When Does Alzheimer’s Disease Really Start? The Role of Biomarkers
Abstract
While Alzheimer’s disease (AD) classical diagnostic criteria rely on clinical data from a stablished symptomatic disease, newer criteria aim to identify the disease in its earlier stages. For that, they incorporated the use of AD’s specific biomarkers to reach a diagnosis, including the identification of Aβ and tau depositions, glucose hypometabolism, and cerebral atrophy. These biomarkers created a new concept of the disease, in which AD’s main pathological processes have already taken place decades before we can clinically diagnose the first symptoms. Therefore, AD is now considered a dynamic disease with a gradual progression, and dementia is its final stage. With that in mind, new models were proposed, considering the orderly increment of biomarkers and the disease as a continuum, or the variable time needed for the disease’s progression. In 2011, the National Institute on Aging and the Alzheimer’s Association (NIA-AA) created separate diagnostic recommendations for each stage of the disease continuum—preclinical, mild cognitive impairment, and dementia. However, new scientific advances have led them to create a unifying research framework in 2018 that, although not intended for clinical use as of yet, is a step toward shifting the focus from the clinical symptoms to the biological alterations and toward changing the future diagnostic and treatment possibilities. This review aims to discuss the role of biomarkers in the onset of AD.
(Appeared originally in Int J Mol Sci 2019, 20 5536)
Alzheimer’s disease (AD) has always been a primarily clinical disease, seeing as its confirmation could only be reached through histopathological post-mortem studies. However, the more its physiopathology is known, the more certainty should be put into diagnosing it. Therefore, scientists have searched for biomarkers to help as diagnostic tools. Nevertheless, the fast rise of biomarkers gave rise to many questions such as, what biomarkers exist today? How can they be used in AD’s diagnosis? When does AD really start? In this review, we aim to answers those questions.
The Classical Diagnostic Criteria
Diagnosing AD has been an absolute challenge since it was described by Alzheimer at the beginning of the 20th century (1]. In the last part of this same century, the first diagnostic criteria were established by the United States National Institute for Communicative Disorders and Stroke—the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (2) and by the Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association (DSM-IV) (3).
The NINCDS-ADRDA-DSM-IV criteria defines AD as a syndrome, and its diagnosis has the following three grades of certainty: probable, possible, and definite AD, the latter usually requiring a post-mortem histopathological confirmation to reach diagnostic certainty. The sensitivity of the NINCDS-ADRDA-DSM-IV diagnostic criteria varied between 65–96% (4–7) and their specificity between 23–88% (6, 7) because other dementias such as Lewy bodies dementia, frontotemporal dementia, and vascular dementia could not be completely excluded (6, 8).
The Inclusion of Biomarkers in the Diagnostic Criteria
As the knowledge on the pathophysiological, molecular and structural changes in AD increased, Dubois et al. (8) revised AD’s classical diagnostic criteria and proposed new ones. These new diagnostic criteria aimed to identify AD in its earlier stages, before the development of a dementia syndrome. They established a specific clinical phenotype of AD, casting aside the diagnosis of exclusion and solving the problem of low diagnostic specificity, while also offering the chance of an early therapeutic intervention (8). Their principal criterion is a failure in episodic memory that appears early in the disease (A, see Table 1). Furthermore, they introduced as a novelty the support criteria which are based on biomarkers (B, C, D, and E). Therefore, the presence of the main criterion together with at least one of the supporting criteria is indicative of AD pathology.
| Criteria | Options |
|---|---|
| Main Criteria for AD Diagnosis (Obligatory) | (A) Early episodic memory failure represented by a gradual or progressive memory dysfunction at the beginning of the disease, informed by the patient or family, lasting more than six months. Associated with objective evidence of significant decline in episodic memory through tests (deferred memory). |
| Support Criteria for AD Diagnosis (At least one present) | (B) Loss of volume of the hippocampus, entorhinal cortex, amygdala or other mesial-temporal structures, evidenced by magnetic resonance imaging (MRI). |
| (C) Abnormality in CSF biomarkers such as - Low concentrations of Aβ; - Increased t-tau or p-tau concentrations; or - A combination of the three. | |
| (D) Specific metabolic pattern evidenced by PET such as hypometabolism of glucose in bilateral temporal parietal regions. | |
| (E) Autosomal dominant family genetic mutations On chromosomes 21 (APP), 14 (PS1), or 1 (PS2). |
TABLE 1. Main proposed criteria for Alzheimer’s disease (AD) diagnosis by Dubois et al. (2007) [8].
With these criteria, Dubois et al. (8) arrive at the diagnosis of AD earlier and in a more specific way than their predecessor, the NINCDS-ADRDA criteria, covering the earliest stages of the disease, when there is still no dementia syndrome, as well as the later stages when the patient is already functionally disabled. The ingenuity resides on the fact that biological biomarkers are included in the criteria for the first time, as a requirement to reach diagnosis. These biomarkers include structural and molecular imaging, cerebrospinal fluid (CSF) analysis, and genetic mutation analysis.
