As accumulating data implicate obstructive sleep apnea in the pathogenesis of systemic disease, particularly in the pathophysiology of cardiovascular disease, the distinction between sleep-disordered breathing and the obstructive sleep apnea syndrome has become increasingly blurred.
Epidemiology
Inconsistencies in definitions of disease and sampling biases contribute to the wide range of prevalence of obstructive sleep apnea reported in the literature. The best evidence of the pervasiveness of obstructive sleep apnea derives from pooled data from 4 large prevalence studies that used similar in-laboratory monitoring, diagnostic criteria, and sampling methods. On the basis of these data, it is estimated that 1 of 5 white adults with an average body mass index of 25 to 28 kg/m2 has an apnea–hypopnea index of 5 or greater (at least “mild disease”) and 1 of 15 has an apnea–hypopnea index of 15 or greater (at least “moderate disease”) (4–8). Up to 5% of adults in western countries probably have the obstructive sleep apnea syndrome (7). Data on the natural history of sleep-disordered breathing from the Wisconsin Sleep Cohort suggest that factors important in progression of disease include baseline obesity, older age, and the presence of snoring (7) (Figure 1).
Longitudinal data from the Wisconsin Sleep Cohort show that among persons with mild obstructive sleep apnea (apnea–hypopnea index, 5 to 15 ) at baseline, a 10% increase in body weight leads to a 6-fold risk for developing moderate or severe obstructive sleep apnea and a 1% change in body weight predicts a concordant 3% change in the apnea–hypopnea index (9). The mechanism underlying the risk imparted by obesity is thought to be related, at least in part, to airway narrowing as a result of excess regional soft tissue. The association between neck circumference and the apnea–hypopnea index supports this theory (10).
Men have a higher risk for obstructive sleep apnea than do women. The reason for this is not entirely clear, but hormonal influences may offer a partial explanation. Postmenopausal women are at higher risk for obstructive sleep apnea than are premenopausal women (7), an effect that hormone replacement therapy may ameliorate (11).
The association between age and obstructive sleep apnea is complex. Several studies have shown a higher prevalence of obstructive sleep apnea in elderly persons compared with middle-aged persons, although daytime symptoms may be less common with advancing age (12). The Sleep Heart Health Study demonstrated that the influence of male sex and body mass index on obstructive sleep apnea tends to wane with age. For unclear reasons, the overall prevalence of obstructive sleep apnea plateaus after 65 years of age (13).
Data are limited on the occurrence of sleep apnea in nonwhite populations. The prevalence among African-American persons, after adjustment for body mass index, seems to be at least equal to and may exceed that among white persons (14). The prevalence among men in urban India and men and women in Korea is similar to that observed in western countries (15, 16).
Clinical presentation
The cardinal symptom of obstructive sleep apnea is excessive daytime sleepiness. It can be difficult to quantify because patients may use varied adjectives to describe sleepiness, and they may confuse sleepiness with fatigue. The challenge for the clinician is to identify sleepiness that warrants further evaluation. Subjective tools, such as the Epworth Sleepiness Scale, are quick and simple to complete but do not correlate well with the severity of the apnea–hypopnea index. It may be the mere presence of sleepiness rather than its quantification that is critical because obstructive sleep apnea correlates with motor vehicle accidents, the risk for which is related to the severity of the apnea–hypopnea index in some (17), but not all (18), studies.
Excessive daytime sleepiness appears to result from sleep fragmentation related to recurrent central nervous system arousals in response to disordered breathing events. However, the relationship among these events may be more complex. Experimental disruption of sleep, as manifested by transient blood pressure or heart rate elevations without visible electroencephalographic alterations that traditionally indicate arousal (so-called autonomic arousals), produces evidence of sleepiness on subsequent objective testing (19). Moreover, although the severity of the apnea–hypopnea index appears to correlate with the degree of subjective sleepiness (20), many people with an apnea–hypopnea index greater than 5 do not report daytime symptoms. Perception and reporting of daytime sleepiness seem to vary greatly among individuals. When a validated sleep questionnaire was used, a subgroup analysis of the Sleep Heart Health Study showed that 35% of persons with severe obstructive sleep apnea (apnea–hypopnea index ≥ 30) reported sleepiness, as did 21% of those without clinical obstructive sleep apnea (apnea–hypopnea index < 5) (20).
Obstructive sleep apnea is strongly associated with snoring. Notwithstanding the limitations of quantifying this subjective finding, data from the Sleep Heart Health Study suggest that snoring is associated with daytime sleepiness independently of the severity of the apnea–hypopnea index (21).
Diagnosis
Several prediction rules have been devised to aid the clinician in determining the pretest probability of obstructive sleep apnea. Such information may allow more efficient use of bed space in sleep laboratories. Validated tools include morphometric examination of the head and neck, which has become more simple and time efficient (22). Patient questionnaires may perform more powerfully when objective data (particularly anthropometric variables and presence or absence of hypertension) are used along with subjective reporting (23).
