What Is an Anxiety Disorder?
Abstract
Initiated as part of the ongoing deliberation about the nosological structure of DSM, this review aims to evaluate whether the anxiety disorders share features of responding that define them and make them distinct from depressive disorders, and/or that differentiate fear disorders from anxious-misery disorders. The review covers symptom self-report as well as on-line indices of behavioral, physiological, cognitive, and neural responding in the presence of aversive stimuli. The data indicate that the anxiety disorders share self-reported symptoms of anxiety and fear; heightened anxiety and fear responding to cues that signal threat, cues that signal no threat, cues that formerly signaled threat, and contexts associated with threat; elevated stress reactivity to aversive stimuli; attentional biases to threat-relevant stimuli and threat-based appraisals of ambiguous stimuli; and elevated amygdala responses to threat-relevant stimuli. Some differences exist among anxiety disorders, and between anxiety disorders and depressive disorders. However, the differences are not fully consistent with proposed subdivisions of fear disorders vs. anxious misery disorders, and comparative data in large part are lacking. Given the high rates of co-morbidity, advances in our understanding of the features of responding that are shared across vs. unique to anxiety and depressive disorders will require dimensional approaches. In summary, the extant data help to define the features of responding that are shared across anxiety disorders, but are insufficient to justify revisions to the DSM nosology at this time.
(This Article is being co-published by Depression & Anxiety and the American Psychiatric Association. This Article first appeared in Depression & Anxiety, 26:1066–1085 (2009).)
Initial deliberations by the DSM-V Anxiety, Obsessive-Compulsive Spectrum, Post-traumatic, and Dissociative Disorders Work Group focused on the nosological structure of the anxiety and depressive disorders. These deliberations were spurred in part by structural modeling studies that highlight a shared internalizing factor (1, 2) and recommendations from Watson (3) for collapsing mood and anxiety disorders into an over-arching class of “internalizing” disorders, with three subclasses: bipolar disorders (bipolar I, bipolar II, and cyclothymia); distress or “anxious-misery” disorders (major depression (MD), dysthymia, generalized anxiety disorder (GAD) and posttraumatic stress disorder (PTSD)); and the fear disorders (panic disorder (PD), agoraphobia, social phobia (SOP) and specific phobia (SP)). The Work Group recognized that deliberations would be aided by knowing whether the anxiety disorders share features of responding that define them and distinguish them from mood disorders, and/or that differentiate between anxious-misery and fear disorders. The nosological recommendations by Watson and others derive from structural analyses of symptom self-report data, which represent estimation of past responding and prediction of future responding. In this review, such data were complemented with measures of “on-line” cognitive, behavioral, psychophysiological, and neural responding in the presence of aversive stimuli.1
This study represents the work of the authors for consideration by the DSM-V Anxiety, Obsessive-Compulsive Spectrum, Post-traumatic, and Dissociative Disorders Work Group. Recommendations provided in this study should be considered preliminary at this time; they do not necessarily reflect the final recommendations or decisions for DSM-V, as the DSM-V development process is still ongoing.
The method of review was based on computer database searches of PubMed and PsychINFO for English language articles from 1994 through 2009, combined with reviews of reference lists from identified manuscripts, as well as the proceedings and/or monographs of the preparatory conference series for DSM-V, particularly the Stress-Induced and Fear Circuitry Disorders. (4) Given the vast number of references produced, this review presents representative rather than complete citations. However, meta-analyses were cited when available, and conclusions were drawn when published findings converged from multiple, independently conducted sources (vs. single and/or dependent sources). Also, whenever the literature was divided on a particular topic, representative citations are presented for each argument.
STRUCTURAL ANALYSES OF SYMPTOM SELF-REPORT DATA
Goals
Diagnosis is based on the self-report of symptoms, whether provided solely by the patient or with the aid of a clinician's appraisal. In this section, we explore the structure of symptom self-report data, to evaluate the degree to which symptom reports share features in common across the anxiety disorders, making them distinct from mood disorders, and whether features of symptom self-report distinguish fear disorders from anxious-misery disorders. Later, we address the behavioral, physiological, cognitive, and neural correlates of symptom reports and diagnoses.
We begin with conceptualizing the terms “fear” and “anxiety,” since these constructs underlie the symptoms of anxiety disorders. Then, we consider whether self-reported fear and anxiety distinctly differ from each other and from depression. Finally, the relationship between symptoms of fear, anxiety and depression and existing diagnostic categories of anxiety and mood disorder is reported.
Defining fear and anxiety
The definition of fear and anxiety varies greatly [for a review, see Reference (5)]. In this review, we use Barlow's (5) concepts, in which; anxiety is a future-oriented mood state associated with preparation for possible, upcoming negative events; and fear is an alarm response to present or imminent danger (real or perceived). This view of human fear and anxiety is comparable to the animal predatory imminence continuum (6). That is, anxiety corresponds to an animal's state during a potential predatory attack and fear corresponds to an animal's state during predator contact or imminent contact.
Lang (7) classified the symptoms of fear and anxiety into a system of three-responses: verbal-subjective, overt motor acts, and somato-visceral activity. In this system, and in accordance with the definitions of anxiety and fear, the symptoms of anxiety include worry (verbal-subjective), avoidance (overt motor acts), and muscle tension (somato-visceral activity). Fear symptoms include thoughts of imminent threat (verbal-subjective), escape (overt motor), and a strong autonomic surge resulting in physical symptoms such as sweating, trembling, heart palpitations, and nausea (somato-visceral) (Table 1). Importantly, these descriptions represent prototypes of fear and anxiety that lie at different places upon a continuum of responding. Along such a continuum, symptoms of fear vs. anxiety are likely to diverge and converge to varying degrees. Zinbarg (8) proposed a similar hierarchical model, with anxiety and fear as higher-order constructs that have effects on lower-order, partially-distinct response systems (i.e., verbal-subjective, overt motor actions, and somato-visceral activity).