After Dubois’ first step in including biomarkers as diagnostic criteria, in 2011, the National Institute on Aging and the Alzheimer’s Association (NIA-AA) defined new diagnostic criteria that separated the disease in three clinical stages, each one with its own diagnostic recommendation. First is the preclinical stage that presents pathologic brain changes, which may be in progress decades prior to disease, without evident clinical symptoms. In this stage, alterations can be seen in CSF and imaging biomarkers although, at present, they cannot predict which of these individuals will develop dementia (9). The second stage is Mild Cognitive Impairment (MCI), which is marked by memory symptoms that are greater than normal for a person’s age and education but that do not interfere with their independence and may or may not progress to Alzheimer’s dementia (10). The final stage is Alzheimer’s dementia, in which symptoms are significant enough to impair a person’s ability to function independently (11).
The definition of a preclinical stage that could be in progress many years before the beginning of symptoms meant that the biomarkers acquired a greater importance in the diagnostic process.
Alzheimer’s Disease Biomarkers
Biomarkers are defined as physiological, biochemical, or anatomical variables that can be measured in vivo and that characterize specific pathological changes of a disease. We can classify AD biomarkers, according to the method of analysis, into biochemical CSF biomarkers or imaging-derived biomarkers (12, 13).
Biomarkers in Cerebrospinal Fluid
CSF biomarkers are, nowadays, widely used in routine clinical practice to support the diagnosis of MCI and AD (14–16). The levels of beta-amyloid peptide (Aβ), total tau (t-tau), and phospho-tau (p-tau) in CSF are used as specific biomarkers of AD, since they reflect the pathologic processes of Aβ42 aggregation and tau’s hyperphosphorylation, respectively, and are included within the support criteria for the clinical diagnosis of probable AD (8).
Aβ42 and Tau as Biomarkers.
There is an inverse correlation between brain amyloid load and CSF Aβ42 levels, with the latter being diminished in AD patients with respect to healthy subjects. The levels of Aβ in CSF reflect the pathologic process of aggregation of this peptide into amyloid plaques. Thus, a reduction on its clearance toward the CSF happens, and its concentration decreases. In early stages of AD, Aβ levels are already altered in the CSF; in fact, there are studies evidencing that CSF Aβ begins to show abnormal levels several years before the appearance of the first subjective memory complaints (17), which makes it the earliest marker that exists today (18).
On the other hand, tau levels in CSF are a reflection of tau’s pathogenesis in the cerebral cortex (19). Although p-tau should be a more specific indicator of AD than t-tau, both t-tau (20) and p-tau (20, 21) behave in a very similar way in AD, increasing their concentrations in CSF. Both increments are associated with the load of neurofibrillary tangles and are indicators of neuronal damage (13, 21–23). Although an increase in CSF tau is not specific to AD, it does correlate with the clinical severity of the disease, increasing its levels at the same time as cognitive failure increases (22).
In early stages of AD, lower levels of Aβ appear in CSF, and it is considered a predictor of the evolution of MCI to AD (23) and, in the same way, high levels of p-tau and t-tau in CSF can predict with good accuracy an incipient AD in patients with MCI (24). These parameter alterations appear even in cognitively normal subjects, where it is possible to detect abnormalities of Aβ and tau in CSF many years before MCI is diagnosed (25–33).
Interestingly, it has been shown that cognitively normal carriers of the Apolipoprotein ε4 allele (APOE4), who are at risk for late-onset AD, also show decreased levels of Aβ in CSF (33, 34). In familial AD, studies have seen that asymptomatic carriers of PSEN1 and APP mutations show alterations in the levels of CSF Aβ and p-tau that precede the clinical onset of the disease by more than 10 years (35–37).
Imaging Biomarkers in AD
PiB-PET.
The Pittsburgh compound B (PiB) is a specific ligand of Aβ that, when used as a tracer in a positron emission tomography (PiB-PET), makes it possible to analyze in vivo both cerebral Aβ load and Aβ spatial distribution. It has been shown that ante-mortem imaging studies with PiB-PET represent a direct measurement of amyloid plaque burden (38–44) and correlate well with post-mortem studies (44, 45). Furthermore, the cerebral load of PiB is inversely related to the levels of CSF Aβ (24, 27, 46).
The conversion of a subject from PiB-negative to PiB-positive occurs at a very early stage of the development of AD (47). When considering cognitive normal subjects, APOE ε4 allele carriers have a higher rate of conversion to PiB-positive that happens years before the clinical onset of AD than those that do not carry the gene. Moreover, asymptomatic carriers of PSEN1 and APP mutations present a higher load of PiB in the cortex and striatum (48–50). Nevertheless, there are also cognitive normal elderly individuals that do not carry genetic risk factors that present a positive PiB signal. Therefore, PiB-PET is only recommended when other clinical evidences of dementia are present (19, 26), even though it is considered a valid marker to help in the diagnosis (51).
Recently, the advent of tau-specific ligands for PET imaging allows the usage of this technique as a biomarker (52–54). It could be thought that this new Tau-PET might compete with the stablished amyloid-PET, but a recent work proposes that combining both could be better to track AD progression (55, 56).
FDG-PET.