Attended, in-laboratory polysomnography is considered the gold standard in the diagnosis of obstructive sleep apnea. Interlaboratory variation in hardware and assessment methods, however, underscores the need for standardization of criteria to allow comparisons with other emerging diagnostic strategies, such as unattended home monitoring.
Nocturnal pulse oximetry is widely used to screen for obstructive sleep apnea, and some data suggest that it may reduce the need for diagnostic polysomnography in certain circumstances (24). However, at present, the problems of cost-effectiveness and constraints on bed space in sleep laboratories remain. If the index of suspicion is high, negative findings on pulse oximetry will require confirmatory polysomnography, and positive findings on oximetry studies will require in-laboratory titration of continuous positive airway pressure (CPAP).
Cardiorespiratory physiology of sleep and obstructive sleep apnea
Neural circulatory control during normal sleep
Normal sleep is broadly characterized as rapid eye movement (REM) sleep, which occurs cyclically and more frequently during the second half of the night, and non-REM sleep, which is subclassified into stages 1 through 4 and constitutes the bulk of sleep time. As non-REM sleep progresses, heart rate, blood pressure, and sympathetic nerve activity (as measured by direct intra-neural recordings) decrease (25). Conversely, REM sleep, which is characterized by increased electrical activity in the brain and sympathetic nerve outflow that can exceed that encountered during relaxed wakefulness, is associated with intermittent and abrupt changes in blood pressure and heart rate (25). Thus, during normal sleep, stage-dependent modulation of cardiac and vascular physiologic processes occurs. In obstructive sleep apnea, this homeostatic control is severely disrupted.
Airway control in normal sleep and in obstructive sleep apnea
Obstructive sleep apnea is almost exclusively a disease of humans. An exception is the brachycephalic English bulldog, which has been used as a model of upper-airway obstruction during sleep (26). Observations in this animal model may be applicable to patients with such craniofacial abnormalities as macroglossia, retrognathia, and acromegaly, which are known to predispose to sleep-disordered breathing. The teleologic basis for species selectivity has been theorized to be acquisition of speech, which is necessarily associated with a more compliant upper airway, as demonstrated in the lack of rigid support of the hyoid bone that is specific to humans (27). That identifiable craniofacial abnormalities account for a minority of patients with obstructive sleep apnea suggests that other pathophysiologic mechanisms are also active.
The inherent collapsibility imparted by the structure of the upper airway is demonstrated by the observation that sleep onset in healthy persons is associated with increased airway resistance (28) and radiographic evidence of upper-airway narrowing (29). During normal sleep, various protective mechanisms maintain partial patency of the upper airway. Tonic and phasic activity of more than 20 skeletal muscles that underlie the pharyngeal mucosa play a role in airway dilation and wall stiffening (30). This activity is further blunted during muscle atonia associated with REM sleep. Chemoreceptor responses to blood oxygen (31) and carbon dioxide (32) tensions, as well as local reflex mechanisms, such as the negative airway pressure associated with forceful inspiration, also modulate muscle activity in the upper airway (33, 34).
Imaging (35) and endoscopic (36) studies have shown that during wakefulness and sleep, patients with obstructive sleep apnea have a smaller-caliber upper-airway lumen compared with normal controls. Volumetric magnetic resonance imaging suggests that most of the responsible soft tissue, which may not be entirely explained by fat deposition, originates from the tongue and lateral pharyngeal walls (37). It appears that this tissue and the narrowing that results render the upper airway of persons with sleep apnea vulnerable to collapse. As a possible compensatory mechanism for this anatomically compromised airway, patients with obstructive sleep apnea have increased electromyographic activity of the pharyngeal dilator muscles during wakefulness (38, 39).
Pathophysiologic mechanisms during sleep in obstructive sleep apnea
Chemoreflex sensitivity and response to hypercapnia, hypoxia, and apnea
Chemoreflexes mediate the ventilatory response to hypercapnia and hypoxemia. Peripheral chemoreceptors, the most important of which are located in the carotid bodies of the internal carotid arteries, primarily respond to blood oxygen tension (40), whereas brainstem central chemoreceptors are most sensitive to carbon dioxide and acid–base balance (41). Even in healthy persons, the chemoreceptor response is blunted during sleep compared with wakefulness, leading to modest changes in blood gas tensions (increase in partial pressure of carbon dioxide of 2 to 6 mm Hg and decreases in oxygen saturation of up to 2%) (42).