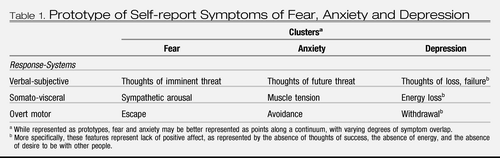 |
Table 1. Prototype of Self-report Symptoms of Fear, Anxiety and Depression
Evidence for differentiating fear from anxiety
There is evidence to support the distinction between self-reported somato-visceral symptoms that are likely influenced by fear and self-reported subjective symptoms that are likely influenced by anxiety. For example, across four different samples of undergraduates, air force academy cadets, and psychiatric outpatients, a two-factor model was a better fit to the data than a one-factor model: the two factors represented physiological arousal (e.g., heart racing) and subjective anxiety (e.g., unable to relax and nervous) (9). Similar findings have been demonstrated with other large community and undergraduate samples (10) and with psychiatric out-patients, (11) and in child and adolescent samples (e.g., (12)). The factors are distinct but highly correlated. These data highlight a distinction between self-report of fear-linked physiological arousal and anxiety-linked subjective distress, but their linkages to the constructs of fear and anxiety, respectively, are only implied.
Differentiating fear, anxiety, and depression: evidence from the tripartite model
The large literature testing the tripartite model of fear, anxiety, and depression (13) provides additional evidence for a distinction between self-reported somato-visceral symptoms of fear and verbal-subjective symptoms of anxiety. The tripartite model proposes that there are symptoms shared across “anxiety” and depression as well as symptoms unique to each. Shared symptoms typically are represented by a negative affect (NA) or general distress factor. Symptoms of anhedonia and the absence of positive affect are specific to depression whereas symptoms of physiological hyper-arousal are specific to “anxiety.”
Using the present terminology, the physiological hyperarousal items would be described as somato-visceral symptoms of fear. The subjective anxiety symptoms often are key markers of general distress. For example, when averaging factor loadings across five different samples (three student samples, an adult sample, and a patient sample), the item “worried a lot about things” had the second largest loading of any item on the general distress factor, (14) and the same was true in a sample of child and adolescent psychiatric inpatients (15).
Tests of the tripartite model demonstrate that somato-visceral symptoms of fear, symptoms of depression, and subjective symptoms of anxiety (along with other general distress symptoms) can be distinguished from each other. Such a distinction has been demonstrated in many samples, clinical and nonclinical, adult and child (e.g., (14–19)). As mentioned, although these factors can be distinguished from one another, they typically are moderately to highly correlated, albeit with some exceptions (e.g., (15)).
Some studies have identified two rather than three factors (e.g., (20)). The two-factor findings have been interpreted as symptoms of “anxiety” vs. symptoms of depression, without distinguishing fear from anxiety symptoms. However, these studies tend to rely on an insufficient number of items for independent measurement of fear and anxiety.
Relationship of DSM disorders to self-report symptoms of fear, anxiety, and depression
Only a handful of studies have examined whether different disorders load differentially on symptoms of fear and anxiety. One study with adult outpatients showed with zero-order correlations that autonomic arousal (somato-visceral symptoms of fear) was positively associated with constructs representing PD, GAD, SOP, obsessive-compulsive disorder (OCD), and MD, and that the correlation was significantly stronger with PD (0.89) than the other constructs (18). Others also report greater correlations between somato-visceral symptoms of fear with PD than with GAD (9, 21) or with MD (9). Thus, whereas somato-visceral symptoms of fear seem to be associated with anxiety disorders in general, they may be of particular relevance to PD. In further support of this premise, when Brown et al. (18) examined unique associations using all disorder constructs and negative affect as covariates, the PD construct was the only one to retain a significant, positive association with autonomic arousal. Also, the unique relationship between GAD and autonomic arousal was significant, but in the negative direction, suggesting a possible division between PD and GAD.
A similar specificity has been found between positive affect and both MD and SOP. That is, when structural models including multiple disorders and symptom constructs are considered, positive affect only displays significant associations with MD and SOP (9, 18). Notably, the similarity between MD and SOP in this regard is at odds with the separation of SOP as a fear disorder from depression as an anxious-misery disorder.
Furthermore, in clinically referred children and adolescents, after controlling for the variance in physiological hyperarousal associated with negative affect, the somato-visceral symptoms of fear were significantly associated with clinical severity ratings for PD only and no other disorders of anxiety or depression (22). Positive affect showed significant, negative associations with MD and SOP, whereas negative affect demonstrated significant, positive relations with PD, GAD, and OCD.
Limitations and future directions
The psychometric distinction between symptoms of fear and anxiety is potentially confounded by the measurement of different response systems, since most studies assess only somato-visceral symptoms of fear and only subjective symptoms of anxiety. For example, studies of SP stimuli typically measure how much fear is expected if a specific stimulus was encountered in the coming week, without any indicators of anxiety about the stimulus (e.g., worry about encountering, or avoidance of situations involving the stimulus). Thus, it is unclear whether symptoms load on different factors because they represent differences between symptoms of fear and anxiety or because they represent differences among response systems (i.e., verbal-subjective, overt motor, and somato-visceral).
