Deconstructing Diabetes and Depression: Clinical Context, Treatment Strategies, and New Directions
Abstract
Depression and diabetes are common, chronic, and frequently comorbid diseases that contribute substantially to global disability and mortality. Their relationship is bidirectional: depression increases the risk of developing type 2 diabetes mellitus (T2DM), and diabetes increases the risk of depression. Unhealthy lifestyles and poor self-care by patients with depression contribute to the increased T2DM risk. The psychosocial burden of a diabetes diagnosis and its eventual complications predispose diabetic patients to depressive symptoms. Neuroendocrine alterations and inflammation may underlie the increased risk of T2DM in depression but are also proposed as common causative factors for both illnesses. Screening for depression is essential in T2DM, and vice versa. Selective serotonin reuptake inhibitors effectively treat depression of patients with diabetes and positively influence glycemic control. Psychological interventions are effective for depressive symptoms, but their effect on glycemic control varies. Novel depression interventions targeting inflammation or insulin resistance underscore the common biological underpinnings of mood and metabolism.
Clinical Context
Both diabetes and depression contribute substantially to the global burden of disease. One in 11 adults worldwide has diabetes, and by 2030 the World Health Organization projects that diabetes will be the seventh-leading cause of death globally (1, 2). Similarly, 350 million people are diagnosed as having depression worldwide, and it is the leading cause of years living with disability (3). Diabetes and depression are interrelated in a multifaceted and bidirectional fashion, and the morbidity associated with their co-occurrence is disproportionately higher than the additive effect of either illness alone (4). Furthermore, the depressive half of the comorbidity is often unrecognized, contributing to the clinical challenge of treating these frequently comorbid illnesses (5).
Definitions
Diabetes mellitus is characterized by chronic hyperglycemia that in the absence of treatment leads to microvascular (e.g., neuropathy, nephropathy, retinopathy) and macrovascular (e.g., myocardial infarction, stroke, amputation) complications (6). Type 1 diabetes (T1DM) is an autoimmune disorder that generally presents before adulthood and leads to a total insulin deficiency. T2DM is a consequence of insulin resistance and a relative insulin deficiency; it is more prevalent and presents later in life (6). People with impaired fasting glucose or glucose tolerance—sometimes referred to as prediabetes—are at high risk of progressing to T2DM (6).
Unlike diabetes, which is a laboratory-based diagnosis, depression is a heterogeneous concept defined by the presence and duration of specific symptoms. Major depressive disorder is a chronic mood disorder characterized by low mood, anhedonia, neurovegetative symptoms (e.g., disturbance of sleep, appetite, energy, and concentration), as well as negative thought processes (e.g., excess guilt, worthlessness, and suicidal ideation) (3). A major depressive episode, occurring when a threshold of these symptoms is reached for a 2-week period, is the hallmark of major depressive disorder. Major depressive events are not unique to major depressive disorder (e.g., major depressive events are also typical of bipolar disorder), and subthreshold depressive symptoms frequently occur among those without major depressive disorder (7).
For research purposes, validated self-report symptom scales (e.g., Beck Depression Inventory, Patient Health Questionnaire–9 [PHQ-9]) are used to screen for depression. The gold standard for diagnosing depression, however, is the Structured Clinical Interview for DSM-IV Axis I Disorders, which is more specific but also more resource-intensive (7). This review refers to clinician-diagnosed depression as major depressive disorder and self-report measures as depressive symptoms whenever the distinction is required.
Coprevalence of Depression and Diabetes
Fifteen years ago, Anderson et al. determined that the presence of diabetes doubles the odds of depression (8). Subsequent studies affirmed the initial findings (8–11). In a 2006 meta-analysis of cross-sectional studies, depression had a prevalence of 17.6% among those with T2DM and 9.8% among those without T2DM (odds ratio [OR]=1.6, 95% confidence interval [CI]=1.2–2.0) (9). However, diabetic patients differed from the nondiabetic patients on other variables known to be associated with an increased risk of depression (e.g., comorbid chronic illnesses) (9). In addition, the prevalence of comorbid depression among patients with diabetes depends on the assessment method, ranging from 11% found with the standardized interviews to 31% with self-report questionnaires (8, 10). Comorbid depression and diabetes are particularly common among women, with female preponderance noted in the 2001 findings (28% for women vs. 18% for men) and 2006 meta-analysis (23.8% vs. 12.8%) (8–10).
For patients with T1DM, meta-analysis of cross-sectional studies showed a 12% prevalence of depression, in comparison with 3.2% among control patients without T1DM, but heterogeneity limited conclusions (12, 13). Subsequent cross-sectional studies support an increased prevalence of depression among adults with T1DM, but the evidence is inconclusive for the under-25-year-old population (11–15).
In addition to female sex, established risk factors for depression are also risk factors for depression in the diabetic population (e.g., older age, poor social support, and low socioeconomic status) (9, 11). Additional risk factors for depression specific to the diabetic population include occurrence of late or acute complications, persistently poor glycemic control, insulin therapy initiation in T2DM, and the perception of deteriorating health (9, 10). A troubling finding is an increasing prevalence of depression in T2DM among hospitalized patients, going from 3.5% in 2001 to 5.8% in 2011 (16).