Compared with persons without sleep apnea, patients with obstructive sleep apnea have heightened peripheral chemoreflex sensitivity, resulting in an increased ventilatory response to hypoxemia (43). This increased response is evident even during normoxia and, by virtue of sympathetic nervous connections with the carotid bodies, contributes to increased sympathetic traffic to skeletal muscle vasculature during daytime wakefulness in persons with obstructive sleep apnea (44). Further evidence of this phenomenon in otherwise healthy persons with sleep apnea is the inhibition of chemoreflex activity with administration of 100% oxygen, which results in decreases in sympathetic activity, blood pressure, and heart rate (45). Enhanced chemoreflex sensitivity in obstructive sleep apnea may explain the exaggerated sympathetic response during hypoxemic episodes.
Apnea-induced bradycardia: the diving reflex
In some persons, hypoxemic stimulation of the peripheral chemoreceptors simultaneously increases sympathetic output to muscle and other vascular beds while activation of cardiac vagal activity results in bradycardia. This phenomenon is called the diving reflex, so-named because of its detailed characterization in diving marine mammals. Features of the diving reflex are peripheral vasoconstriction, which preserves blood flow to the brain and heart vessels, and profound bradycardia as a means of limiting cardiac oxygen demand. This protective mechanism allows homeostasis during prolonged periods of apnea and may be activated in some patients with obstructive sleep apnea, manifesting as severe bradyarrhythmias during apneic events.
Baroreflex activation
In healthy persons, there are interactions between the chemoreflex and baroreflex responses. Activation of the baroreflex attenuates ventilatory (46), sympathetic (47), and bradycardic (48) responses to peripheral chemoreflex excitation. Baroreflex dysfunction, such as may occur in hypertension or heart failure, may therefore lessen this buffer effect. This disinhibition of chemoreflex responses may have relevance when cardiovascular disease coexists with obstructive sleep apnea (48–50).
Variations in intrathoracic pressure
A physiologic hallmark of obstructive sleep apnea is marked reductions in pleural pressure that are related to respiratory efforts against a narrowed or collapsed airway. The sometimes profoundly negative pleural pressures have important acute mechanical, neural, and circulatory effects (25). These effects have been simulated experimentally in humans by use of the Müller maneuver, in which the research participant attempts to inspire against a closed glottis. The maneuver (to pleural pressures of −30 cm H2O) reduces cardiac output and blood pressure, an effect that is more pronounced and prolonged in those with heart failure than in those with normal cardiac function (51). An experimental canine model of chronic upper airway obstruction during sleep has demonstrated a decrease in left ventricular systolic performance over 1 to 3 months (52).
Neural circulatory control during obstructive apneas
Figure 2 shows some of the pathophysiologic pathways that are activated during an obstructive apneic event. Chemoreflex-mediated vascular sympathetic activation and vasoconstriction intensify as apnea progresses, in association with increases in blood pressure.
Resumption of breathing appears to instantaneously suppress sympathetic activity, for at least 2 reasons. First, lung ventilation activates vagally mediated stretch-sensitive thoracic mechanoreceptors, resulting in sympathetic inhibition. In addition, the surge in blood pressure activates baroreflexes to suppress sympathetic drive. Prevention of repetitive obstructive apneas by use of CPAP attenuates the chemoreflex-mediated sympathetic activation and surges in blood pressure (53).
Pathophysiologic mechanisms during wakefulness in obstructive sleep apnea
Sympathetic activity and impaired cardiovascular variability
In addition to heightened daytime levels of sympathetic drive, other abnormalities in cardiovascular regulation are observed during resting normoxic daytime wakefulness in patients with obstructive sleep apnea. Compared with controls who have similar blood pressure, patients with obstructive sleep apnea have faster heart rates, blunted heart rate variability, and increased blood pressure variability (54). The importance of these factors in obstructive sleep apnea is yet to be determined, but large studies have shown them to be markers of increased cardiovascular risk in the general population (55).
Vascular dysfunction
Impaired endothelial function, which has been demonstrated experimentally as an attenuated vasomotor response to vasoactive stimuli, may be an independent marker of future risk for cardiovascular events (56). The physiologic stressors associated with apneas cause elaboration of endogenous vasoactive substances, of which the most important may be endothelin, a potent, long-lasting vasoconstrictor. After about 4 hours of disordered breathing events during sleep, endothelin levels and blood pressure increase (57). Use of CPAP reverses the increases in endothelin levels and blood pressure for several hours. Furthermore, levels of nitric oxide, a powerful vasodilator, may be decreased in obstructive sleep apnea (58) Overnight use of CPAP has been shown to increase circulating levels of nitric oxide (59).
Systemic inflammation
Serum levels of the inflammatory markers C-reactive protein (60) and serum amyloid A (61) appear to be increased in persons with sleep apnea and may be pathophysiologically related to sleep deprivation or intermittent hypoxemia (62, 63). Moreover, there is evidence of oxidative stress in obstructive sleep apnea (64). These inflammatory pathways are thought to play a role in the development and progression of cardiovascular disease (65) and may be important mediators of disease in obstructive sleep apnea.