Another limitation is that most studies employ nonstimulus-specific items. For example, physiological hyperarousal (e.g., heart pounding) items usually refer to the frequency or intensity of symptoms over a specified period (past week or two), without reference to an eliciting stimulus. Individuals who experience fear symptoms in the presence of circumscribed stimuli (e.g., air travel) may not encounter such stimuli over the specified period of time, and thus would score low on fear items. Similarly, even though they may worry about particular circumscribed stimuli as they become more probable (e.g., days before air travel), such individuals may not typically worry about those stimuli and hence would score low on symptoms of anxiety. In contrast, individuals with PD would be more likely to score high on items of fear, as would individuals with GAD be more likely to score high on items of anxiety, because they experience fear and anxiety, respectively, more frequently. In other words, nonstimulus-specific measures appear to represent frequency of fear and anxiety. However, frequency does not take into account the magnitude of response in the presence of specific stimuli or the frequency of exposure to specific triggering stimuli.
Hence, non-stimulus-specific items (as a measure of frequency) may be best complemented by stimulus-specific symptom items (as a measure of magnitude). For example, social anxiety symptom items might include: “I avoid parties” (overt motor acts), “when alone I worry about meeting new people” (verbal-subjective), and “I get tense and irritable from worrying about social situations” (somato-visceral). Social fear items could include: “I try to leave social situations as soon as possible” (overt motor acts), “when speaking in public, I think I look like a fool” (verbal-subjective), and “my palms get sweaty when eating in front of others” (somato-visceral). Patterns of covariation among such items would reveal whether or not a future-oriented social anxiety factor, indicated by avoidance of and worry about potential social situations, was separable from a present-focused social fear factor, indicated by escape from social situations as well as fearful thoughts and physiological arousal during social situations. With this kind of measurement, the extent to which each disorder is marked by symptoms of fear vs. symptoms of anxiety could be determined.
Summary and implications for DSM
The existing literature reveals that self-reported somato-visceral symptoms of fear (e.g., breathlessness) are separable from, yet related to, verbal-subjective symptoms of anxiety (e.g., worry a lot). Although verbal-subjective symptoms of anxiety are not always separable from other general distress symptoms, anxiety symptoms and fear symptoms generally are separable from, yet related to, symptoms representing anhedonia or the absence of positive affect (i.e., depression). The extent of the relationship between factors of fear, anxiety, and depression tends to be moderate-to-large, but can be quite modest depending on item content. While there is some evidence to indicate that several anxiety disorders are significantly, positively related to somato-visceral symptoms of fear, the relationship of these symptoms to PD seems to be stronger than with GAD. These findings lend support to a distinction between fear-based disorders and anxious-misery disorders. Symptoms of anhedonia are especially linked to both MD and SOP, thereby at odds with the proposed categorization of SOP as a fear disorder vs. anxious-misery disorder.
However, as noted, the limitations to existing self-report methodologies weaken the implications for DSM. That is, most studies are limited by the measurement of different response systems to assess fear (i.e., somato-visceral) vs. anxiety (i.e., verbal-subjective) symptoms as well as by restriction to nonstimulus-specific symptoms of fear and anxiety. Even when studies employ stimulus-specific items, they tend to address either fear or anxiety symptoms, but not both. Therefore, it is recommended that self-report items are developed that would better assess fear and anxiety symptoms across all three response modalities as well as in response to a range of stimuli.
EXPERIMENTAL PARADIGMS
Goals
This section reviews aversive conditioning, stress reactivity, and information processing (attention, memory, and appraisal) paradigms to evaluate commonalities and differences in behavioral, psychophysiological, and cognitive responding to aversive stimuli across anxiety and mood disorders. Symptom self-report (reviewed in the earlier section) entails retrospective estimation of how one has responded in the past or prediction of how one might respond in the future. In contrast, in this section we evaluate features of verbal-subjective, overt motor acts, and somato-visceral activity responding as they occur in the presence of specific stimuli. Discordances often exist between symptom self-report on the one hand and online measurement of behaviors and physiology on the other hand.2 As before, our goal is to evaluate the extent to which these indices of on-line responding are shared across anxiety disorders and differ from mood disorders.
Pavlovian aversive conditioning: explicit threat cue (fear) vs. context (anxiety)
Pavlovian fear conditioning has long been applied as an etiological model for anxiety disorders (23). In a typical Pavlovian fear conditioning paradigm, a neutral stimulus is paired with an innately aversive stimulus (unconditional stimulus, US, such as a painful shock) a sufficient number of times for the neutral stimulus to become a reliable predictor of the aversive, and therefore capable of eliciting a conditional response (CR) in the absence of the US. The CR typically is measured by an increase in arousal (e.g., galvanic skin conductance response) and an increase in negative valence (e.g., startle blink reflex). The CR is even acquired to a CS that is masked to prevent conscious awareness (i.e., subliminal), at least with stimuli judged to be “fear-relevant.”3 Pavlovian conditioning also occurs through observation, as when observing others react fearfully in the presence of a given stimulus. Olsson and Phelps (24) found that observational conditioning in humans was measurable even to subliminal presentations of the CS. Furthermore, verbal instructions to expect a shock without actual shock delivery have been shown to elicit similar CRs, although only to supraliminal presentations of the CS (e.g., (24)). Thus, the Pavlovian conditioning model posits that fears are acquired through associations with aversive events, experienced directly, vicariously, or through informational transmission.