Recently, scales such as the Diabetes Distress Scale and Problem Areas in Diabetes Scale have measured diabetes distress, which reflects the emotional response to the burden of diabetes and its complications, as well as the prolonged psychological distress during adjustment to the diagnosis (8). Diabetes distress presents in its severe form to 10%−30% of people with diabetes (8). Measures of diabetes distress correlate strongly with self-reports of depressive symptoms (coefficient: 0.22–0.63) but less so with standardized diagnostic interviews (coefficient: 0.04–0.15) (8, 15). In the T1DM population, use of the PHQ-9 has led to a high false-positive rate (52%−71%) compared with use of the Structured Clinical Interview for DSM-IV Axis I Disorders, suggesting that some of what was considered depression among adults with diabetes might represent diabetes distress (15). Although depression and diabetes distress are partially overlapping constructs, they are not interchangeable.
A 2015 meta-analysis examined the prevalence of T2DM among patients with diagnosed major depressive disorder (17). Patients with major depressive disorder were more likely to have T2DM (OR=1.49, CI=1.29–1.72), even when analyses controlled for age and sex (OR=1.36, CI=1.28–1.44) (17). In the largest of the included studies (143,943 primary care patients with major depressive disorder), T2DM was present in 9.3% of the major depressive disorder population, in comparison with 4.3% of those without depression (18).
Depression as a Risk Factor for Diabetes
Building on the cross-sectional associations, longitudinal studies inform on the temporal relationship between diabetes and depression in order to determine whether one might be a risk factor for the development of the other, or vice versa. In 2006, a meta-analysis of nine longitudinal studies found that patients with either a diagnosis of depression or depressive symptoms were at 37% increased risk of developing T2DM, with a pooled relative risk of 1.26 (CI=1.13–1.39) (19). This finding was echoed in 2008, when a meta-analysis of 13 longitudinal studies found that baseline major depressive disorder diagnosis or presence of depressive symptoms was associated with a 60% increased risk of T2DM (pooled relative risk of 1.60, CI=1.37–1.88) (20). A 2014 meta-analysis reaffirmed these findings, noting a pooled relative risk of 1.32 (CI=1.18–1.47) (21). For patients with depression the risk of developing T1DM remains unclear, owing to the natural history of the two illnesses (with the onset of T1DM preceding the onset of major depressive disorder in most cases).
The increased risk of T2DM for patients with depression may relate to behavioral factors (22–25) (see Figure 1). Depression is associated with higher body mass index, poor diet, less physical activity, and smoking, all of which are risk factors for T2DM (22). This hypothesis posits that the symptoms of depression (e.g., anhedonia, sleep and appetite dysregulation, fatigue) lead to an unhealthy lifestyle and poor self-care, which in turn increases the risk of obesity and T2DM (23). In support of this, a 2008 study found higher rates of new-onset T2DM among those with elevated depressive symptoms, but after analyses controlled for lifestyle factors such as smoking, daily caloric intake, physical activity, and alcohol use, the trend for increased T2DM risk was no longer significant (26).
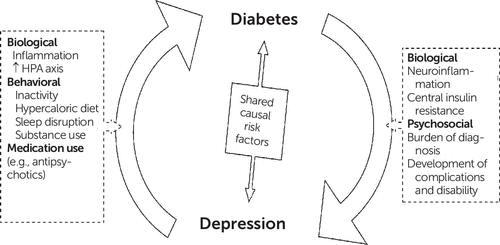
Figure 1. The Bidirectional Relationship of Diabetes and Depressiona
a HPA, hypothalamic-pituitary-adrenal axis
A second hypothesis explaining the increased T2DM vulnerability of patients with depression involves the biochemical changes associated with depression (see Figure 1). Depression is associated with dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system, particularly in the severe or melancholic subtypes of depression (27, 28). Excess production of glucocorticoids by the adrenal glands can contribute to metabolic syndrome, insulin resistance, and T2DM (27, 28); therefore, overactivation of the HPA axis might explain the T2DM-depression relationship. In addition, evidence from meta-analysis demonstrates that depression is associated with higher circulating levels of proinflammatory cytokines such as tumor necrosis factor and interleukin-6 (29). Raised concentrations of inflammatory cytokines, specifically interleukin-6, have been associated with increased risk of T2DM (30). Lastly, depression may have a direct impact on insulin resistance (24). A recent meta-analysis found a significant association between depression and insulin resistance (effect size=0.19, CI=0.11–0.27), with a larger effect size when standardized interviews were used for diagnosis (31). Furthermore, some drugs that reduce insulin resistance, such as pioglitazone, improve depressive symptoms (24). However, the relationship between depressive symptoms and other glycemic traits (e.g., fasting glucose, glucose tolerance) has been inconclusive (24).
Antidepressant medication is a potential confounding factor in the relationship between T2DM and depression, because antidepressant use has been associated with increased risk of physician-diagnosed T2DM (32, 33). However, antidepressant use is not associated with changes in fasting glucose or glucose tolerance, nor is it associated with undiagnosed diabetes (32, 33). The finding that antidepressants are associated with T2DM diagnosis but not increased glucose concentrations suggests a noncausal relationship, with the detection/ascertainment bias a proposed explanation (e.g., increased contact with medical care leads to improved detection, or diabetes may be detected when ruling out endocrine causes of depression) (33).
Diabetes as a Risk Factor for Depression
Multiple meta-analyses of longitudinal studies have determined that people with T2DM have an elevated risk of depression. According to a 2008 meta-analysis of seven studies, the pooled relative risk for incident depression in the population with baseline diabetes was 1.15 (CI=1.02–1.30), corresponding to a 15% increased risk (20). In 2010, analysis of 11 studies found a pooled relative risk of 1.24 (CI=1.09–1.40) (34); a 2012 meta-analysis of 16 studies reported an adjusted relative risk of 1.25 (CI=1.10–1.44) (35). Of note, the increased risk was significant in studies measuring depression with diagnostic interview or antidepressant usage but not in studies using questionnaires (35). To date, no meta-analysis has reported a relative risk of depression in T1DM.