Metabolic dysregulation
After adjustment for body weight, patients with obstructive sleep apnea have a higher prevalence of insulin resistance and diabetes mellitus than do healthy persons (66). The relationship among these conditions may be critical when making associations with cardiovascular disease. The etiologic mechanism of insulin resistance may be linked to sleep deprivation or sympathetic activation in patients with obstructive sleep apnea (67). Abolition of sleep-disordered breathing by the use of CPAP mitigates glucose intolerance in both the short term (68) and the long term (69). This effect wanes with increasing body weight (68).
Regulation of leptin, the adipocyte-derived protein product of the ob/ob gene, also appears to be abnormal in patients with obstructive sleep apnea (70). Leptin is known to act in the central nervous system as an appetite suppressant, and its blood concentrations correlate closely with overall adipocyte mass. Hyperleptinemia in obstructive sleep apnea may reflect target tissue resistance to the weight-reducing effects of leptin (70).
The complex interaction between obstructive sleep apnea and obesity is underscored by the strong predisposition to weight gain in the year leading up to diagnosis (70). Recent data suggest that treatment of obstructive sleep apnea with CPAP will decrease not only leptin levels but also central obesity, independent of any overall change in body weight after treatment (71) (Figure 3).
Cardiovascular diseases related to obstructive sleep apnea
For nearly 3 decades, research studies have suggested an association between sleep apnea and cardiovascular disease and have implicated obstructive sleep apnea in the causality of systemic disease. However, in early reports, this association was often confounded by other comorbid conditions, most notably obesity. Case–control and cross-sectional studies were limited by the inability to prove that obstructive sleep apnea preceded the development of cardiovascular disease. With the recognition of these limitations have come more rigorous methods, including longitudinal cohort studies and interventional clinical trials.
Hypertension
The most compelling evidence that obstructive sleep apnea is causally related to cardiovascular disease comes from the Wisconsin Sleep Cohort Study (72). In normotensive persons who were followed for 4 years after an initial sleep study, worsening severity of obstructive sleep apnea was independently associated with progressively increasing risk for new hypertension (Figure 4). Even persons with very mild abnormalities in the apnea–hypopnea index (0.1 to 4.9) had 42% greater odds of developing hypertension at follow-up than did those with an apnea–hypopnea index of 0. Thus, the aforementioned physiologic mechanisms that contribute to acute increases in blood pressure during sleep-related breathing events may also affect daytime blood pressure.
The greater question may be whether treatment of obstructive sleep apnea has efficacious antihypertensive effects. Small randomized studies of therapeutic versus sham CPAP in normotension and mild hypertension have shown a small but significant effect in decreasing nighttime and daytime blood pressure (73, 74).
Heart failure
Available evidence suggests that at least 10% of patients with heart failure have clinically significant obstructive sleep apnea (75). Some data show that nocturnal upper-airway edema in patients with congestive heart failure may predispose to or worsen obstructive sleep apnea by narrowing the airway lumen (76). For unclear reasons, patients with coexisting obstructive sleep apnea and heart failure may be less likely to report substantial daytime sleepiness (77).
Because hypertension is a common cause of heart failure, the association between obstructive sleep apnea and hypertension (72) may be a powerful indirect risk factor for congestive heart failure in patients with obstructive sleep apnea. Although large-scale randomized trials of treatment of obstructive sleep apnea in patients with heart failure are lacking, smaller studies have suggested improvement in surrogates of outcome, such as left ventricular ejection fraction (Figure 5) and blood pressure (77), as well as decreases in urinary cate-cholamine levels (78). Whether the mortality rate is reduced, however, remains unknown.
Cardiac arrhythmias
Use of a validated screening tool suggests a high prevalence of obstructive sleep apnea in patients with atrial fibrillation (79) and, among patients who underwent cardioversion for atrial fibrillation, the recurrence rate in those with untreated obstructive sleep apnea was nearly double that seen in those treated with CPAP during 1 year of follow-up (80). Further research is needed to determine the independent effects of obstructive sleep apnea on atrial fibrillation, because both conditions share common associated diseases, including hypertension and heart failure.
Marked bradycardia, asystolic episodes, and ventricular arrhythmias have been documented in patients with obstructive sleep apnea, even in the setting of normal cardiac electrical conduction (81). The clinical importance of these arrhythmias, and whether they are related to sudden cardiac death, remains to be determined.
Management
Because a decrease in body weight of as little as 10% has been associated with clinically significant improvement in the apnea–hypopnea index (82), weight loss should be recommended to overweight patients with obstructive sleep apnea. Although the amount of weight loss seems to correlate directly with reduction in sleep-disordered breathing (7), studies have demonstrated modest improvement in rather than normalization of the apnea–hypopnea index. The long-term effects of increasingly popular methods of weight loss, such as bariatric surgery and carbohydrate-restricted diets, on the severity of obstructive sleep apnea must be assessed in longitudinal studies.