Developments in the basic science of fear conditioning have converged on a distinction between two types of learning, explicit threat cue vs. context learning. Explicit threat cues refer to the specific CSs that predict the US, whereas context refers to the location within which the US is presented. Like the CS, the context becomes predictive of the US and capable of eliciting a CR. Explicit threat cues are presumed to elicit phasic fear responses to certain or imminent threat, whereas contextual cues are presumed to elicit a more sustained anxious response to less certain threat (since the context is a reminder of threat but does not signal the precise timing of its occurrence). In this way, explicit threat cue vs. context learning map onto different defensive responses tied to proximal vs. distal threat on the predator imminence continuum, (6) for a related discussion also see Reference (25) and parallel the distinctions between self-reported fear and anxiety. Davis and colleagues (26) have demonstrated that two distinct neural substrates underlie these responses: phasic fear response to imminent threat (explicit threat cue) is mediated by the amygdala; and anxiety response to uncertain threat (context) is mediated by the bed nucleus of the stria terminalis, BNST (extended amygdala) (Table 2). Another body of research has established the role of the hippocampus in context conditioning relative to cue conditioning, (27) and perhaps more so for complex vs. simple contextual information (28).
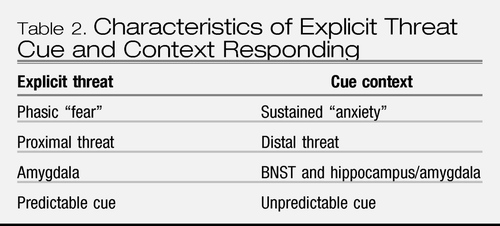 |
Table 2. Characteristics of Explicit Threat Cue and Context Responding
In humans, the context may be the screen on which the CS and US are presented, ambient lighting, or the room itself. Several studies have investigated context conditioning in humans via the presentation of unpredictable USs.4 Greater context conditioning is observed following unpredictable than predictable shocks in healthy controls, as measured by startle reflexes during baseline conditions (conditions of anticipation prior to the delivery of an experimental procedure) and during intertrial intervals. In one study, (29) the effect of unpredictable shocks was tied to an elevated expectancy for the US, perhaps akin to the state of vigilance and preparation for threat that is believed to be characteristic of anxiety (5). In another study, (30) participants were less likely to subsequently explore the unpredictable vs. the predictable context for monetary reward; lack of exploration was interpreted as an index of anxious avoidance and further evidence of context conditioning.
In summary, the findings with nonclinical human samples (albeit small in number) are remarkably consistent with the findings from nonprimate samples, in differentiating explicit threat cue learning from contextual learning. In addition, human research has identified the role of the amygdala in cued fear conditioning (e.g., (31)) and initial findings indicate the role of the right anterior hippocampus and bilateral amygdala in context conditioning (32, 33). The next question is how well these features of explicit threat cue fear learning and contextual anxiety learning map onto the anxiety disorders vs. mood disorders.
Anxiety disorders and explicit threat cue conditioning: simple (CS+) and differential (CS+/CS−).
The clinical literature to date has emphasized explicit threat cue conditioning, with only a few studies examining context conditioning. Within explicit threat cue conditioning, the paradigms tested include simple conditioning (CS+ is paired with a US) or differential or discrimination conditioning (CS+ is paired with a US, and CS− is never paired with the US). The CS− can be viewed as a “safety stimulus” in comparison to the danger CS+, since the CS− signals the absence of the US.5
Simple conditioning.
From their 2005 meta-analysis, Lissek et al. (34) concluded that anxiety disordered adults show elevated responding to CS+s in simple conditioning paradigms, relative to healthy controls (weighted mean ES = .42). These studies included samples of patients with “neurotic/anxiety” states (four studies), PTSD (two studies), and SOP (one study). Also, the effects of extinction training (albeit with fewer studies) were weaker in anxious adults vs. controls (34) (weighted mean ES = .39), although possibly due to elevated acquisition levels. Aside from studies of eye blink conditioning,6 there have been no additional studies of simple conditioning in relation to anxiety disorders since 2005.
Differential conditioning.
Studies involving differential conditioning in Lissek et al.'s (34) meta-analysis included samples of “neurotic/anxious” patients (two studies), PTSD (five studies), SOP (two studies), GAD (two studies), PD (one study), and OCD (one study). Weighed mean ES for strength of discrimination between the CS+ and CS− were lower for differential conditioning than simple conditioning because anxiety disordered adults showed elevated responding to reinforced trials as well as nonreinforced trials, some-times leading to nondiscriminant responding to the CS+ vs. the CS− relative to controls. Thus, anxious individuals were characterized by elevated responding to the CS+ and CS− during acquisition and extinction.
Seven additional studies were identified since 2005, three in adult and four in youth samples. In the adult samples, one study with PTSD individuals showed stronger responding during acquisition to both the CS+ and CS− than healthy controls7 (35) and one study with PD did not; (36) the remaining PTSD study found no differences in comparison to traumatized non-PTSD individuals. (37) The first two studies found stronger responding during extinction to both the CS+ and CS− than healthy controls (35, 36). In child samples, mixed anxious groups showed stronger responding during acquisition to the CS+ and CS− in three of the four studies (35, 39) although one was limited to self report. (40) Lack of effects in the fourth study (41) may be attributable to confounding influences.8 Also, responding was elevated during extinction in the anxious group in three studies (38, 39, 41) but not when measurement was limited to self-report (40).
Summary.
