Sleep Matters: Sleep Functioning and Course of Illness in Bipolar Disorder
Abstract
Background: Few studies have prospectively examined the relationships of sleep with symptoms and functioning in bipolar disorder. Methods: The present study examined concurrent and prospective associations between total sleep time (TST) and sleep variability (SV) with symptom severity and functioning in a cohort of DSM-IV bipolar patients (N = 468) participating in the National Institute of Mental Health Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD), all of whom were recovered at study entry. Results: Concurrent associations at study entry indicated that shorter TST was associated with increased mania severity, and greater SV was associated with increased mania and depression severity. Mixed-effects regression modeling was used to examine prospective associations in the 196 patients for whom follow-up data were available. Consistent with findings at study entry, shorter TST was associated with increased mania severity, and greater SV was associated with increased mania and depression severity over 12 months. Discussion: These findings highlight the importance of disrupted sleep patterns in the course of bipolar illness.
(Reprinted with permission from Journal of Affective Disorders 2011; 134:416–429)
1. Introduction
Converging lines of evidence suggests the importance of sleep disturbance in bipolar disorder (e.g., Ehlers et al., 1988; Harvey, 2009). Sleep disturbance is an important symptom of bipolar disorder across mood phases. During manic episodes, there is a reduced need for sleep, whereas episodes of depression in bipolar disorder are characterized by either insomnia or hypersomnia (American Psychiatric Association, 2000). Sleep is also impaired in bipolar disorder even when patients are not actively symptomatic. In fact, 70% of euthymic bipolar patients report clinically significant levels of sleep disturbance (Harvey et al., 2005). Cross-sectional research suggests that both short (<6 h) and long (≥9 h) sleep durations are associated with impaired functioning and quality of life in bipolar disorder (Gruber et al., 2009).
There is evidence that sleep disturbance may be causally related to recurrence. Disturbance in sleep is a common prodrome of both manic and depressive episodes (Jackson et al., 2003). Experimentally-induced sleep deprivation has been associated with a worsening of both depressive and manic symptom severity the subsequent day in patients with bipolar disorder in some studies (e.g., Colombo et al., 1999; Kasper and Wehr, 1992; Wu and Bunney, 1990), but with improvement in others (e.g., Barbini et al., 1998). Few studies, however, have prospectively monitored sleep quality and mood episode status. One study found that shorter sleep duration predicted greater depressive, but not manic, symptoms over 6 months in patients with bipolar I disorder (Perlman et al., 2006). Barbini et al. (1996) monitored 34 patients with mania over three days for sleep duration and manic symptoms and found significant correlations between decreased sleep duration and increased manic symptoms the subsequent day. Finally, Bauer et al. (2006) recorded patterns of mood, sleep and bedrest over a minimum of 100 (M = 169, SD = 59) days in 59 inpatients that were currently manic. They found that a decrease in sleep or bedrest was followed by a shift towards hypomania/mania on the next day, whereas an increase in sleep or bedrest was followed by a shift towards depression the next day. Taken together, these studies find longitudinal associations between sleep disturbance and mood changes in bipolar disorder, although the nature of the association— whether sleep disturbance is more related to manic or depressive symptoms—is less consistent.
The current study addressed two aims. The first was to examine whether there was an association between sleep functioning and mood symptoms in recovered (i.e., euthymic) patients with bipolar disorder upon study entry into the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD), a multi-site longitudinal study. Prior cross-sectional and experimental evidence suggests that shortened total sleep duration is associated with increased depressive and manic symptom severity (e.g., Bauer et al., 2006; Colombo et al., 1999; Kasper and Wehr, 1992; Perlman et al., 2006; Wu and Bunney, 1990) as well as impaired functioning (e.g., Gruber et al., 2009). This aim focused on whether such associations are observable in a remitted bipolar sample. We hypothesized that lower Total Sleep Time (TST) and greater Sleep Variability (SV) would be associated with increased symptom severity and decreased functioning at study entry.
The second aim was to investigate whether there is an association between sleep functioning with symptom severity and functioning across a 12-month prospective period. This association has not been investigated in patients who are minimally symptomatic (no more than two threshold mood symptoms) at study entry. Our second hypothesis was that greater SV would be associated with increased mania severity, increased depression severity, and decreased global functioning. This hypothesis is based upon the supposition that irregularities in social rhythms, one of which includes sleep, is associated with increased risk for mania and depression symptom severity as well as poorer treatment outcome (e.g., Frank et al., 2005; Goldstein et al., 2008).