Continuous positive airway pressure remains the most effective therapy for obstructive sleep apnea. It eliminates upper-airway flow limitation in almost any patient and is associated with improvement in daytime symptoms (83) and objective measures of sleepiness (84) in patients with mild or severe abnormalities in the apnea–hypopnea index. Use of CPAP mitigates or reverses many of the acute pathophysiologic responses outlined previously that result from sleep-disordered breathing. Currently, however, few data support use of CPAP in the absence of daytime symptoms. Randomized clinical trials suggest that sleepiness may be an important manifestation of systemic disease. For example, use of CPAP to treat sleep-disordered breathing has been shown to have little effect on blood pressure in patients who are not sleepy (85).
On the basis of its established effectiveness in ameliorating sleep-disordered breathing and reversal of daytime symptoms, a trial of CPAP therapy is warranted in most patients with the obstructive sleep apnea and associated daytime symptoms. Universal adherence to this therapy is hampered by intolerance or refusal in a substantial proportion of patients. Nonadherence is often related to nasal congestion and irritation, which may be ameliorated by in-line heated humidification and local treatments, such as moisturizers or corticosteroids.
The role of surgery in the treatment of obstructive sleep apnea remains ill defined and is controversial. The most appropriate indication is clearly reversible causes of upper-airway obstruction, such as adenotonsillar hypertrophy or mass lesions. Application of surgical therapy to general populations with obstructive sleep apnea is limited by a dearth of randomized, controlled trials to support its routine use. Uvulopalatopharyngoplasty (also known as UPPP), a commonly performed inpatient procedure involving excision of soft palatal tissue, results in an apnea–hypopnea index less than 20 in a minority of patients (86). The suboptimal performance of uvulopalatopharyngoplasty may be explained in part by the role of anatomic factors other than soft palatal tissue. Imaging studies (29) and airway pressure measurements (76) have revealed that the oropharyngeal and velopharyngeal areas, which are dorsal to the soft palate and tongue base, respectively, are the regions most vulnerable to collapse during sleep.
Even in patients in whom sleep-disordered breathing seems to improve in the early postoperative period after uvulopalatopharyngoplasty, these effects may be short-lived. Thus, polysomnography should be repeated within 4 to 6 months.
Mandibular advancement devices, which induce protrusion of the mandible during sleep and thereby reduce retroglossal airway collapse, appear to be effective in the treatment of mild apnea or nonapneic snoring and have been shown to improve daytime symptoms and modestly decrease the apnea–hypopnea index (87, 88).
Summary
Obstructive sleep apnea remains an important public health problem because of its neurocognitive sequelae. Detailed characterization of physiologic pathways and cardiovascular disease mechanisms suggest an important role of obstructive sleep apnea in systemic disease, particularly of the cardiovascular system. Rigorous studies with a refined diagnostic approach to obstructive sleep apnea are needed to identify the relative importance of apneas, hypopneas, blood gas abnormalities, arousals, and sleep deprivation in the initiation of cardiovascular disease. An added challenge will be disentangling the influence of multiple comorbid conditions that are often present in patients with obstructive sleep apnea.
Small interventional studies suggest that CPAP reverses some of the important pathophysiologic pathways in obstructive sleep apnea, but this finding has not been clinically validated by large-scale randomized, controlled trials. Further clarification of physiologic and pathologic pathways may help to identify novel mechanism-based therapies that interrupt or delay progression of pathophysiologic processes related to obstructive sleep apnea.