In summary (Table 3), the meta-analysis conducted by Lissek et al. (34) and most subsequent studies suggest that, compared to healthy controls, those with anxiety disorders exhibit stronger responding to explicit threat cues during acquisition and extinction, with larger effects seen in simple conditioning. In differential conditioning, the results mostly, but not always, indicate an elevated response to both the explicit threat cue (CS+) and to the “safety” cue that is not predictive of aversive events (CS−) during acquisition and especially during extinction. The effects during extinction were found despite lack of group differences during acquisition in two studies (36, 41) suggesting that extinction effects were not due solely to elevated acquisition responding.
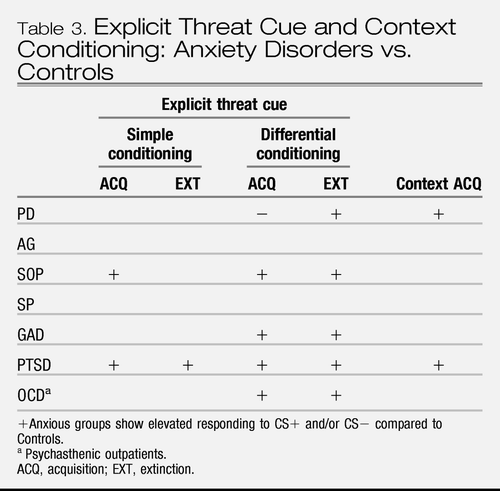 |
Table 3. Explicit Threat Cue and Context Conditioning: Anxiety Disorders vs. Controls
Potential mechanisms of elevated responding to CS+ and CS−.
In associative models (e.g., (42)), elevated responding to the CS+ is attributed to enhanced excitatory fear mechanisms. Lissek et al. (34) outline a number of mechanisms for why anxious individuals also show elevated responding to the CS− (Table 4). One possibility is reduced contingency awareness (i.e., awareness of CS-US pairings), because lack of awareness has been shown to be associated with enhanced reactivity to CS−. However, Lissek et al. (34) reported that anxious individuals were, on average, as aware of the CS/US contingency as nonanxious individuals, and studies since 2005 indicate the same results whether participants who reported lack of awareness of the contingency were included or excluded from analyses (e.g., (35)).
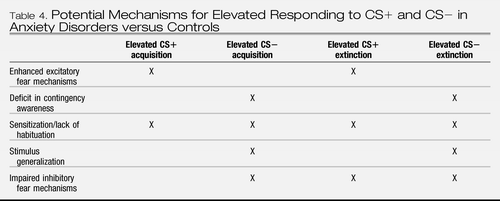 |
Table 4. Potential Mechanisms for Elevated Responding to CS+ and CS− in Anxiety Disorders versus Controls
Another possibility is sensitization and lack of habituation. That is, responses to the CS+ and CS− may be elevated by being more negatively impacted and thereby sensitized by the US (indicated by a larger UR), leading to slowed habituation to the CSs. However, the evidence is limited and available results are contradictory: elevated physiological UR to the delivery of the US (e.g., (38)) vs. equivalence in subjective ratings of anxiety for the US (e.g., (35)). Yet another explanation is that anxious individuals show greater stimulus generalization due to deficits in processing of perceptual information that distinguishes the CS+ from the CS−. Although direct evidence is lacking, evidence pertaining to attentional bias to threat and limited attentional control reviewed in a later section may be relevant here, since over attention to threat may disrupt processing of nonthreatening stimuli.
Davis's (43) model, in which pathological anxiety is attributed to abnormalities in inhibitory fear mechanisms, or the failure to inhibit fear in response to safety signals, offers another explanation for elevated responding to the CS−. It also explains elevated responding during extinction in simple and differential conditioning paradigms, since inhibitory processes are heavily involved in extinction (44). That is, extinction is associated with neuronal activity that is involved in inhibition of CRs, (45–47) mostly within the medial prefrontal cortex (see the following section). In a study of PTSD, the extinction phase of training was associated with decreased function in the orbito-frontal and medial prefrontal cortex, visual association cortex, and other areas, indicative of impaired inhibitory regulation, whereas controls demonstrated an increase in the medial prefrontal cortex (48). Finally, as extinction does not reflect destruction of the CS-UCS association learned during conditioning, but rather, represents the formation of a new association that renders the meaning of the CS+ essentially ambiguous, (44) impaired inhibitory responding during extinction may be associated with a failure to reappraise the CS-UCS association as new information comes to hand. Biases in appraisal of ambiguous information (see later section) may contribute to such failures.
Comparing explicit threat cue conditioning across disorders.
Only two studies were located in which an anxiety disorder was compared to another disorder. One study compared children with attention deficit/hyperactivity disorder (ADHD) with and without overanxious disorder9 (49). There were no between group difference in terms of strength of discrimination between CS+ and CS− trials during acquisition or extinction (absolute levels of responding were not reported). In an fMRI study, four individuals with “criminal psychopathy,” four individuals with SOP and seven controls underwent differential conditioning (50). Healthy controls showed a larger skin conductance to the CS+ than the CS−, whereas the other two groups did not differentiate between the two. Absolute levels of responding to the CS+ and CS− were not reported. The three groups did not differ in their response to the US itself. Clearly, there is a need for more comparative research.
Anxiety disorders and context conditioning.