One explanation is that the psychological burden of a diagnosis predisposes patients to depression. The psychological burden is a multifaceted construct, encompassing disease-related cognitions (e.g., perceived disability, awareness of illness) and the interaction of increased self-care demands with social support and coping style (36). A 2011 meta-analysis found that individuals with undiagnosed diabetes had a significantly lower risk of depression than did those with previously diagnosed diabetes (OR=0.57, CI=0.45–0.74) (37). In addition, the risk of depression did not differ between individuals with undiagnosed diabetes and those with either impaired glucose metabolism or normal glucose metabolism (37). This suggests that the diagnosis itself, rather than the biochemical changes leading up to diagnosis, underlies the increased depression risk. Two studies have noted an increase in antidepressant prescription in the first year after T2DM diagnosis, consistent with an acute worsening of mental health following T2DM diagnosis (38).
A second possibility is that the structural and neurochemical changes associated with diabetes induce depressive symptoms (22, 24, 36). The neuroinflammatory changes induced by diabetes and the direct impact of altered insulin metabolism on the brain are biologically plausible mechanisms by which those with diabetes could be predisposed to depression (39–41). Diabetes-related biochemical changes induce cognitive disturbances and can adversely affect emotional processing and reward circuitry (41–43).
In advanced diabetes, the complications of the illness (and resulting self-care needs or disability) might cause an increased risk of depressive symptoms (4, 44). Some of the complications of diabetes—such as cardiac disease, cerebrovascular disease, kidney disease, or poor vision—are themselves independent risk factors for depression (44). Thus, the increased risk of major depressive disorder in diabetes is mainly attributed to psychosocial factors, although there is increasing evidence for a biochemical mechanism (see Figure 1).
Impact of Comorbidity on Outcomes
Depression in diabetes leads to suboptimal self-care and poorer glycemic control, leading to an increased risk of complications and mortality (45). There is a modest yet significant association between depressive symptoms and hyperglycemia among diabetic patients, supported by meta-analysis of cross-sectional studies and subsequent longitudinal studies (11, 45, 46). However, recent prospective studies suggest that diabetes distress, rather than depressive symptoms, might be a better predictor of suboptimal glycemic control (15, 45, 47). Among patients with diabetes, those with comorbid depressive symptoms are less active, more likely to smoke, less likely to eat healthfully, and less likely to adhere to treatment (including glucose monitoring and antidiabetes medication) (48). A 2008 meta-analysis of 47 studies found a significant association between depression and nonadherence to diabetes treatment; the effect size was highest for missed appointments and composite measures of self-care (49). In this case, depressive symptoms had a stronger negative relationship with self-care than did diabetes distress (50). In a recent longitudinal study, persisting depressive symptoms or worsening depressive symptoms at 5-year follow-up were associated with worse adherence to diet and exercise regimens, whereas patients with baseline depression who had clinical improvement in their depression were no different from the nondepression group with regard to diet and exercise adherence (51). Higher levels of depressive symptoms or diabetes distress are associated with negative beliefs about insulin among insulin-naïve T2DM patients, suggesting that depression might delay the initiation of insulin in T2DM (52).
Among patients with diabetes, depression has been associated with a higher prevalence of both microvascular and macrovascular complications (53). Depression among patients with diabetes has been associated with a higher incidence of blindness, diabetic foot ulcers, coronary artery disease, end-stage renal disease, and amputations (45).
Evidence also suggests that diabetes among patients with depression leads to poorer depression outcomes (54). When diabetes is comorbid, the course of depression appears to be of longer duration, with more recurrences and greater severity (55). Even with successful treatment, relapse may occur with as much as 80% of patients with diabetes with major depressive disorder (55). Poor metabolic control can exacerbate depression or diminish response to antidepressant medication (56). In addition, those with depression notice the symptoms of diabetes more and perceive a greater burden of illness (57).
Patients with depression and diabetes are at greater risk of dementia (hazard ratio [HR]=4.84, CI=4.21–5.55), and the excess risk to those with both conditions is higher than the additive risks of the individual diagnoses (58). Cognitive dysfunction results from both depression and diabetes, and mild cognitive impairment is more likely to proceed to Alzheimer’s dementia if either of the two diagnoses is present (59). There is a complex neurobiological relationship between psychiatric disorders (e.g., major depressive disorder) and metabolic changes (e.g., T2DM), which leads to synergistically adverse changes in cognitive processes (e.g., alterations in brain connectivity and impairment of brain plasticity) (39, 41–43).
Patients with both diabetes and depression have an 82% increased risk of myocardial infarction (HR=1.82, CI=1.69–1.97) in comparison with those with neither condition (60). Notably, a recent meta-analysis of 16 prospective studies demonstrated that baseline depression among patients with diabetes was associated with nearly 50% increased risk of all-cause mortality (HR=1.46, CI=1.29–1.66), mainly attributed to cardiovascular mortality (HR=1.39, CI=1.11–1.73) (61).
Treatment Strategies for Depression in Diabetes
Given the frequency with which T2DM and depression co-occur, as well as the augmented deleterious effects on morbidity and mortality, it is essential to recognize and treat both diseases effectively. The bidirectional relationship between depression and T2DM underscores the conceptual importance of treating diabetes and depression together rather than as separate entities. Fortunately, there is a growing body of evidence to inform on screening strategies, pharmacological interventions, and psychosocial interventions for depression and diabetes (62).