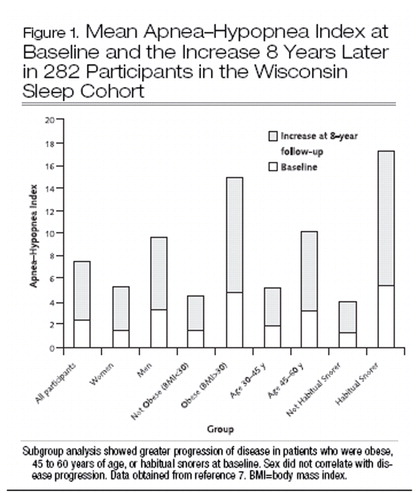
Figure 1. Mean Apnea–Hypopnea Index at Baseline and the Increase 8 Years Later in 282 Participants in the Wisconsin Sleep Cohort
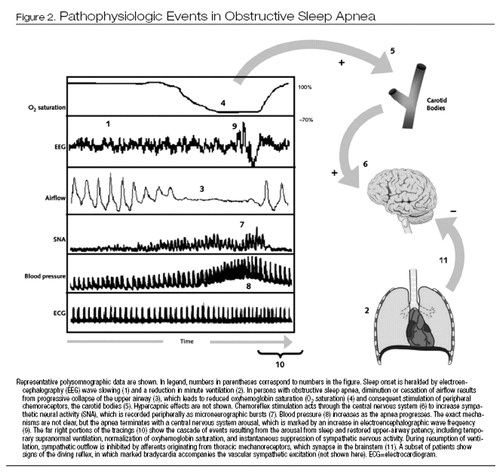
Figure 2. Pathophysiologic Events in Obstructive Sleep Apnea
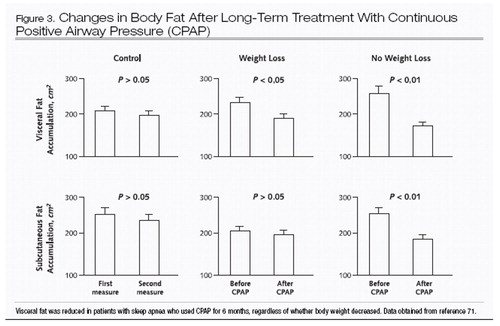
Figure 3. Changes in Body Fat After Long-Term Treatment With Continuous Positive Airway Pressure (CPAP)
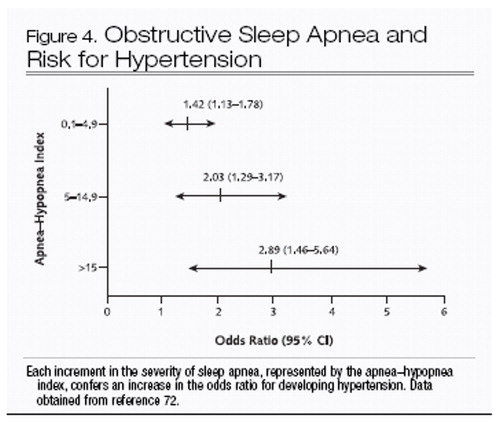
Figure 4. Obstructive Sleep Apnea and Risk for Hypertension
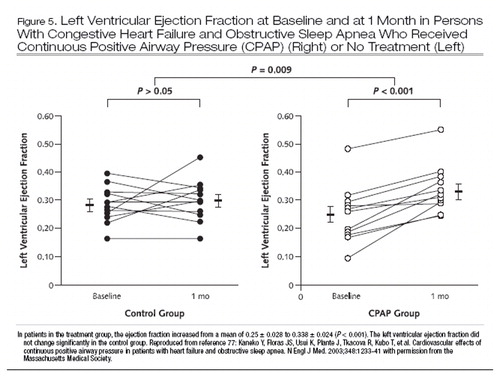
Figure 5. Left Ventricular Ejection Fraction at Baseline and at 1 Month in Persons With Congestive Heart Failure and Obstructive Sleep Apnea Who Received Continuous Positive Airway Pressure (CPAP) (Right) or No Treatment (Left)
1 Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PMID: 10450601]Crossref, Google Scholar
2 Redline S, Kapur VK, Sanders MH, Quan SF, Gottlieb DJ, Rapoport DM, et al. Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment. Am J Respir Crit Care Med. 2000;161:369–74. [PMID: 10673173]Crossref, Google Scholar
3 Hosselet J, Ayappa I, Norman RG, Krieger AC, Rapoport DM. Classification of sleep-disordered breathing. Am J Respir Crit Care Med. 2001;163:398–405. [PMID: 11179113]Crossref, Google Scholar
4 Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. [PMID: 11254512]Crossref, Google Scholar
5 Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. [PMID: 9445292]Crossref, Google Scholar
6 Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. [PMID: 11254524]Crossref, Google Scholar
7 Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. [PMID: 11991871]Crossref, Google Scholar
8 Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993; 328:1230–5. [PMID: 8464434]Crossref, Google Scholar
9 Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. [PMID: 11122588]Crossref, Google Scholar
10 Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992; 47:101–5. [PMID: 1549815]Crossref, Google Scholar
11 Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–92. [PMID: 12531779]Crossref, Google Scholar
12 Young T. Sleep-disordered breathing in older adults: is it a condition distinct from that in middle-aged adults? [Editorial] Sleep. 1996;19:529–30. [PMID: 8899930]Crossref, Google Scholar
13 Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. [PMID: 11966340]Crossref, Google Scholar
14 Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. [PMID: 9001310]Crossref, Google Scholar
15 Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169:168–73. [PMID: 14604837]Crossref, Google Scholar
16 Kim J, In K, Kim J, You S, Kang K, Shim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. [PMID: 15347562]Crossref, Google Scholar
17 Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–51. [PMID: 10080847]Crossref, Google Scholar
18 Barbe, Pericas J, Munoz A, Findley L. Automobile accidents in patients with sleep apnea syndrome. An epidemiological and mechanistic study. Am J Respir Crit Care Med. 1998;158:18–22. [PMID: 9655701]Crossref, Google Scholar
19 Martin SE, Wraith PK, Deary IJ, Douglas NJ. The effect of nonvisible sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1997;155:1596–601. [PMID: 9154863]Crossref, Google Scholar
20 Gottlieb DJ, Whitney CW, Bonekat WH, Iber C, James GD, Lebowitz M, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–7. [PMID: 9927364]Crossref, Google Scholar
21 Gottlieb DJ, Yao Q, Redline S, Ali T, Mahowald MW. Does snoring predict sleepiness independently of apnea and hypopnea frequency? Am J Respir Crit Care Med. 2000;162:1512–7. [PMID: 11029370]Crossref, Google Scholar
22 Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:1427–32. [PMID: 12738600]Crossref, Google Scholar
23 Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. [PMID: 10507956]Crossref, Google Scholar
24 Chiner E, Signes-Costa J, Arriero JM, Marco J, Fuentes I, Sergado A. Nocturnal oximetry for the diagnosis of the sleep apnoea hypopnoea syndrome: a method to reduce the number of polysomnographies? Thorax. 1999;54:968–71. [PMID: 10525553]Crossref, Google Scholar
25 Somers VK, Dyken ME, Skinner JL. Autonomic and hemodynamic responses and interactions during the Mueller maneuver in humans. J Auton Nerv Syst. 1993;44:253–9. [PMID: 8227959]Crossref, Google Scholar