PET imaging with 2-deoxy-2 [18F] fluoro-D-glucose tracer (FDG-PET) measures the cerebral metabolism of glucose and is an indicator of neuronal and glial function. In AD, FDG-PET signal decreases, which is consistent with glucose hypometabolism and synaptic dysfunction, and it also has a specific topographic distribution pattern (57). In addition, it correlates with decreased levels of synaptophysin found post-mortem that indicate a loss of synaptic activity (58, 59). Furthermore, a bilateral reduction in FDG uptake in the temporal and parietal regions and especially in the cingular cortex is described in individuals with AD (60, 61). Moreover, FDG uptake is inversely related to the cognitive deficit, that is to say, less uptake correlates with greater cognitive damage throughout the entire clinical spectrum of the disease (62) with a sensitivity and a specificity greater than 80% (63, 64). Cognitively normal APOE4 carriers also have glucose hypometabolism (65, 66), which is worse in homozygotes than in heterozygotes (67), and can be detected as early as the third decade of life (68).
Finally, it is now possible to assess the regional distribution and the total tau burden in vivo with new PET imaging radiotracers. In this regard, it has been shown that tau-PET is a more sensitive marker for the detection of the earliest cognitive changes in AD than Aβ PET and cortical thickness measures (69).
Structural and Functional Magnetic Resonance Imaging (MRI).
Cerebral atrophy, specifically in mesial-temporal structures, can be quantified using structural magnetic resonance imaging (MRl) (60, 70) and can be detected before the appearance of the first clinical symptoms. In fact, many studies defend this biomarker as a reliable diagnostic tool (71) and as a neurodegeneration marker (61, 72). Indeed, it is included in both the Dubois (8) and the NIA-AA (16) diagnostic criteria and its specificity and sensitivity as an AD marker is greater than 85% (60).
In early AD, the first detectable signs that can be observed in a structural MRI are atrophy in the middle temporal lobe (affecting specially the hippocampus), and a decrease in the thickness of the cerebral cortex in regions that are vulnerable to AD (73–81). In asymptomatic carriers of APP mutations, a decrease in hippocampal volume can be identified 2–3 years before the onset of dementia (82) and, in elderly people, this alteration can be detected up to six years before (72–77). Moreover, in cognitively normal elders, CA1 region abnormalities represent an early predictor of the development of dementia (74). In addition, the loss of volume in the entorhinal cortex precedes cognitive decline by four years and has a predictive power of up to 90% (75).
When Does AD Really Start?
With the advent of biomarkers came a conceptual change of the disease. We moved from a “static and defensive” view of the pathogenesis of AD to a “dynamic and compensatory” point of view. In the first viewpoint, the brain lesions that lead to neuronal and synaptic loss and finally to cognitive deterioration depend on the degree of external aggression and on the structural reserve that each person has. The current view considers an inter-individual variability in the response to these initial aggressions, as well as differences in the severity of the pathological process and in the efficiency and evolution over time of the cerebral compensatory mechanisms (83–85). Therefore, the idea that AD’s main pathological processes have already taken place before we can clinically diagnose MCI has been established, and this is reinforced by the fact that these lesions begin even decades before the appearance of the earliest symptoms, when the subject is still cognitively normal (86). Therefore, this change in perspective increasingly supports the need for early therapeutic action in order to compensate for those biological processes that are already compromised before the onset of the cognitive failure (87).
AD is now considered a neurodegenerative disease with a very long evolution that starts silently decades before the onset of symptoms and advances gradually and slowly until it compromises the person’s cognition. Therefore, we moved from a static vision of AD in which a person is affected or not by the disease, to a dynamic concept of AD, in which dementia is considered the final stage of a set of pathological changes that occur in a chronic and gradual manner.
In this progression, which may take years, biomarkers can anticipate the clinical manifestations of dementia and, as the new diagnostic criteria introduced biomarkers in a supporting role in AD’s diagnosis, many laboratories worldwide have already started using them. With this in mind, and based on determinations made in different populations, Jack and collaborators proposed a model for the evolution of AD over time, known as “the dynamic biomarker cascade model” (16). In this model, biomarkers do not increase all at once but do so in an orderly manner, a concept that is reinforced in the work by Dubois and collaborators (18). The model presents three phases along the continuum of the disease—first, the cognitively normal asymptomatic phase, then the MCI phase that begins to show clinical affectation, and finally the dementia phase. All over this spectrum, the biomarkers described previously would present abnormal levels as the disease evolves and, eventually, correlate with the clinical symptoms presented by the patients.
Jack and collaborators propose as the initial event the abnormally increased levels of Aβ that would lead to the formation of cerebral amyloid plaques. This would be reflected in the decreased levels of Aβ in CSF and in the increased amyloid load in PiB-PET, and these alterations would appear while the individuals are still cognitively normal. Afterward, there would be an increase in CSF tau abnormalities followed by alterations in FDG-PET. These are biomarkers of neuronal dysfunction and neurodegeneration and correlate with the severity of clinical symptoms. Lastly, in advanced stages, structural brain changes would appear such as cortical atrophy and decreased hippocampal volume that could be detected by MRI (16).
A very recent work by Petrella and collaborators (88) developed a mathematical causal model of the dynamic biomarker cascade theory in AD, which might help to explain how these biomarkers interact and evolve over time and could potentially help patients, researchers, and medical personnel. This is a great advancement in the knowledge of the disease, but there is still a long way to go. Although, biomarkers could have a role in predicting whether a patient could convert from MCI to AD, there is not a consensus on which biomarkers could assume that role (89, 90).