There are only a few studies of context conditioning in clinical samples. One study reported that baseline startle reflexes (i.e., the waiting period before delivery of experimental procedures) increased in PTSD veterans from the first to the second laboratory session, after having received an aversive event in the first session, relative to veterans without PTSD (51). Such elevations were not present when veterans with PTSD knew that no aversive stimuli would be presented during a second experimental procedure, (52) and other studies fail to find elevations in baselines when aversive stimuli are either mild or never presented (e.g., (53)). Thus, elevation of startle reflexes during baseline is viewed as an index of contextual anxiety in the place where strong aversive events are possible, albeit uncertain in terms of their precise timing. These results imply that PTSD involves elevated sensitivity to contexts associated with threat compared to controls. In a study of PD, (54) startle reflexes measured during intertrial intervals (as a measure of context) increased to a greater degree from neutral to predictable to unpredictable conditions in PD relative to controls. These findings imply that, like individuals with PTSD, individuals with PD are more sensitive to contexts of threat than are healthy controls.
Threat of shock paradigms (i.e., shock is expected but not delivered) are purported to induce contextual anxiety by inducing uncertain threat. Grillon and colleagues demonstrated that adults with PD and PTSD show sustained elevations in startle reflexes throughout experiments involving threat of shock relative to controls (e.g., (51)). One study demonstrated different effects for PD when it was comorbid with depression (55). Specifically, PD without depression showed a facilitation of startle reflexes relative to controls whereas PD with depression showed an attenuation of startle. Clearly, there is need for more evaluation, given the possibility of basic differences in responding to threat of an aversive stimulus between those with anxiety with and without depression.
Moreover, another line of research shows that children and adolescents at risk for emotional dis-orders, by virtue of parental/grandparental anxiety or depression, or the temperamental trait of neuroticism, also show elevated startle reflexes in anticipation of experiments involving threat (at least in females) (56) and in anticipation of threat within the experiment (57). These findings suggest that contextual anxiety may not only be characteristic of anxiety disorders but also could be a marker of risk for emotional disorders in general.
In summary (Table 3), the findings from context conditioning suggest that anxiety disorders that have been tested (PTSD and PD) are characterized by hypersensitivity to contexts in which threat will/may be delivered. However, the degree to which this hyper-sensitivity discriminates anxiety disorders from mood disorders, or discriminates fear disorders from other anxiety disorders, is not yet known.
Summary of explicit threat cue and context conditioning and implications for DSM.
Whereas elevated sensitivity to explicit threat cues and safety cues (including cues during extinction) may be characteristic of anxiety disorders, it is unknown whether it is more characteristic of some anxiety disorders vs. other anxiety disorders, and whether it is exclusive to anxiety disorders relative to depression. Similarly, extant data suggest that certain anxiety disorders are characterized by hypersensitivity to contexts in which threat will/may be delivered, but the degree to which this hypersensitivity discriminates anxiety disorders from other disorders, or discriminates fear disorders from anxious-misery disorders is not known. Thus, the literature pertaining to aversive conditioning helps to define features of responding that are common to anxiety disorders, but is insufficient to justify revisions to the organizational structure of the DSM nosology.
Anxiety disorders and stress reactivity
Another extensive body of research measures psy-chophysiological response to a variety of different aversive stimuli, without testing the learning of an association with neutral stimuli. Such “stress reactivity” measurement illuminates differences between anxious groups and controls, and between anxiety disorders. Anticipatory baseline responding in studies of “stress reactivity” can be viewed as an index of contextual anxiety. The acute response measured during actual delivery of stressors can be viewed as an UR (when using generic stressors, such as shock), or a response to personally relevant stimuli (when using disorder-specific stressors, such as trauma reminders for PTSD). Stress reactivity paradigms typically measure skin conductance, heart rate, respiration, muscle tension, or startle reflex to index the somato-visceral response component of fear and anxiety. The most thorough evaluation would entail assessment of generic stressors and disorder-specific stressors across different anxiety disorders and mood disorders. However, instead, almost every study pertains to a single anxiety disorder vs. healthy controls, and usually tests either generic or disorder-specific stressors. Results are summarized in Table 5. Notably, this review does not cover pharmacological probes that exert central nervous system effects, such as meta-chlorophenylpiperazine or yohimbine, but rather is restricted to assessment of reactivity to inherently aversive stimuli that may occur in the natural environment.
Combining the psycho-physiological results from the studies of individual anxiety disorders and the few studies that address more than one anxiety disorder (Table 5), it may be concluded that PTSD and PD show elevations in baseline anticipation of generic stressors, (58, 59) and perhaps more so in PTSD than PD, (60) but do not show elevations in acute response to generic stressors relative to controls (58, 59). Also, individuals with PD show a stronger baseline response (e.g., (61)) but not a stronger physiological acute response to disorder-specific stressors (e.g., (62, 63)), although there are occasional exceptions (e.g., (64)). The disorder-specific stressors for PD have consisted mostly of carbon dioxide inhalation paradigms.10,11 The majority of PTSD individuals additionally exhibit stronger acute physiological response to trauma reminders compared to controls (e.g., (69)] although not always (e.g., (70, 71)). SOP (nongeneralized) and SP may possess stronger baseline and acute physiological response to disorder-specific stimuli compared to healthy controls (e.g., (72–75)), although baseline and acute response have not been evaluated to highly aversive generic stressors such as shock, and the findings remain tentative. Evidence pertaining to GAD and OCD also is limited by insufficiently aversive generic stressors. Available evidence provides an inconsistent picture regarding baseline differences between GAD and healthy controls (76, 77) and for non-anxious individuals to sometimes have stronger autonomic responses to acute generic stressors than individuals with GAD (e.g., (77)), which may be due to tonic inhibitory effects in GAD (e.g., (78)). Individuals with OCD did not differ from controls during baseline recordings (79) although this may be due to the use of mild stressors; they show an elevated autonomic acute response when confronted with relevant stimuli, such as contaminated objects, relative to baseline conditions, although comparisons have not been made with controls (e.g., (80)).
 |
Table 5. Stress Reactivity (Psychophysiology): Anxiety Disorders vs. Controls
Comparing stress reactivity across disorders.