2. Method
2.1. Participants
Eligibility in STEP-BD required that all participants were at least 15 years of age and met criteria for bipolar I disorder, bipolar II disorder, bipolar disorder not otherwise specified (NOS), schizoaffective disorder bipolar type, or cyclothymia. In the present study, we focused on a subset of 196 study participants (BD I = 64.3%; BD II = 27.4%; BD NOS = 8.3%) who met the following criteria: (a) data on sleep functioning parameters were available at study entry (N = 2024), (b) were currently recovered at study entry (n = 469) and (c) had sufficient data on symptom severity and global functioning across the 12-month monitoring period (final n = 196).
2.2. Procedure
In the present study, only STEP-BD patients who met lifetime criteria for bipolar disorder types I, II, or NOS were included. Exclusion criteria included inability to provide informed consent or current inpatient status. Prior to study entry, oral and written informed consent was obtained. At study entry, the Affective Disorder Evaluation (ADE; Sachs et al., 2003) and the Clinical Monitoring Form (CMF; Sachs et al., 2002) were administered. The CMF was re-administered during routine clinical care over the subsequent year where patients received open, naturalistic treatment, guided by model practice procedures. The present study includes data from a 12-month follow-up, which enabled examination of symptom change in bipolar disorder (Tohen et al., 1990). The mean interval between the baseline evaluation and a 12-month follow-up was 343.49 (SD = 53.05) days. The average number of psychiatric visits was 8.61 (SD = 4.73) visits, and mean visit frequency was 52.85 (SD = 33.78) days. Participation was voluntary and no financial compensation was given.
2.3. Measures
2.3.1. Diagnostic evaluation
At study entry, all participants were administered the Affective Disorder Evaluation by a STEP-BD psychiatrist (ADE; Sachs et al., 2003). The ADE is a valid and reliable semistructured interview adapted from the mood and psychosis modules of the Structured Clinical Interview for DSM-IV, Patient Version (SCID; First et al., 1996). A second certified clinical psychologist, psychiatrist, social worker, or psychiatric nurse independently interviewed the same participant using the Mini-International Neuropsychiatric Interview (version 5.0; Sheehan et al., 1998). Final diagnoses were based on consensus between the two interviewers.
2.3.2. Symptom severity
Symptom severity was measured at baseline and each visit during the 12-month follow-up period using the CMF (Sachs et al., 2002). Severity of depressive and manic symptoms over the past week included the depression (SUM-D) and mood elevation (SUM-M) subscales of the CMF with scores ranging from 0 to 19.8 and 0 to 13.5, respectively. Higher scores on both subscales reflect greater symptom severity. Both the SUM-D (α = .85) and SUM-ME (α = .80) scales demonstrated accept-able internal consistency.
2.3.3. Clinical status
Recovered clinical status at study entry or baseline was verified using the CMF. This included two or fewer threshold symptoms of depression or mood elevation for at least two months (e.g., Gruber et al., 2009).
2.3.4. Global functioning
The Global Assessment of Functioning (GAF; Axis V; DSM-IV) Scale was used to assess global functioning in the past week. The GAF assesses overall psychological, social, and occupational functioning on a scale from 1 (lowest level of functioning) to 100 (highest level of functioning).
2.3.5. Sleep functioning
We used two previously published variables to ascertain sleep functioning from the CMF, which included a single score for the maximum sleep in hours per night over the past week and a single score for the minimum sleep in hours slept per night over the past week (cf. Gruber et al., 2009). These variables were used to calculate (1) average total sleep time (TST) in the past week by averaging across the maximum and minimum sleep hours and (2) sleep variability (SV), calculated as the maximum minus the minimum sleep hours. If a participant was missing a score for either the maximum or minimum hours of sleep, neither TST nor SV were calculated for that time point.