However, the scientific community’s efforts go beyond designing computational models to determine the behaviour of different biomarkers in the evolution of the disease. Models have been designed for many different aspects of the disease, such as a model based on the amyloid cascade hypothesis, showing the effects of pathological processes such as oxidative stress, inflammation or cerebrovascular disease in the kinetic of Aβ aggregation (91). Moreover, another model focused on synaptic loss and compensation by the reinforcement of the remaining connections (92) and, more recently, Ding et al. (2018) designed a hybrid computational approach for a more accurate disease severity classification (93).
Nevertheless, as scientists started to better understand AD’s pathophysiology, the biggest challenge became designing computational models capable of predicting the efficacy of a specific treatment. To reach this objective, models have been created analysing potential treatments. Anastasio (2013) incorporated the role of estrogens in Aβ regulation into a model that can generate therapeutic predictions and the possible benefits of this therapy (94). This model showed that estrogen could reduce Aβ and that non-steroidal anti-inflammatory drugs could provide a small additional benefit.
Furthermore, immunotherapy, probably the most promising treatment for AD at this moment, was also analysed by computational models. Diem et al. (2016), have incorporated this therapy’s possible complications into their model and concluded that a failure in periarterial drainage seems to be an important mechanism (95). Another computer simulation model pointed out that immunotherapy against Aβ might not be effective, unless it is used during early stages of AD (96) or combined with other therapies. However, a more recent model simulated the differential impact of Aβ oligomers on glutamate and nicotinic neurotransmission while under different treatments, including a passive vaccination with the monoclonal antibody solanezumab, the use of the beta-secretase inhibitor verubecestat, and of the gamma-secretase inhibitor semagacestat. They predicted a cognitive worsening in people with low Aβ baseline and an improvement in those with moderate to high Aβ levels (97).
Computational models analyzing neurotransmitters have also been created. One such model has been implemented using preclinical data available on receptor pharmacology of cholinergic and catecholamine neurotransmitters and clinical data, to predict the effects of memantine, an N-Methyl-D-aspartic acid (NMDA) inhibitor, in different phases of AD pathology (98).
Finaly, Stefanovski et al. (2019) created a computational multi-scale brain model, using the Virtual Brain Platform, and including PET and electroencephalogram, to simulate regional neural activity and hyperexcitability in AD and how it relates to Aβ. This model reveals a potential functional reversibility of large-scale alterations in AD after memantine treatment (99).
Abnormalities in Biomarkers Precede Clinical Symptoms
In the “dynamic biomarker cascade model,” each biomarker reaches its maximum effect at a certain moment in the progression of the disease, and that happens in an orderly manner over time. Interestingly, the maximum levels can be detected in a person before any clinical symptom. In fact, several studies have shown that 20–40% of cognitively normal old present Aβ deposits in their cerebral tissue (22, 100, 101). Moreover, in post-mortem samples from non-demented elderly people, Aβ plaques were also present (102, 103). Therefore, deposits of amyloid plaques alone, even in significant quantities, are not enough to produce dementia (16, 26, 27, 30). Not only Aβ but also tangles may be present in subjects without cognitive decline. Nonetheless, in asymptomatic patients, the presence of neurofibrillary tangles tends to be limited to the entorhinal cortex (stages of Braak I–II), while in symptomatic subjects, tangles are much more widespread (102, 103).
On the other hand, data obtained through imaging studies with PiB-PET suggests that Aβ deposits may appear up to two decades before the onset of clinical manifestations of dementia (86). This concurs with the fact that, after the diagnosis of AD, the levels of Aβ in CSF do not change significantly, since it has already reached a plateau, and also with the pattern of PiB retention, which is not modified throughout the disease’s spectrum (86). Consistent with the previous studies, investigations on familiar AD have shown that these alterations in Aβ and p-tau precede by more than 10 years the clinical onset of the disease (104). Very recently, sequential changes in normal older adults, from Aβ to tau to cognition, have been described using repeated tau-PET and amyloid-PET measures to detect the earliest AD pathologic changes (105).
These studies are all in line with a final concept that this model postulates—the existence of a latent phase of variable duration between plaque formation and the onset of the neurodegenerative cascade. This could be due to differences in the processing of Aβ, to the capacity of resistance to pathological damage derived from the toxicity of Aβ and to compensatory mechanisms (106).
There is an increasing idea that AD pathology would trigger cerebral compensatory mechanisms all across the AD spectrum, and it would be in the preclinical phase that these mechanisms would begin to appear. However, there is still no consensus regarding the role that compensatory mechanisms might play in cognitively healthy subjects at risk of AD with positive biomarkers, and also regarding the influence they could have on the conversion to dementia. Lazarczyk et al. (104) suggest that the compensatory mechanisms would be divided into two categories: the passive ones (matching the cognitive reserve concept) and the active ones. The former would delay conversion to dementia, and the latter could stop disease progression in the preclinical phase and effectively prevent conversion to dementia.
Anatomically, this compensatory mechanism can be seen in structural changes found in asymptomatic people carrying the presenilin 1 mutation when compared to age-matched controls (107). These individuals present a cortical thickening mainly in temporal and parietal areas, extending into precentral and postcentral cortex and pars triangularis, and also in structures of the posterior midline, such as precuneus and posterior cingulate. No areas of cortical thinning are observed in asymptomatic carriers, unlike those found in symptomatic people. Furthermore, structural changes are not limited to subjects with mutations in AD determinant genes, since they are also seen in the sporadic form of the disease. Some studies have shown that healthy subjects with evidence of initial deposits of Aβ (108) present greater volume and thickness in AD related cortical regions. With AD progression, these areas suffer a progressive thinning of gray and also white matter reaching the atrophy observed in more extensive regions in the symptomatic stage (109).