In terms of comparisons across anxiety disorders, the most extensive body of research compares response to carbon dioxide inhalations in PD relative to other disorders. However, as noted, differences between PD and other disorders reside almost always in subjective measures of distress and rarely appear in psychophysiological measurement. Otherwise, research comparing stress reactivity across anxiety disorders, let alone anxiety and depression, is very limited. Only two studies were located. Cuthbert et al. (81) compared autonomic and startle reflex among groups with PD, SOP, SP, or PTSD. Participants listened to and imagined neutral scenes, standardized fear scenes (generic), and personalized fear scenes (disorder-specific). Baseline physiology (e.g., heart rate) was higher in PD relative to controls and SOP, and PTSD looked most similar to PD but did not differ significantly from any other group. In terms of acute response, SP and SOP showed the highest physiological response to personal fear scenes, whereas physiological reactivity was limited in PTSD and PD. The fact that the latter two groups had higher rates of co-morbidity including depression led the authors to posit that generalized negative affect is associated with elevated baseline responding and lessened physiological reactivity to specific fear cues. In the second study, Blechert et al. (60) compared groups of PD, PTSD, and controls during baseline and during threat of shock (generic). The PTSD group showed higher levels of sympathetic arousal during baseline but lower levels during threat of shock relative to PD. Clearly, further comparative research is needed, using a comprehensive array of psycho-physiological measures, in order to further our understanding of commonalities and differences among the anxiety disorders and in contrast to mood disorders.
Child/adolescent samples.
Results from child samples are mixed. In terms of baseline response, one study showed elevated responding in 9–18 year olds with PD, SOP, overanxious disorder, and separation anxiety disorder compared to controls, before receiving carbon dioxide inhalations; (82) another study showed no differences in children with GAD, separation anxiety, SP or SOP compared to children with ADHD, before a stressful arithmetic test (83). In terms of acute response, two studies show stronger responses to carbon dioxide inhalations in adolescents with separation anxiety disorder vs. controls (or SOP) (84) and to stressful arithmetic tests in children with GAD, separation anxiety, SP or SOP vs. ADHD (83). Two other studies show no differences in acute response to carbon dioxide inhalation compared to controls (82) or to the Trier Stress Test in children with GAD, SOP, separation anxiety, and/or SP compared to children with recurrent abdominal pain (85).
Summary of stress reactivity and implications for DSM.
Findings from stress reactivity studies suggest that some anxiety disorders are characterized by elevated anxiety in anticipation of generic threat (PTSD and PD), and some anxiety disorders are characterized by elevated acute fear to disorder-specific threat (PTSD, specific phobia (SP), and nongeneralized SOP). However, investigations to date are insufficiently comprehensive to draw conclusions regarding groupings of anxiety disorders (i.e., fear vs. anxious-misery) let alone distinctions between anxiety and mood disorders. Thus, there is no evidence to justify revisions to the diagnostic nosology for anxiety disorders based on stress reactivity.
Information processing bias
Information processing refers to cognitive processes of attention, memory, and appraisal. An extensive body of research has evaluated these processes in relation to anxiety and depression. In this section, we evaluate the extent to which indices of information processing are shared across anxiety disorders and differ from mood disorders.
Attentional bias and anxiety.
Numerous studies support the presence of an attentional bias toward threat-related stimuli across a range of anxiety disorders, using the emotional Stroop task, the probe detection task, as well as visual search paradigms, and eye tracking. Furthermore, these effects persist for all anxiety disorders when using subliminal presentations or conditions that restrict conscious awareness of threat stimuli (e.g., (86)). Whereas cognitive biases are common to all anxiety disorders, the content of these biases becomes relatively specific, presumably as a result of past history and learning experiences.
For example, an attentional bias toward personally threat-relevant stimuli is characteristic of individuals with GAD (e.g., (87)), and SOP (e.g., (88)). Also, individuals with SOP show an attentional bias toward angry faces, perhaps followed by an avoidant diversion of attention away from such stimuli (e.g., (89)) consistent with a “vigilance-avoidance” pattern. An attentional bias toward phobic stimuli in SP has been observed using several different paradigms (e.g., (90)). Also, individuals with PD preferentially allocate attentional resources to stimuli that represent physical and mental threat such as “fatality” and “insane” (e.g., (88)). In PTSD, an attentional bias toward trauma cues has been observed in several studies (e.g., (91)). The one anxiety disorder where the results are unclear is OCD: several studies that failed to find an attentional bias (e.g., (92)) may have used stimuli that were insufficiently relevant. Another study indicated that even though patients with OCD did not show an attentional bias on response latency measures in an emotional Stroop task, they did show a specific neural response during color naming of relevant words (93).
It is posited that anxious individuals initially show rapid orienting of attention toward (90) and engagement in/or difficulty disengaging from (94) threat stimuli, followed by eventual direction of attention away from threat in an effort to avoid anxiety-provoking situations and to reduce subjective distress and/or perceived danger (86). Such a “vigilance-avoidance” pattern of cognitive bias is viewed as an attempt to regulate negative emotion that is maladaptive because it may enhance sensitization and interfere with habituation, thereby maintaining anxiety in the long-term. In contrast, non-anxious adults attend to threat based on features such as objective stimulus threat value (e.g., (90, 95)).