Screening
Depression is underdetected among diabetic patients (54, 63, 64). In accordance with this, multiple clinical guidelines advocate for increased screening for depression among patients with diabetes (65–67). Routine screening for anxiety, eating disorders, and cognitive impairment is also recommended (67). Similarly, screening for T2DM (e.g., measuring fasting glucose, measuring hemoglobin A1c) and identification of those at risk (e.g., glucose tolerance testing, monitoring for metabolic syndrome) should be fundamental aspects of treatment for depression, as indicated by recent guidelines (68, 69). Screening for depression typically utilizes depression self-report questionnaires with validated cut-off scores. The Beck Depression Inventory, the Center for Epidemiologic Studies Depression Scale, the Hospital Anxiety and Depression Scale, and the PHQ-9 have been validated in diabetic populations (70–72). Nonetheless, the PHQ-9 has the strongest evidence of validity (71).
Unfortunately, these scales are limited in specificity and have a tendency to overestimate rates of depression, particularly given the potential overlap between diabetes-related symptoms and depression symptoms (70, 71, 73). For example, cognitive dysfunction may be a primary complication of diabetes or a symptom of depression, and other symptoms such as fatigue or appetite changes can present in both disorders (74). Furthermore, there is considerable overlap between depression and diabetes distress, and recent efforts to differentiate the two have led to the development of scales such as the Diabetes Distress Scale and Problem Areas in Diabetes Scale (7). Optimization of screening protocols is underway to improve specificity while maintaining adequate sensitivity (71). One solution is to increase the scales’ cut-off values. For example, the best cut-off score for the PHQ-9 in a diabetic population is 12 (rather than 10 in the general population) (75).
Regardless of the screening tool used, positive screens require a subsequent diagnostic interview with the intention of confirming or refuting the diagnosis. Primary care providers frequently diagnose and treat major depressive events within their scope of practice, so referral to a psychiatrist is often unnecessary. However, it is essential to have adequate follow-up and well-defined treatment pathways, because the evidence has shown that screening for depression on its own does not improve outcomes (76, 77). Screening is only beneficial when adequate follow-up and effective treatments are available (77). Overall, care for those with diabetes and depression must be integrative and multidisciplinary; collaborative care leads to better treatment adherence, greater improvement of depression and glycemic control, and better quality of life (78, 79).
Treatment
After confirming diagnosis, clinicians should use evidence-based treatment targeting both depressive symptom severity and glycemic control in the depressed diabetic population (62, 80). Many clinical trials have evaluated the effect of interventions on both sets of outcomes (psychological and medical) (64). Categorically, the interventions can be grouped into psychological interventions (e.g., problem-solving techniques, counseling, cognitive-behavioral therapy [CBT]), psychopharmacological treatments (e.g., antidepressants, anti-inflammatories, insulin sensitizers), and lifestyle modification (e.g., diet, exercise) (64).
Psychopharmacological treatments.
A 2015 systematic review identified 12 clinical trials assessing the effect of antidepressants with persons with diabetes (62). Seven of the placebo-controlled trials assessed the efficacy of selective serotonin reuptake inhibitors (SSRIs), including paroxetine, fluoxetine, and sertraline (81–85). On the basis of a meta-analysis of five trials, the pooled antidepressant effect size (standard mean difference in depression severity scores) of SSRIs in comparison with placebo was −0.39, indicative of a moderate effect size that is similar to the effect of SSRIs in the general population (62, 64). SSRIs also had a significant impact on glycemic control; the change in glycemic control versus placebo indicated a moderate effect size of −0.38 (64). Although it is likely that the antidepressant effect of these medications can be generalized, the effect on glycemic control might not be similarly generalizable (86). The antidepressants that induce significant weight gain (e.g., paroxetine, mirtazapine, tricyclic antidepressants) could decrease glycemic control by increasing insulin resistance (78). Conversely, bupropion is associated with weight loss; it also does not cause sexual side effects, which are of particular importance given the frequency of erectile dysfunction in the diabetic population (78). Atypical antipsychotics, increasingly used for depression, increase risk for metabolic syndrome and should be avoided in the diabetic population whenever possible (68, 69). Given the variable effects on weight gain, insulin resistance, and other metabolic parameters, the effect of pharmacological interventions on glycemic control must be evaluated on an individual basis (85).
A recent trial compared the antidepressant effects of sertraline with that of a 12-week course of diabetes-specific CBT (87). At the end of the 12-week trial, both interventions improved depression symptom severity with a similar, moderate effect size (87). However, sertraline was more efficacious than was CBT for relapse prevention at one-year follow-up for persons who remitted with treatment (87). Of note, neither intervention had a significant impact on glycemic control in this trial (87).
Several novel treatments for depression in subjects with diabetes or prediabetes are currently under investigation. The use of anti-inflammatory agents to treat depression in the general population has been of great interest (88). A recent meta-analysis showed a moderate antidepressant effect size of adjunctive anti-inflammatory agents in comparison with conventional treatment alone in the general population (89). Given the role of inflammation in both diabetes and depression, anti-inflammatory agents may be of particular benefit in the treatment of depression with comorbid diabetes (62, 89). There is also a theoretical basis for the use of antidiabetes medications for the treatment of depression among insulin-resistant patients, because central insulin resistance facilitates the cognitive and mood disturbances in diabetes and depression (90). Accordingly, pioglitazone (which reduces insulin resistance) has been shown to have an antidepressant effect in two randomized controlled trials (91, 92). Furthermore, intranasal insulin and liraglutide (insulin secretagogue) are currently under investigation to evaluate their antidepressant effects (93, 94).