26 Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med. 1996;153:776–86. [PMID: 8564132]Crossref, Google Scholar
27 Davidson TM. The Great Leap Forward: the anatomic basis for the acquisition of speech and obstructive sleep apnea. Sleep Med. 2003;4:185–94. [PMID: 14592320]Crossref, Google Scholar
28 Hudgel DW, Hendricks C. Palate and hypopharynx—sites of inspiratory narrowing of the upper airway during sleep. Am Rev Respir Dis. 1988;138:1542–7. [PMID: 3202504]Crossref, Google Scholar
29 Trudo FJ, Gefter WB, Welch KC, Gupta KB, Maislin G, Schwab RJ. State-related changes in upper airway caliber and surrounding soft-tissue structures in normal subjects. Am J Respir Crit Care Med. 1998;158:1259–70. [PMID: 9769290]Crossref, Google Scholar
30 Fouke JM, Teeter JP, Strohl KP. Pressure-volume behavior of the upper airway. J Appl Physiol. 1986;61:912–8. [PMID: 3093456]Crossref, Google Scholar
31 Onal E, Lopata M, O’Connor TD. Diaphragmatic and genioglossal electromyogram responses to isocapnic hypoxia in humans. Am Rev Respir Dis. 1981; 124:215–7. [PMID: 6792954]Google Scholar
32 Seelagy MM, Schwartz AR, Russ DB, King ED, Wise RA, Smith PL. Reflex modulation of airflow dynamics through the upper airway. J Appl Physiol. 1994;76:2692–700. [PMID: 7928902]Crossref, Google Scholar
33 Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991;436:31–44. [PMID: 2061834]Crossref, Google Scholar
34 Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–7. [PMID: 11779733]Crossref, Google Scholar
35 Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148:1385–400. [PMID: 8239180]Crossref, Google Scholar
36 Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–26. [PMID: 9104871]Crossref, Google Scholar
37 Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. [PMID: 12746251]Crossref, Google Scholar
38 Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA, et al. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–30. [PMID: 11739130]Crossref, Google Scholar
39 Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest. 1992;89:1571–9. [PMID: 1569196]Crossref, Google Scholar
40 Lugliani R, Whipp BJ, Seard C, Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med. 1971;285:1105–11. [PMID: 5095735]Crossref, Google Scholar
41 Gelfand R, Lambertsen CJ. Dynamic respiratory response to abrupt change of inspired CO2 at normal and high PO2. J Appl Physiol. 1973;35:903–13. [PMID: 4765831]Crossref, Google Scholar
42 Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax. 1982;37:840–4. [PMID: 7164002]Crossref, Google Scholar
43 Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–9. [PMID: 10069786]Crossref, Google Scholar
44 Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. [PMID: 7560081]Crossref, Google Scholar
45 Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998; 97:943–5. [PMID: 9529260]Crossref, Google Scholar
46 Heistad DD, Abboud FM, Mark AL, Schmid PG. Interaction of baroreceptor and chemoreceptor reflexes. Modulation of the chemoreceptor reflex by changes in baroreceptor activity. J Clin Invest. 1974;53:1226–36. [PMID: 4825222]Crossref, Google Scholar
47 Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–7. [PMID: 2040688]Crossref, Google Scholar
48 Somers VK, Dyken ME, Mark AL, Abboud FM. Parasympathetic hyperresponsiveness and bradyarrhythmias during apnoea in hypertension. Clin Auton Res. 1992;2:171–6. [PMID: 1498563]Crossref, Google Scholar
49 Hedner JA, Wilcox I, Laks L, Grunstein RR, Sullivan CE. A specific and potent pressor effect of hypoxia in patients with sleep apnea. Am Rev Respir Dis. 1992;146:1240–5. [PMID: 1443878]Crossref, Google Scholar
50 Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–12. [PMID: 3391673]Crossref, Google Scholar
51 Bradley TD, Hall MJ, Ando S, Floras JS. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest. 2001;119:1827–35. [PMID: 11399711]Crossref, Google Scholar
52 Parker JD, Brooks D, Kozar LF, Render-Teixeira CL, Horner RL, Douglas Bradley T, et al. Acute and chronic effects of airway obstruction on canine left ventricular performance. Am J Respir Crit Care Med. 1999;160:1888–96. [PMID: 10588602]Crossref, Google Scholar
53 Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–5. [PMID: 10587337]Crossref, Google Scholar
54 Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–7. [PMID: 9736593]Crossref, Google Scholar