3. Results
3.1. Preliminary analyses
Demographic and clinical characteristics are listed in Table 1. We first examined skewness and kurtosis indices of our primary independent variables (TST and SV). Given that both variables mirrored a normal distribution, we did not transform variables. Second, we examined whether demographic variables (gender, age, ethnicity, and education) were associated with TST or SV at baseline. Gender, education, and ethnicity were not associated with TST or SV (ps >.05). Age was weakly but significantly negatively correlated with TST, r (179) = −0.18, p<.05, suggesting that older patients slept fewer hours than younger patients. Third, TST was negatively correlated with illness duration, r(175) = −0.24, p<.01, and SV was negatively correlated with age at onset, r(459) = −0.11, p<.05, suggesting that patients with earlier ages at onset and longer illness durations slept less and with more variability.
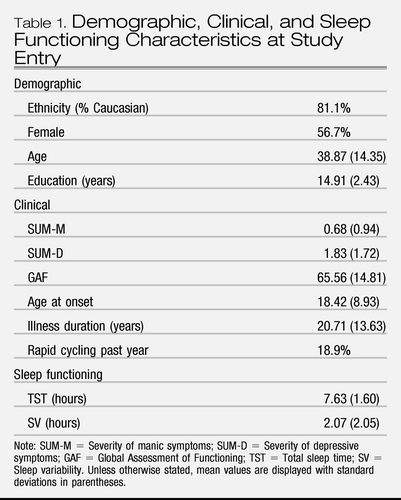 |
Table 1. Demographic, Clinical, and Sleep Functioning Characteristics at Study Entry
3.2. Concurrent associations between sleep with symptom severity and functioning
We conducted bivariate correlations between SV at baseline with mania severity (SUM-M), depressive symptom severity (SUM-D), and global functioning (GAF) at baseline. Given the significant correlation between TST and age, partial correlations between TST and SUM-M, SUM-D, and GAF at baseline were conducted including age as a covariate. Lower TST was associated with increased mania severity scores (r = −0.31) and greater SV was associated with increased mania (r = 0.18) and depression (r = 0.14) severity scores (ps <.01). Neither TST nor SV was associated with global functioning (r = −0.12 and −0.08, respectively).
3.3. Prospective associations between sleep with symptom severity and functioning
Mixed-effects regression models examined the relation of sleep disturbance (TST and SV) to symptom severity (SUM-D and SUM-M) or role functioning scores (GAF) during the same interval longitudinally across the 12-month period. All analyses controlled for the patient's age and number of psychiatric visits. The regression models conducted using PROC MIXED in SAS (Littell et al., 1996) considered multiple follow-up visits simultaneously and missing data was excluded listwise. Unlike standard repeated measures analyses of variance, mixed effects models with random subject effects permit the analysis of repeated measurements while controlling for the within-subject correlation of the dependent measure (e.g., SUM-D, SUM-M, or GAF). These methods assume that data are missing at random. Below is a model representation of our analytic procedure with the time-varying covariate, whereby a significant coefficient denotes a significant association between TST (or SV) and one of our three dependent measures (e.g., SUM-D, SUM-M, or GAF).
For symptom severity, results from the mixed-effects linear modeling across the 12-month period demonstrated that, over time, lower TST was associated with higher SUM-M scores [F(1, 1397) = 71.46, p<.0001] (see Fig. 1). TST was unrelated over time to SUM-D scores [F(1, 1384) = 3.18, p = 0.07]. Moreover, mixed-effects linear modeling demonstrated that increases in SV over time were associated with increases in SUM-D [F(1, 1393) = 31.39, p <.001] and SUM-M [F(1, 1360) = 10.03, p < .01] scores (see Fig. 2).


For global functioning, mixed-effects linear modeling did not demonstrate a relationship over time between TST and GAF scores [F(1, 1313) = 0.72, p = .40]. Increases in SV over time were associated with increases in GAF scores [F(1, 1330) = 7.99, p < .01], although this association was no longer significant once SUM-M and SUM-D scores were covaried [F(1, 1268) = 0.34, p>.10].
4. Discussion
For some time sleep disturbance has been implicated as important in bipolar disorder (e.g., Ehlers et al., 1988) and evidence now supports these claims (e.g., Harvey, 2009). Few studies have prospectively examined the relationships of sleep with symptoms and functioning, especially in a larger sized bipolar cohort. Our results revealed significant concurrent and prospective associations between SV and TST with both mood symptoms and functioning in bipolar disorder.