Psychological interventions.
A recent systematic review identified 13 clinical trials assessing psychological interventions in comorbid diabetes and depression (62). Interventions were highly variable in terms of quantity of time, delivery method (e.g., face to face, telephone, Internet based), content (e.g., CBT, psychoeducation), and provider of the intervention (e.g., physician, nurse, psychotherapist). Given the heterogeneity of methods and outcomes, a 2014 meta-analysis did not pool data to determine an overall effect size, but instead reported a range of the mean differences in effect on depressive symptoms (−1.47 to −0.14) (64). Of the interventions studied, CBT has the most robust evidence to support its use (62, 65). Four randomized controlled trials of CBT interventions demonstrated a moderate to large antidepressant effect (95–98). Also, CBT is most effective when supplemented by additional psychosocial interventions, including stress management strategies, coping skills training, case management, and motivation interviewing (78, 99). A 2010 meta-analysis noted larger effects on depressive symptoms from psychotherapy combined with diabetes self-management education when compared with pharmacotherapy alone (99).
In a recent meta-analysis, the effect of psychological interventions on glycemic control was highly variable, with a mean difference range in effect size ranging from −0.97 to 0.47 (59). A prior meta-analysis did note a moderate improvement in glycemic control with psychological interventions (99). Overall, heterogeneity has limited the accrual of meta-analytic evidence for an effect on glycemic control of psychological interventions, but the majority of studies have shown some benefit (62).
Lifestyle modification.
Lifestyle modification is a fundamental intervention for both depression and diabetes (62, 100–102). Physical exercise, diet modification, sleep hygiene, and smoking cessation simultaneously improve both medical and psychological outcomes. Exercise, in particular, has robust evidence as an intervention to improve both glycemic control and depressive symptoms (103). Recent research has shown that a healthy dietary pattern is associated with a reduced likelihood of depressive symptoms, especially for those with T2DM (104). In addition, a recent meta-analysis found that sleeping either six or fewer hours per night or eight or more hours per night was associated with an increased risk of T2DM in comparison with those who sleep seven hours per night (suggesting that interventions targeting insomnia or hypersomnia due to depression could reduce T2DM risk) (105).
Questions and Controversy: Shared Biological Underpinnings
There is mounting evidence to support the theory that shared biological mechanisms underlie major depressive disorder and T2DM (23, 24, 39). Depression and T2DM have a small and not statistically significant genetic correlation (r=0.19, CI=0.00–0.46), although the two diseases independently associate with common single nucleotide polymorphisms (24, 106). Adaptation to an adverse in utero environment (through “metabolic ageing”) could predispose patients to depression and diabetes via epigenetic mechanisms (e.g., DNA methylation), and low birthweight is associated with both T2DM and depression (24). Moreover, environmental exposures (e.g., childhood abuse and neglect, chronic adult work stress) and lifestyle (e.g., smoking, inactivity, poor diet), collectively comprising an individual’s allostatic load, predispose patients to both diabetes and depression (24) (see Figure 2).
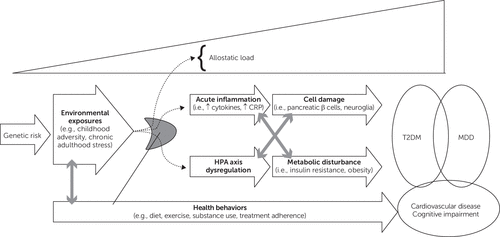
Figure 2. A Common Cause Model of Diabetes and Depressiona
a H-P-A, hypothalamic-pituitary-adrenal axis; CRP, C-reactive protein; T2DM, type 2 diabetes mellitus; MDD, major depressive disorder
One proposed biological pathway is overactivation of the innate immune system. Cytokine-induced inflammatory response can lead to β-cell apoptosis in the pancreas—causing insulin resistance—and oxidative stress in the brain—leading to reduced serotonin production via activation of the tryptophan-kynurenine pathway (23, 24, 39). Inflammatory markers predict onset of both T2DM and major depressive disorder (29, 105). Moreover, anti-inflammatory medications can ameliorate both depressive symptoms (e.g., celecoxib) and glycemic control (e.g., nonsteroidal anti-inflammatory drugs, interleukin-1 receptor agonists) (107–110).
Increased serum cytokines also activate the HPA axis, another biological pathway that might subserve both disorders (23, 24, 39). Chronic hypercortisolemia contributes to metabolic syndrome, insulin resistance, and T2DM (23, 24). Excess cortisol also hinders hippocampal neurogenesis, a region of the brain implicated in both major depressive disorder and T2DM (23, 24). Early-life adversity affects later-life HPA response; women exposed to childhood abuse have a hyperactive HPA response, and early stress leads to high glucocorticoid receptor density in the amygdala and hippocampus (23, 24).
Insulin resistance might be an independent path to both disorders. Depression and insulin resistance are linked, although meta-analysis has found that factors such as central adiposity attenuate this relationship (31). Obesity is an independent risk factor for both T2DM and major depressive disorder (23). Weight loss by obese individuals decreases depressive symptoms along with diabetes risk (23, 111). In addition, reduction of insulin resistance with pioglitazone has shown great promise in treating depressive symptoms in a myriad of settings (91, 92, 112–114).