It would be interesting to have more studies evaluating these compensatory mechanisms, to understand it better and, if possible, to add it to the current computational models evaluating AD’s pathology and possible treatments, as they seem to be present since the very early stages of the disease.
Influence of Risk Factors in Biomarker Dynamics
In 2013, Jack and Holtzman proposed a new model (17) where the subjects are classified by the time needed by a subject to progress through the entire spectrum of the disease and not by clinical stage. However, this time varies for each individual. According to this model, people with high risk of developing the disease would show greater abnormality in the biomarkers when compared to those individuals with low risk at the same age. High-risk subjects would be those carrying genetic allele mutations such as APP/PSEN1, as well as those with low cognitive reserve and those whose lifestyles increase the probability of cognitive damage. Low-risk subjects with a protective genetic profile, a high cognitive reserve, no pathological brain comorbidities, and a low-risk lifestyle for dementia could maintain a normal cognitive function despite developing AD pathology (Figure 1). Interestingly, Aβ-positive patients with greater cognitive reserve show attenuated clinical progression in pre-dementia stages of AD but accelerated cognitive decline after the onset of dementia (110).
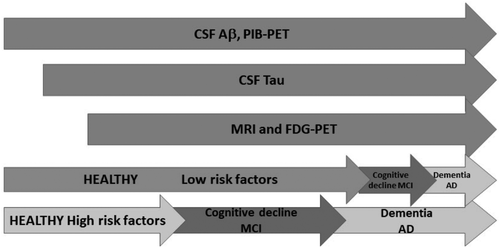
FIGURE 1. Evolution of the different AD biomarkers along time. Cerebrospinal fluid (CSF) Aβ and Pittsburgh compound B positron emission tomography (PiB-PET) abnormalities appear first followed by an increase in CSF tau levels and finally by structural alterations evidenced by magnetic resonance imaging (MRI) and fluoro-D-glucose tracer positron emission tomography (FDG-PET) (blue). The presence of risk factor determines the onset of the disease. People with low risk factors will present cognitive decline later than those with high risk factor (green).
Recently, McDade et al. (111) carried out a longitudinal study with high-risk subjects carrying autosomal dominant mutations and showed that the sequence and temporal dynamics of the biomarkers match the theoretical model. Another very recent study with more than 3600 participants showed two patterns of changes in biomarkers, one in APOE4 carriers and another in non-carriers. Subjects without the APOE4 allele showed an initial increase and then a decrease in CSF Aβ42 with a progression of CSF tau, while subjects with at least one APOE4 allele showed only a decrease in CSF Aβ42 associated with a progression of tau pathology, and both markers became positive with the progression of the disease (112).
New AD Classification Based on Biomarkers
Biomarkers provide a powerful tool for the clinical practice as well as for research, especially in human clinical trials, by improving diagnosis. This knowledge leads to an increase in clinical trials evaluating possible drugs that could cure, ease or control AD, but the efforts are still challenging.
Since the gap between the pathological processes and the cognitive symptoms has proven itself quite large, and the risk of generating hypotheses that were not based on the pathophysiological changes of AD was a reality, the NIA-AA (113) proposed a new framework for the use of biomarkers in observational and interventional research. Therefore, the A/T/N system was described classifying subjects according to the number of positive biomarkers they presented. This system includes a binary system (positive or negative) depending on the measured biomarker. “A” refers to the value of a β-amyloid biomarker (amyloid PET or CSF Aβ42), “T” to the value of a tau biomarker (CSF p-tau, or tau PET), and “N” to biomarkers of neurodegeneration or neuronal injury (18FDG–PET, structural MRI, or CSF total-tau) (114). A person with a positive “A” biomarker is classified as being in the “Alzheimer’s continuum”, that denotes either Alzheimer’s pathologic changes or AD, and those with positive biomarkers in both “A” and “T” categories are classified as having AD (113). The possible outcomes of this classification system are illustrated in Table 2.
| A/T/N Profiles | Biomarker Outcome | Diagnosis | ||
|---|---|---|---|---|
| A+ | T+ | N+ | AD | AD SPECTRUM |
| N– | AD | |||
| T– | N+ | AD and suspected non-AD pathologic change | ||
| N– | Alzheimer’s pathological change | |||
| A– | T+ | N+ | Non AD pathological change | No AD |
| N– | Non AD pathological change | |||
| T– | N+ | Non AD pathological change | ||
| N– | Normal BIOMARKERS | |||
TABLE 2. Outcomes on the biomarker’s A/T/N classification [113]. A: amyloid. N: Neurodegeneration. T: Tau.
A recent study analysing the prevalence of biologically defined AD compared to clinically defined probable AD reports that the former is more prevalent than the latter, especially at age 85 years, a difference that is mostly driven by asymptomatic individuals with biological Alzheimer disease, that could be diagnosed after the A/T/N description (115).
Therefore, this research framework defines AD by its pathological alterations that could be documented by biomarkers and not by its clinical consequences. Although, at the moment, it is intended only for research and not routine clinical care (113, 116), this new viewpoint might improve the selection of subjects and the conception of new therapies for clinical trials.