We note that the results of the present study need to be interpreted within the confines of several limitations. First, the existing sleep variables in the STEP-BD database were limited in several ways. Specifically, measures of sleep functioning from the CMF were measured using single score estimates of the maximum and minimum sleep averaged across the previous week, unlikely to fully reflect the central tendency of sleep patterns and variability in over this time period. For example, estimates of both SV and TST might be susceptible to retrospective memory biases as compared to daily sleep diaries, actigraphy, and polysomnography. Furthermore, it was not possible to make formal diagnoses of insomnia or hypersomnia based on the CMF and so future work is needed to replicate these findings when formal insomnia and hypersomnia diagnoses are made. Second, our measure of global functioning may not have been sensitive enough to capture sleep-related impairment Third, the medication regimens of patients were variable and changed over the 12-month follow-up period. Research indicates that frequently prescribed medications for bipolar disorder may influence sleep architecture (e.g., Eidelman et al., 2010). Future studies should systematically assess medication influences on leep duration and variability.
Considering these limitations, the present study represents a step forward in examining sleep functioning in bipolar disorder. More specifically, our first aim examined concurrent associations between sleep at study entry (i.e., baseline) with mood symptoms and functioning. Interestingly, lower TST was associated with more severe mania symptoms, whereas greater SV was associated with more severe mania and depression symptoms. Taking TST first, this finding is consistent with research documenting cross-sectional associations between short sleep durations and increased mania or hypomania (Bauer et al., 2006; Colombo et al., 1999; Gruber et al., 2009; Kasper and Wehr, 1992; Wehr et al., 1987). For SV, the results suggest that greater variability in sleep may be associated with more severe mood symptoms. These findings are broadly consistent with the finding that disruptions in social rhythms, including sleep, are associated with greater depressive symptomatology in adolescents (Goldstein et al., 2008). Moreover, disrupted social rhythms have been associated with increased risk for manic and depressive symptom severity and poorer treatment outcome (Frank et al., 2005).
Our second aim examined prospective associations between sleep functioning with mood symptoms and functioning across a 12-month period using mixed-effects modeling. Consistent with concurrent associations at baseline, TST was associated with increased mania severity though TST was not associated with depression severity over time. This is inconsistent with the notion that extended sleep duration, or hypersomnia, is associated with greater symptoms of depression (Kaplan and Harvey, 2009). However, the relationship is likely more complicated, given that depression can be characterized by either insomnia or hypersomnia (American Psychiatric Association, 2000).
Increased sleep variability was associated with greater manic and depressive symptom severity across the 12-month period. These results are consistent concurrent associations between SV and mood symptoms in prior work (e.g., Perlman et al., 2006). A tendency towards an inconsistent sleep pattern in bipolar disorder may precipitate the onset of mood episodes. Alternatively, prodromal mood symptoms may aggravate sleep regularity leading to the exacerbation of mood symptoms. A third possibility is that a bi-directional escalating vicious cycle is operating whereby sleep and mood progressively feed into the other worsening (Wehr et al., 1987). Either way, there is likely to be value in exploring specific sleep variables in future research.
In sum, the present study suggests that longer total sleep duration was associated with more severe concurrent and prospective mania, but not depression, symptom severity in bipolar disorder. Furthermore, greater variability in sleep was associated with worsened concurrent and prospective mania and depression symptom profiles. No associations between sleep duration or variability emerged with global functioning. The present findings represent an important initial step towards identifying aspects of sleep functioning that may contribute to clinical impairment in bipolar disorder. Furthermore, this research underscores the importance of incorporating interventions that target sleep functioning aimed at promoting illness stabilization.