Additional theorized pathways include circadian rhythm disruption (implicated in both T2DM and major depressive disorder) and alterations in brain-derived neurotropic factor (which has trophic effects in the endocrine and neurological systems and modulates hormones such as insulin and leptin) (23, 24, 39). By conceptualizing these two diseases in the framework of common biological underpinnings, we emphasize that the two disorders should not be treated in isolation. Rather than allowing the mind/body dualism to persist, clinicians must see depression as part of diabetes, and vice versa. The depression-diabetes phenotype predisposes individuals to severe sequelae, from cognitive dysfunction to cardiovascular death. Interventions targeting common biological factors have the great potential to reduce the global burden of disease.
Recommendations
We offer several recommendations to practitioners. First, with the knowledge that diabetes is a risk factor for the development of depression, screen with validated tools and follow positive screens with a diagnostic interview. Depression is likewise a risk factor for development of type 2 diabetes. Screen patients with depression for diabetes as well as for other metabolic parameters (fasting blood sugar, weight, waist circumference, lipid profile).
Second, treatment for comorbid diabetes and depression should target both medical and psychological outcomes. Prioritize treatments that are likely to improve both glucose control and depressive symptoms, such as weight-neutral antidepressants or CBT with psychoeducation. Emphasize lifestyle modification, including sleep hygiene, healthy diet, exercise, and avoidance of substance use.
Third, consider diabetes and depression as overlapping constructs, with common etiological factors (inflammation and the HPA axis) and synergistic effects on adverse outcomes (including cognitive impairment and cardiovascular disease).
1 Global status report on noncommunicable diseases 2014. Geneva, Switzerland, World Health Organization, 2014Google Scholar
2 : Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3:e442Crossref, Google Scholar
3 Depression. Fact sheet no 369. Geneva, World Health Organization, 2015. Available at http://www.who.int/mediacentre/factsheets/fs369/en/. Accessed Nov 29, 2015Google Scholar
4 : Chronic diseases and risk for depression in old age: a meta-analysis of published literature. Ageing Res Rev 2010; 9:131–141Crossref, Google Scholar
5 : Dialogue on diabetes and depression: dealing with the double burden of co-morbidity. J Affect Disord 2012; 142:S1–S3Crossref, Google Scholar
6 Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33:S62–S69Crossref, Google Scholar
7 : Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol 2015; 3:450–460Crossref, Google Scholar
8 : The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24:1069–1078Crossref, Google Scholar
9 : Epidemiology of depression and diabetes: A systematic review. J Affect Disord 2012; 142:S8–S21Crossref, Google Scholar
10 : The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2006; 23:1165–1173Crossref, Google Scholar
11 : Prevalence of comorbid depression is high in out-patients with type 1 or type 2 diabetes mellitus. Results from three out-patient clinics in the Netherlands. Diabet Med 2010; 27:217–224Crossref, Google Scholar
12 : The prevalence of co-morbid depression in adults with type 1 diabetes: systematic literature review. Diabet Med 2006; 23:445–448Crossref, Google Scholar
13 : Prevalence of depression among young people with type 1 diabetes: a systematic review. Diabet Med 2013; 30:199–208Crossref, Google Scholar
14 : Prevalence and correlates of depression in individuals with and without type 1 diabetes. Diabetes Care 2009; 32:575–579Crossref, Google Scholar
15 : Prevalence of depression in type 1 diabetes and the problem of over-diagnosis. Diabet Med (Epub Oct 3, 2015). Available at doi:
16 : Trends in the prevalence of depression in hospitalized patients with type 2 diabetes in Spain: analysis of hospital discharge data from 2001 to 2011. PLoS One 2015; 10:e0117346Crossref, Google Scholar
17 : Type 2 diabetes in patients with major depressive disorder: a meta-analysis of prevalence estimates and predictors. Depress Anxiety 2015; 32:763–773Crossref, Google Scholar
18 : Depression and multimorbidity: a cross-sectional study of 1,751,841 patients in primary care. J Clin Psychiatry 2014; 75:1202–1208, quiz 1208Crossref, Google Scholar
19 : Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006; 49:837–845Crossref, Google Scholar
20 : Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008; 31:2383–2390Crossref, Google Scholar
21 : Depression and risk for diabetes: a meta-analysis. Can J Diabetes 2015; 39:266–272Crossref, Google Scholar
22 : Cardiac risk factors in patients with diabetes mellitus and major depression. J Gen Intern Med 2004; 19:1192–1199Crossref, Google Scholar
23 : Depression and type 2 diabetes: a causal association? Lancet Diabetes Endocrinol 2014; 2:236–245Crossref, Google Scholar
24 : The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol 2015; 3:461–471Crossref, Google Scholar
25 : The bidirectional relationship of depression and diabetes: a systematic review. Clin Psychol Rev 2011; 31:1239–1246Crossref, Google Scholar
26 : Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008; 299:2751–2759Crossref, Google Scholar
27 : Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011; 73:114–126Crossref, Google Scholar
28 : A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev 2007; 3:252–259Crossref, Google Scholar
29 : A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67:446–457Crossref, Google Scholar
30 : Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2013; 36:166–175Crossref, Google Scholar
31 : A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care 2013; 36:480–489Crossref, Google Scholar
32 : Use of antidepressant medication and risk of type 2 diabetes: results from three cohorts of US adults. Diabetologia 2012; 55:63–72Crossref, Google Scholar
33 : Antidepressant medication use and risk of hyperglycemia and diabetes mellitus: a noncausal association? Biol Psychiatry 2011; 70:978–984Crossref, Google Scholar