Conclusions
As we have seen in this article, our understanding of AD’s pathophysiology has changed considerably over the last few years. The disease is now considered a continuum, were the first stage begins decades before clinical symptoms are evident. This stage can be detected only with the help of specific biomarkers that identify the pathological process that is already in progress. Different models of the biomarkers’ progress have been created that consider their presence, the time they take to rise, their correlation to the individual’s cognitive status, and other factors that may contribute to an individual’s cognitive resilience despite the pathologic load. The last advance in this field was the new research framework defined by the NIA-AA in 2018. Although not indicated for use in clinical settings, this framework unifies all the biomarkers and their use and identifies them as the most important diagnostic factors. This vision ultimately changes the idea of AD as a clinical disease into a biological disease that, in reality, begins decades before any symptom start. This creates many new future possibilities, not only in the search for a more accurate and early diagnosis, but also in the search for more specific and efficient treatments.
1. : Über eigenartige Krankheitsfälle des späteren Alters. Z Gesamte Neurol Psychiatr 1911; 4:356385.Crossref, Google Scholar
2. : The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer’s disease: A clinicopathologic study of 57 cases. Neurology 1988; 38:359–364.Crossref, Google Scholar
3.
4. : Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc 1999; 47:564–569.Crossref, Google Scholar
5. : Accuracy of clinical criteria for A Din the Honolulu-Asia Aging Study, a population-based study. Neurology 2001; 57:226–234.Crossref, Google Scholar
6. : Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer’s disease and fronto temporal dementia. J Neurol Neurosurg Psychiatry 1999; 66:184–188.Crossref, Google Scholar
7. : Clinicopathologic correlates in Alzheimer disease: Assessment of clinical and pathologic diagnostic criteria. Alzheimer Dis Assoc Disord 1993; 7:152–164.Crossref, Google Scholar
8. : Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol 2007; 6:734–746.Crossref, Google Scholar
9. : Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 2011; 7:280–292.Crossref, Google Scholar
10. : The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 2011; 7:270–279.Crossref, Google Scholar
11. : The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 2011; 7:263–269.Crossref, Google Scholar
12. : Biomarkers and surrogate endpoints: Developing common terminology and definitions. Jama 2016; 315:1107–1108.Crossref, Google Scholar
13. : Identifying and validating biomarkers for Alzheimer’s disease. Trends Biotechnol 2011; 29:26–32.Crossref, Google Scholar
14. : Biomarkers of Alzheimer’s disease. Neurobiol Dis 2009; 35:128–140.Crossref, Google Scholar
15. : Biological markers of amyloid β-related mechanisms in Alzheimer’s disease. Exp Neurol 2010; 223:334–346.Crossref, Google Scholar
16. : Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 2010; 9:119–128.Crossref, Google Scholar
17. : Biomarker modeling of Alzheimer’s disease. Neuron 2013; 80:1347–1358.Crossref, Google Scholar
18. : Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement 2016; 12:292–323.Crossref, Google Scholar
19. : CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 2012; 78:1568–1575.Crossref, Google Scholar
20. : Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 2009; 66:382–389.Crossref, Google Scholar
21. : CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 2006; 129:3035–3041.Crossref, Google Scholar
22. : Cerebro spinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 2009; 65:403–413.Crossref, Google Scholar
23. : Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 2012; 69:98–106.Crossref, Google Scholar
24. : Cerebrospinal fluid tau and ptau (181) increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009; 1:371–380.Crossref, Google Scholar
25. : CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009; 302:385–393.Crossref, Google Scholar
26. : Decreased cerebrospinal fluid A beta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol 2009; 65:176–183.Crossref, Google Scholar
27. : Cerebrospinal fluid tau/beta-amyloid (42) ratio as a prediction of cognitive decline in non demented older adults. Arch Neurol. 2007; 64:343–349.Crossref, Google Scholar
28. : Alzheimer’s Disease Neuroimaging Initiative Investigators. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Aβ1-42. Ann Neurol 2010; 68:825–834.Crossref, Google Scholar
29. : Preclinical evidence of Alzheimer changes: Convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol 2009; 66:632–637.Crossref, Google Scholar
30. : CSF tau/Abeta 42 ratio for increased risk of mild cognitive impairment: A follow-upstudy. Neurology 2007; 69:631–639.Crossref, Google Scholar
31. : Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: A population-based study in 85-year-olds. Dement Geriatr Cogn Disord 2003; 15:169–176.Crossref, Google Scholar
32. : Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry 2007; 78:461–464.Crossref, Google Scholar
33. : CSF T-Tau/Aβ42 predicts white matter microstructure in healthy adults at risk for Alzheimer’s disease. PLoS ONE 2012; 7:e37720.Crossref, Google Scholar
34. : Cerebrospinal fluid beta-amyloid 1-42 and tau in control subjects at risk for Alzheimer’s disease: The effect of APOE epsilon 4 allele. Biol Psychiatry 2004; 56:670–676.Crossref, Google Scholar
35. : Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology 2008; 71:85–92.Crossref, Google Scholar
36. : Proteomic changes in cerebrospinal fluid of presymptomatic and affected persons carrying familial Alzheimer disease mutations. Arch Neurol 2012; 69:96–104.Crossref, Google Scholar
37. : Familial Alzheimer disease: Decreases in CSF A beta 42 levels precede cognitive decline. Neurology 2005; 65:323–325.Crossref, Google Scholar
38. : Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004; 55:306–319.Crossref, Google Scholar
39. : Molecular imaging with Pittsburgh Compound B confirmed at autopsy: A case report. Arch Neurol 2007; 64:431–434.Crossref, Google Scholar
40. : Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 2007; 62:229–234.Crossref, Google Scholar
41. : PIB is a non-specific imaging marker of amyloid-beta (A beta) peptide-related cerebral amyloidosis. Brain J Neurol. 2007; 130:2607–2615.Crossref, Google Scholar
42. : In vivo fibrillar β-amyloid detected using [11c] pib positron emission tomography and neuropathologic assessment in older adults. Arch Neurol 2011; 68:232–240.Crossref, Google Scholar
43. : Correspondence between in vivo 11C-PiB PET amyloid imaging and post-mortem, region-matched assessment of plaques. Acta Neuropathol 2012; 124:823–831.Crossref, Google Scholar
44. : Antemortem amyloid imaging and β-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol Aging 2012; 33:878–885.Crossref, Google Scholar
45. : Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain J Neurol 2008; 131:1630–1645.Crossref, Google Scholar
46. : Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid A beta 42 in humans. Ann Neurol 2006; 59:512–519.Crossref, Google Scholar
47. : Amyloid-beta plaque growth in cognitively normal adults: Longitudinal [11C]Pittsburgh compound B data. Ann Neurol. 2011; 70:857–861.Crossref, Google Scholar
48. : Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci Off J Soc Neurosci 2007; 27:6174–6184.Crossref, Google Scholar
49. : High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol 2009; 66:1537–1544.Crossref, Google Scholar
50. : Glucose metabolism and PIB binding in carriers of a His163Tyr presenilin 1 mutation. Neurobiol Aging 2011; 32:1388–1399.Crossref, Google Scholar
51. : Imaging β-amyloid burden in aging and dementia. Neurology 2007; 68:1718–1725.Crossref, Google Scholar
52. : Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 2013; 79:1094–1108.Crossref, Google Scholar
53. : Discovery of 6-(Fluoro-(18)F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([(18)F]-MK-6240): A positron emission tomography (PET) imaging agent for quantification of neurofibrillary tangles (NFTs). J Med Chem 2016; 59:4778–4789.Crossref, Google Scholar
54. : Tau PET imaging in neurodegenerative tauopathies—Still a challenge. Mol Psychiatry 2019; 24:1112–1134.Crossref, Google Scholar
55. : Imaging Aβ and tau in early stage Alzheimer’s disease with [18 F] AV45 and [18 F] AV1451. EJNMMI Res 2018; 8:19.Crossref, Google Scholar
56. and
57. : What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 2007; 69:871–877.Crossref, Google Scholar
58. : Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science 1979; 205:723–725.Crossref, Google Scholar
59. : An Energy Budget for Signaling in the Grey Matter of the Brain. J Cereb Blood Flow Metab 2001; 21:1133–1145.Crossref, Google Scholar
60. : Alzheimer’s disease. Lancet 2011; 377:1019–1031.Crossref, Google Scholar
61. ; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016; 388:505–517.Crossref, Google Scholar
62. : Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997; 42:85–94.Crossref, Google Scholar
63. : Longitudinal change in CSF Tau and Aβ biomarkers for up to 48 months in ADNI. Acta Neuropathol 2013; 126:659–670.Crossref, Google Scholar
64. : Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. NeuroImage Clin 2013; 4:45–52.Crossref, Google Scholar
65. : Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 1996; 334:752–758.Crossref, Google Scholar
66. : Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 1995; 273:942–947.Crossref, Google Scholar
67. : Correlations between apolipoprotein E epsilon 4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci USA 2005; 102:8299–8302.Crossref, Google Scholar
68. : Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA 2004; 101:284–289.Crossref, Google Scholar
69. : Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology 2019; 92:e601–e612.Crossref, Google Scholar
70. : The histological validation of postmortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience 2000; 95:721–725.Crossref, Google Scholar
71. : The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol 2010; 6:67–77.Crossref, Google Scholar
72. : The critical need for defining preclinical biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2014; 10:S196–S212.Crossref, Google Scholar
73. : Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry 2006; 63:57–62.Crossref, Google Scholar
74. : Preclinical detection of Alzheimer’s disease: Hippocampal shape and volume predict dementia onset in the elderly. Neuroimage 2005; 25:783–792.Crossref, Google Scholar
75. : Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol Aging 2010; 31:1099–1106.Crossref, Google Scholar
76. : Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology 2003; 229:691–696.Crossref, Google Scholar
77. : Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 2000; 55:484–489.Crossref, Google Scholar
78. : Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging 2010; 31:1077–1088.Crossref, Google Scholar
79. : Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 2011; 76:1395–1402.Crossref, Google Scholar
80. : The cortical signature of Alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex 2009; 19:497–510.Crossref, Google Scholar
81. : Alzheimer’s Disease Neuroimaging Initiative. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology 2012; 78:84–90.Crossref, Google Scholar
82. : Imaging of onset and progression of Alzheimer’s disease with voxel-compression mapping of serial magnetic resonance images. Lancet Lond Engl 2001; 358:201–205.Crossref, Google Scholar
83. : β-Amyloid and the Pathogenesis of Alzheimer’s Disease. N Engl J Med 1991; 325:1849–1857.Crossref, Google Scholar
84. : Alzheimer’s diseaseas a presumptive threshold phenomenon. Neurobiol Aging 1987; 8:552–554.Crossref, Google Scholar
85. : The Pathogenesis and Progression of the Pathological Changes of Alzheimer’s disease. Ann Med. 1989; 21:133–136.Crossref, Google Scholar
86. : Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain J Neurol 2009; 132:1355–1365.Crossref, Google Scholar
87. : Anti-Aβ Therapeutics in Alzheimer’s disease: The Need for a Paradigm Shift. Neuron 2011; 69:203–213.Crossref, Google Scholar
88. : Computational Causal Modeling of the Dynamic Biomarker Cascade in Alzheimer’s disease. Comput Math Methods Med 2019.Crossref, Google Scholar
89. : Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol Aging 2011; 32:2322-e19.Crossref, Google Scholar
90. : Conversion of amyloid positive and negative MCI to AD over 3 years: An 11C-PIB PET study. Neurology 2009; 73:754–760.Crossref, Google Scholar
91. : Data driven modelling of Alzheimer’s disease pathogenesis. J Theor Biol 2011; 290:60–72.Crossref, Google Scholar
92. : Neural network modeling of memory deterioration in Alzheimer’s disease. Neural Comput 1993; 5:736–749.Crossref, Google Scholar
93. : A hybrid computational approach for efficient Alzheimer’s disease classification based on heterogeneous data. Sci Rep 2018; 8:9774.Crossref, Google Scholar
94. : Exploring the contribution of estrogen to amyloid-beta regulation: A novel multifactorial computational modelling approach. Front Pharmacol 2013; 4:16.Crossref, Google Scholar
95. : A simulation model of periarterial clearance of amyloid-β from the brain. Front Aging Neurosci 2016; 8:18.Google Scholar
96. : Investigating interventions in alzheimer’s disease with computer simulation models. PLoS ONE 2013; 8:e73631.Crossref, Google Scholar
97. : Impact of amyloid-beta changes on cognitive outcomes in Alzheimer’s disease: Analysis of clinical trials using a quantitative systems pharmacology model. Alzheimers Res Ther. 2018; 10:14.Crossref, Google Scholar
98. : Simulations of symptomatic treatments for Alzheimer’s disease: Computational analysis of pathology and mechanisms of drug action. Alzheimers Res Ther 2012; 4:50.Crossref, Google Scholar
99. : Alzheimer’s Disease Neuroimaging Initiative. Linking Molecular Pathways and Large-Scale Computational Modeling to Assess Candidate Disease Mechanisms and Pharmacodynamics in Alzheimer’s Disease. Front Comput Neurosci 2019; 13:54.Crossref, Google Scholar
100. : 11C]PIB in a non-demented population: Potential antecedent marker of Alzheimer disease. Neurology 2006; 67:446–452.Crossref, Google Scholar
101. : Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008; 65:1509–1517.Crossref, Google Scholar
102. : Age, neuropathology, and dementia. N Engl J Med 2009; 360:2302–2309.Crossref, Google Scholar
103. : Neuropathology of cognitively normal elderly. Journal of Neuropathology and Experimental. Neurology 2003; 62:1087–1095.Google Scholar
104. : Preclinical Alzheimer’s disease: Identification of cases at risk among cognitively intact older individuals. BMC Med 2012; 10:1–13.Crossref, Google Scholar
105. : Association of Amyloid and Tau With Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurol 2019; 76:915–924.Crossref, Google Scholar
106. : The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat Rev Drug Discov 2011; 10:698–712.Crossref, Google Scholar
107. : The molecular and geneticsbasis of AD: The end of the beginning. The 2000 Watenberg lecture. Neurology 2000; 54:2045–2054.Crossref, Google Scholar
108. : Cognitively preserved subjects with transitional cerebrospinal fluid ß-amyloid 1-42 values have thicker cortex in Alzheimer’s disease vulnerable áreas. Biol Psychiatry 2011; 70:183–190.Crossref, Google Scholar
109. : Changes in White matter integrity before conversión from mild cognitive impairment to alzheimer’s disease. PLoS ONE 2014; 9:e106062.Crossref, Google Scholar
110. : Alzheimer’s Disease Neuroimaging Initiative. Cognitive reserve and clinical progression in Alzheimer disease: A paradoxical relationship. Neurology 2019; 93:e334–e346.Crossref, Google Scholar
111. : Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 2018; 91:e1295–e1306.Crossref, Google Scholar
112. : Biomarker profiles of Alzheimer’s disease and dynamic of the association between cerebrospinal fluid levels of β-amyloid peptide and tau. PLoS ONE 2019; 14:e0217026.Crossref, Google Scholar
113. : NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018; 14:535–562.Crossref, Google Scholar
114. : A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016; 87:539–547.Crossref, Google Scholar
115. : Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging-Alzheimer’s Association Research Framework. JAMA Neurol. 2019; e191971.Google Scholar
116. : NIA commentary on the NIA-AA Research Framework: Towards a biological definition of Alzheimer’s disease. Alzheimer’s Dement 2018; 14:576–578.Crossref, Google Scholar