American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision. APA, Washington, DC.Google Scholar
Barbini, B., Bertelli, S., Colombo, C., Smeraldi, E., 1996. Sleep loss, a possible factor in augmenting manic episode. Psychiatry Research 65, 121–125.Crossref, Google Scholar
Barbini, B., Colombo, C., Benedetti, F., Campori, E., Bellodi, L., Smeraldi, E., 1998. The unipolar-bipolar dichotomy and the response to sleep deprivation. Psychiatry Research 79 (1), 43–50.Crossref, Google Scholar
Bauer, M., Grof, P., Rasgon, N., Bschor, T., Glenn, T., Whybrow, P.C., 2006. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disorders 8 (2), 160–167.Crossref, Google Scholar
Colombo, C., Benedetti, F., Barbini, B., Campori, E., Smeraldi, E., 1999. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Research 86, 267–270.Crossref, Google Scholar
Ehlers, C.L., Frank, E., Kupfer, D.J., 1988. Social zeitgebers and biological rhythms: a unified approach to understanding the etiology of depression. Archives of General Psychiatry 45, 948–952.Crossref, Google Scholar
Eidelman, P., Talbot, L.S., Gruber, J., Hairston, I.S., Harvey, A.G., 2010. Sleep architecture as a correlate and predictor of mood symptoms and impairment in inter-episode bipolar disorder: taking on the challenge of medication effects. Journal of Sleep Research 19, 516–524.Crossref, Google Scholar
First, M.B., Spitzer, R.L., Gibbon, M., Williams, J.B.W., 1996. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Biometrics Research, New York State Psychiatric Institute, NY.Google Scholar
Frank, E., Kupfer, D.J., Thase, M.E., Mallinger, A.G., Swartz, H., Fagiolini, A.M., Grochocinski, V., Houck, P., Scott. J., Thompson, W., Monk, T., 2005. Two year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Archives of General Psychiatry 62, 996–1004.Crossref, Google Scholar
Goldstein, T.R., Bridge, J.A., Brent, D.A., 2008. Sleep disturbance preceding completed suicide in adolescents. Journal of Consulting and Clinical Psychology 76 (1), 84–91.Crossref, Google Scholar
Gruber, J., Harvey, A.G., Wang, P.W., Brooks, J.O., Thase, M.E., Sachs, G.S., Ketter, T.A., 2009. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Journal of Affective Disorders 114, 41–49.Crossref, Google Scholar
Harvey, A.G., 2009. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. American Journal of Psychiatry 165, 820–829.Crossref, Google Scholar
Harvey, A.G., Schmidt, D.A., Scarna, A., Semler, C.N., Goodwin, G.M., 2005. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. American Journal of Psychiatry 162, 50–57.Crossref, Google Scholar
Jackson, A., Cavanagh, J., Scott, J., 2003. A systematic review of manic and depressive prodromes. Journal of Affective Disorders 74, 209–217.Crossref, Google Scholar
Kaplan, K.A., Harvey, A.G., 2009. Hypersomnia across mood disorders: a review and synthesis. Sleep Medicine Reviews 13, 275–285.Crossref, Google Scholar
Kasper, S., Wehr, T.A., 1992. The role of sleep and wakefulness in the genesis of depression and mania. Encephale 18, 45–50.Google Scholar
Littell, R., Miliken, G., Stroup, W., Wolifinger, R., 1996. SAS System for Mixed Models. SAS Institute, Inc., Cary, NC.Google Scholar
Perlman, C.A., Johnson, S.L., Mellman, T.A., 2006. The prospective impact of sleep duration on depression and mania. Bipolar Disorders 8 (3), 271–274.Crossref, Google Scholar
Sachs, G.S., Guille, C., McMurrich, S.L., 2002. A clinical monitoring form for mood disorders. Bipolar Disorders 4 (5), 323–327.Crossref, Google Scholar
Sachs, G.S., Thase, M.E., Otto, M.W., Bauer, M., Miklowitz, D., Wisniewski, S.R., et al., 2003. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biological Psychiatry 53, 1028–1042.Crossref, Google Scholar
Sheehan, D.V., Lucrubier, Y.K., Harnett-Sheehan, K., Amorim, P., Weiller, E.J., Hergueta, T., et al., 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic interview for LSD-IV and ICD-10. Journal of Clinical Psychiatry 59 (Suppl. 20), 22–23.Google Scholar
Tohen, M., Waternaux, C.M., Tsuang, M.T., 1990. Outcome in mania: a 4-year prospective follow-up of 75 patients utilizing survival analysis. Archives of General Psychiatry 47, 1106–1111.Crossref, Google Scholar
Wehr, T.A., Sack, D.A., Rosenthal, N.E., 1987. Sleep reduction as a final common pathway in the genesis of mania. American Journal of Psychiatry 144, 201–204 (correction, 144, 542).Crossref, Google Scholar
Wu, J.C., Bunney, W.E., 1990. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. American Journal of Psychiatry 147, 14–21.Crossref, Google Scholar