34 : Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia 2010; 53:2480–2486Crossref, Google Scholar
35 : Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res Clin Pract 2013; 99:98–104Crossref, Google Scholar
36 : A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care 2000; 23:1556–1562Crossref, Google Scholar
37 : Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care 2011; 34:752–762Crossref, Google Scholar
38 : Antidepressant use before and after initiation of diabetes mellitus treatment. Diabetologia 2009; 52:425–432Crossref, Google Scholar
39 : Depression and type 2 diabetes: inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neurosci Biobehav Rev 2012; 36:658–676Crossref, Google Scholar
40 : Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol 2015; 11:701–711Crossref, Google Scholar
41 : Brain volume abnormalities and neurocognitive deficits in diabetes mellitus: points of pathophysiological commonality with mood disorders? Adv Ther 2010; 27:63–80Crossref, Google Scholar
42 : Diabetes mellitus and disturbances in brain connectivity: a bidirectional relationship? Neuromolecular Med 2014; 16:658–668Crossref, Google Scholar
43 : Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci 2015; 16:660–671Crossref, Google Scholar
44 : Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci 2011; 13:7–23Google Scholar
45 : Adverse effects of depression on glycemic control and health outcomes in people with diabetes: a review. Endocrinol Metab Clin North Am 2013; 42:529–544Crossref, Google Scholar
46 : Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000; 23:934–942Crossref, Google Scholar
47 : Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010; 33:23–28Crossref, Google Scholar
48 : Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care 2007; 30:2222–2227Crossref, Google Scholar
49 : Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care 2008; 31:2398–2403Crossref, Google Scholar
50 : Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia 2008; 51:1822–1825Crossref, Google Scholar
51 : The relationship between changes in depression symptoms and changes in health risk behaviors in patients with diabetes. Int J Geriatr Psychiatry 2010; 25:466–475Crossref, Google Scholar
52 : Symptoms of depression and diabetes-specific emotional distress are associated with a negative appraisal of insulin therapy in insulin-naïve patients with type 2 diabetes mellitus. A study from the European Depression in Diabetes [EDID] Research Consortium. Diabet Med 2009; 26:28–33Crossref, Google Scholar
53 : Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001; 63:619–630Crossref, Google Scholar
54 : Quality of depression care in a population-based sample of patients with diabetes and major depression. Med Care 2004; 42:1222–1229Crossref, Google Scholar
55 : Depression in adults with diabetes. Semin Clin Neuropsychiatry 1997; 2:15–23Google Scholar
56 : Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications 2005; 19:113–122Google Scholar
57 : Depression and diabetes. Psychiatry 2009; 8:203–207Crossref, Google Scholar
58 : Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA Psychiatry 2015; 72:612–619Crossref, Google Scholar
59 : Depression, diabetes and dementia. Key Issues Ment Health 2015; 179:42–53Crossref, Google Scholar
60 : Increased risk of myocardial infarction in depressed patients with type 2 diabetes. Diabetes Care 2011; 34:1729–1734Crossref, Google Scholar
61 : Depression and risk of mortality in people with diabetes mellitus: a systematic review and meta-analysis. PLoS One 2013; 8:e57058Crossref, Google Scholar
62 : Depression and diabetes: treatment and health-care delivery. Lancet Diabetes Endocrinol 2015; 3:472–485Crossref, Google Scholar
63 : Prevalence and correlates of undiagnosed depression among U.S. adults with diabetes: the Behavioral Risk Factor Surveillance System, 2006. Diabetes Res Clin Pract 2009; 83:268–279Crossref, Google Scholar
64 : Psychological and pharmacological interventions for depression in patients with diabetes mellitus: an abridged Cochrane review. Diabet Med 2014; 31:773–786Crossref, Google Scholar
65 International Diabetes Foundation Clinical Guidelines Taskforce: Global guidelines for type 2 diabetes. Brussels, Belgium, International Diabetes Foundation, 2012. Available at https://www.idf.org/sites/default/files/IDF-Guideline-for-Type-2-Diabetes.pdf. Accessed Nov 29, 2015Google Scholar
66 : Psychosocial factors and diabetes mellitus: evidence-based treatment guidelines. Curr Diabetes Rev 2005; 1:255–270Crossref, Google Scholar
67 : Executive summary: standards of medical care in diabetes—2014. Diabetes Care 2014; 37(Suppl 1):S5–S13Crossref, Google Scholar
68 : Managing medical and psychiatric comorbidity in individuals with major depressive disorder and bipolar disorder. Ann Clin Psychiatry 2012; 24:163–169Google Scholar
69 : The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid metabolic disorders. Ann Clin Psychiatry 2012; 24:69–81Google Scholar
70 : Key concepts in screening for depression in people with diabetes. J Affect Disord 2012; 142:S72–S79Crossref, Google Scholar
71 : Screening tools used for measuring depression among people with type 1 and type 2 diabetes: a systematic review. Diabet Med 2012; 29:164–175Crossref, Google Scholar
72 : The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry 2010; 32:345–359Crossref, Google Scholar
73 : Comparison of depressive symptoms in type 2 diabetes using a two-stage survey design. Psychosom Med 2013; 75:791–797Crossref, Google Scholar
74 : Associations between anxiety and depression symptoms and cognitive testing and neuroimaging in type 2 diabetes. J Diabetes Complications 2016; 30:143–149.Crossref, Google Scholar
75 : Validation of the PHQ-9 as a screening instrument for depression in diabetes patients in specialized outpatient clinics. BMC Health Serv Res 2010; 10:235Crossref, Google Scholar
76 : Limited effect of screening for depression with written feedback in outpatients with diabetes mellitus: a randomised controlled trial. Diabetologia 2011; 54:741–748Crossref, Google Scholar