55 Tsuji H, Larson MG, Venditti FJ Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–5. [PMID: 8941112]Crossref, Google Scholar
56 Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. [PMID: 12588755]Crossref, Google Scholar
57 Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17:61–6. [PMID: 10100095]Crossref, Google Scholar
58 Schulz R, Schmidt D, Blum A, Lopes-Ribeiro X, Lucke C, Mayer K, et al. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax. 2000;55:1046–51. [PMID: 11083891]Crossref, Google Scholar
59 Ip MS, Lam B, Chan LY, Zheng L, Tsang KW, Fung PC, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–71. [PMID: 11112132]Crossref, Google Scholar
60 Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. [PMID: 12034649]Crossref, Google Scholar
61 Svatikova A, Wolk R, Shamsuzzaman AS, Kara T, Olson EJ, Somers VK. Serum amyloid a in obstructive sleep apnea. Circulation. 2003;108:1451–4. [PMID: 12952844]Crossref, Google Scholar
62 Tamura DY, Moore EE, Partrick DA, Johnson JL, Offner PJ, Silliman CC. Acute hypoxemia in humans enhances the neutrophil inflammatory response. Shock. 2002;17:269–73. [PMID: 11954825]Crossref, Google Scholar
63 Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. [PMID: 14975482]Crossref, Google Scholar
64 Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–9. [PMID: 11934717]Crossref, Google Scholar
65 Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. [PMID: 12551853]Crossref, Google Scholar
66 Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82. [PMID: 11874813]Crossref, Google Scholar
67 Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. [PMID: 10543671]Crossref, Google Scholar
68 Harsch IA, Schahin SP, Radespiel-Troger M, Weintz O, Jahreiss H, Fuchs FS, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. [PMID: 14512265]Crossref, Google Scholar
69 Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–5. [PMID: 7989475]Google Scholar
70 Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–7. [PMID: 10899061]Crossref, Google Scholar
71 Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–12. [PMID: 10449691]Crossref, Google Scholar
72 Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342:1378–84. [PMID: 10805822]Crossref, Google Scholar
73 Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. [PMID: 11812555]Crossref, Google Scholar
74 Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. [PMID: 12515745]Crossref, Google Scholar
75 Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. [PMID: 9626176]Crossref, Google Scholar
76 Shepard JW Jr, Thawley SE. Localization of upper airway collapse during sleep in patients with obstructive sleep apnea. Am Rev Respir Dis. 1990;141:1350–5. [PMID: 2339852]Crossref, Google Scholar
77 Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. [PMID: 12660387]Crossref, Google Scholar
78 Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–6. [PMID: 14597482]Crossref, Google Scholar
79 Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004; 110:364–7. [PMID: 15249509]Crossref, Google Scholar
80 Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. [PMID: 12743002]Crossref, Google Scholar
81 Shepard JW Jr, Garrison MW, Grither DA, Dolan GF. Relationship of ventricular ectopy to oxyhemoglobin desaturation in patients with obstructive sleep apnea. Chest. 1985;88:335–40. [PMID: 2411477]Crossref, Google Scholar
82 Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–5. [PMID: 3933396]Crossref, Google Scholar
83 Ballester E, Badia JR, Hernandez L, Carrasco E, de Pablo J, Fornas C, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:495–501. [PMID: 9927363]Crossref, Google Scholar
84 Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–5. [PMID: 10382693]Crossref, Google Scholar
85 Barbe F, Mayoralas LR, Duran J, Masa JF, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. A randomized, controlled trial. Ann Intern Med. 2001;134:1015–23. [PMID: 11388814]Crossref, Google Scholar
86 Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. [PMID: 8855039]Crossref, Google Scholar
87 Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:743–8. [PMID: 12204875]Crossref, Google Scholar
88 Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. [PMID: 11371418]Crossref, Google Scholar