77 : Screening and case-finding instruments for depression: a meta-analysis. CMAJ 2008; 178:997–1003Crossref, Google Scholar
78 : Co-occurrence of diabetes and depression: conceptual considerations for an emerging global health challenge. J Affect Disord 2012; 142:S56–S66Crossref, Google Scholar
79 : Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010; 363:2611–2620Crossref, Google Scholar
80 : Treatment of depression in diabetes: an update. Curr Opin Psychiatry 2009; 22:211–217Crossref, Google Scholar
81 : Quality of life and metabolic status in mildly depressed women with type 2 diabetes treated with paroxetine: a single-blind randomised placebo controlled trial. BMC Fam Pract 2003; 4:7Crossref, Google Scholar
82 : Quality of life and metabolic status in mildly depressed patients with type 2 diabetes treated with paroxetine: a double-blind randomised placebo controlled 6-month trial. BMC Fam Pract 2007; 8:34Crossref, Google Scholar
83 : Fluoxetine for depression in diabetes: a randomized double-blind placebo-controlled trial. Diabetes Care 2000; 23:618–623Crossref, Google Scholar
84 : Effect of pharmacological treatment of depression on A1C and quality of life in low-income Hispanics and African Americans with diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Care 2009; 32:2156–2160Crossref, Google Scholar
85 : Glycemic control improvement through treatment of depression using antidepressant drugs in patients with diabetes mellitus type 1. Neuroendocrinol Lett 2010; 31:801–806Google Scholar
86 : Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care 2013; 36:3337–3345Crossref, Google Scholar
87 : Cognitive behavioral therapy versus sertraline in patients with depression and poorly controlled diabetes: the Diabetes and Depression (DAD) study: a randomized controlled multicenter trial. Diabetes Care 2015; 38:767–775Crossref, Google Scholar
88 : Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 2014; 53:23–34Crossref, Google Scholar
89 : Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014; 71:1381–1391Crossref, Google Scholar
90 , Carvalho A, McIntyre RS: Towards a “metabolic” subtype of major depressive disorder: shared pathophysiological mechanisms may contribute to cognitive dysfunction. CNS Neurol Disord Targets 2014; 13:1693–1707Google Scholar
91 : Does pioglitazone improve depression through insulin-sensitization? Results of a randomized double-blind metformin-controlled trial in patients with polycystic ovarian syndrome and comorbid depression. Psychoneuroendocrinology 2013; 38:767–776Crossref, Google Scholar
92 : Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology 2012; 37:2093–2100Crossref, Google Scholar
93 University Health Network, Toronto: Cognitive dysfunction and glucagon-like peptide-1 agonists. ClinicalTrials.gov Identifier NCT02423824. Bethesda, MD, National Library of Medicine, 2015. Available at https://clinicaltrials.gov/ct2/show/NCT02423824?term=Cognitive+Dysfunction+and+Glucagon-like+Peptide-1+Agonists.&rank=1. Accessed Nov 29, 2015Google Scholar
94 University Health Network, Toronto: Effect of intranasal insulin on depressive symptoms in major depressive disorder. ClinicalTrials.gov Identifier NCT00570050. Bethesda MD, National Library of Medicine, 2015. Available at https://clinicaltrials.gov/ct2/show/NCT00570050?term=Effect+of+Intranasal+Insulin+on+Depressive+Symptoms+in+Major+Depressive+Disorder&rank=1. Accessed Nov 29, 2015Google Scholar
95 : Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1998; 129:613–621Crossref, Google Scholar
96 : A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care 2014; 37:625–633Crossref, Google Scholar
97 : A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Med Care 2011; 49:641–648Crossref, Google Scholar
98 : Web-based depression treatment for type 1 and type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2011; 34:320–325Crossref, Google Scholar
99 : Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: a systematic review and meta-analysis. Gen Hosp Psychiatry 2010; 32:380–395Crossref, Google Scholar
100 : Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. V. Complementary and alternative medicine treatments. J Affect Disord 2009; 117(Suppl 1):S54–S64Crossref, Google Scholar
101 : Exercise improves cardiorespiratory fitness in people with depression: a meta-analysis of randomized control trials. J Affect Disord 2016; 190:249–253Crossref, Google Scholar
102 : (4) Foundations of care: education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Diabetes Care 2015; 38(Suppl):S20–S30Crossref, Google Scholar
103 : Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr 2014; 19:496–508Crossref, Google Scholar
104 : The association between dietary patterns, diabetes and depression. J Affect Disord 2015; 174:215–224Crossref, Google Scholar
105 : Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2015; 38:529–537Crossref, Google Scholar
106 : A test for common genetic and environmental vulnerability to depression and diabetes. Twin Res Hum Genet 2011; 14:169–172Crossref, Google Scholar
107 : From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9:46–56Crossref, Google Scholar
108 : Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007; 356:1517–1526Crossref, Google Scholar
109 : Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2014; 48:79–85Crossref, Google Scholar
110 : Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med 2013; 159:1–12Crossref, Google Scholar
111 : Intentional weight loss and changes in symptoms of depression: a systematic review and meta-analysis. Int J Obes 2011; 35:1363–1376Crossref, Google Scholar
112 : Adjuvant pioglitazone for unremitted depression: clinical correlates of treatment response. Psychiatry Res 2015; 230:846–852Crossref, Google Scholar
113 : Pioglitazone is an effective treatment for patients with post-stroke depression combined with type 2 diabetes mellitus. Exp Ther Med 2015; 10:1109–1114Crossref, Google Scholar
114 : Use of insulin sensitizers for the treatment of major depressive disorder: a pilot study of pioglitazone for major depression accompanied by abdominal obesity. J Affect Disord 2012; 136:1164–1173Crossref, Google Scholar