A related body of research addresses attention to bodily states, or interoception, mostly in the context of PD. PD is associated with heightened awareness of, or ability to detect, bodily sensations of arousal compared to controls (e.g., (96–99)). Discrepant findings (e.g., (100, 101)) exist, but have been attributed to methodological artifact (97).
Attentional bias across anxiety disorders.
Only a few studies have directly compared attentional biases across anxiety disorder groups, using the emotional Stroop task. Maidenberg et al. (88) found more attentional bias for social threat words than control words in SOP, and for social, panic, and general threat words than control words in PD, suggesting a more circumscribed attentional bias in SOP (102). Kampman et al. (102) found no differences on Stroop performance between PD and OCD samples. van den Heuvel et al. (93) compared patients with OCD, PD, and hypochondriasis: attentional bias was observed in PD patients for both OCD-related and PD-related words whereas neither type of word elicited a bias in OCD patients. Clearly, more comparative research is needed.
Attentional bias in youth samples.
Whereas some studies find that anxious children attend preferentially to threat stimuli relative to controls (e.g., (103)), a similar number of studies indicate that a bias for threat stimuli is common to both anxious children and controls (e.g., (104)). Inconsistencies may relate to age and developmental level, since group differences are absent in 8–12 year olds and present in 9–19 year olds (105). That the magnitude of attentional bias appears to persist in anxious children but subside in non-anxious children with advancing age may reflect that with increasing maturity and development, non-anxious children learn to inhibit the processing of threat information whereas anxious children fail at this learning (106). Controlled studies of younger and older anxious youth are required in order to track the developmental progression of this bias as a function of anxiety status.
Attentional bias in anxiety vs. depression.
Only a few studies have directly compared anxiety and depression. In one study, (107) a PD group showed a significant interference in color naming of supraliminal threat words (presented on cards), as well as depression words, whereas a depressed group did not. In another study, faster eye movements toward threatening faces were observed in GAD than a depressed group, most of whom met criteria for comorbid GAD as well (95). Finally, depressed patients showed an attentional bias to SOP facial expressions presented for 10000 ms, whereas patients with GAD did not show an attentional bias to SOP, angry, or happy faces (108). Clearly, conclusions are limited from so few studies.
Still, based on results from independent investigations of anxiety and depression, Mathews and Macleod (109) concluded that depression is characterized by selective attention to cues that are consistent with negative affect when presented at long durations of 1 sec or more (results at shorter durations are inconsistent or absent (e.g., (108)) suggesting the involvement of strategic control processes. In contrast, only anxiety is characterized by selective attention to threat cues at shorter durations of 500 ms or less and under masked conditions, (86) suggesting that selective attention to-ward threatening cues represents a more automated process, not dependent on conscious awareness in anxiety disorders. Hence, there may be basic differ-ences in the attention given to threat relevant information in anxiety vs. depression.
Memory bias and anxiety.
Given the enhanced processing of threat stimuli, a memory bias, particularly implicit memory (i.e., effects of prior exposure to information on later behavior without intention to remember), for threat cues might be expected in anxiety disorders. However, there is no consistent evidence for such memory biases, with the possible exception of PD (e.g., (110)). Some studies even suggest poorer explicit memory of threat-related material in anxiety disorders relative to controls (e.g., (111)). Furthermore, when enhanced memory is occasionally found, the effects have been attributed to superior encoding of emotionally relevant information rather than conceptual and/or retrieval based processes that characterize the memory biases seen in depression (109).
Memory bias in anxiety vs. depression.
Memory bias in depression is comprised of an overgeneral autobiographical memory (e.g., (112)); that is, when instructed to recall a specific incident from one's past in relation to a cue word or phrase, a general class of events tends to be provided. This bias has been attributed to the retrieval strategy used, since it can be changed by being instructed to use a different strategy to retrieve past incidents (109). Another type of memory bias in depressed individuals is to recall more negative self-descriptive adjectives than controls (e.g., (113)). The same occurs in depressed children (e.g., (114)). This relatively enhanced memory for negative information has been attributed to conceptual processing of (or rumination upon) negative information (109).
Thus, in comparison to the rapid attentional bias to threat and limited memory bias seen in anxiety disorders, depression is associated with a delayed attention to threat and greater “rumination” upon and recall of negative information. However, there are no direct comparisons of memory bias in anxiety disorders vs. depression, although lower recall of positive adjectives was associated with dimensional measures of depression symptom levels but not with anxiety symptom levels in an inpatient sample of children and adolescents (115).
Appraisal bias and anxiety.
Studies of appraisal biases indicate that individuals with anxiety disorders appraise situations as more threatening compared to controls. For example, PD individuals are more likely to resolve ambiguous stimuli related to physical sensations in a threat-congruent fashion (116). Persons with PTSD are faster to respond to threat meanings of ambiguous words and to complete sentences with threatening meanings (e.g., (117)). SOP is associated with tendencies to judge negative social events to be more likely and positive social events to be less likely than controls, and to interpret ambiguous social events as more negative and mildly negative social events as more catastrophic than other anxious patients or controls (e.g., (118)). Individuals with SPs overestimate negative outcomes, dangers and the extent of harm or injury in relation to their phobic objects (e.g., (119)), and compulsive washing correlates with estimates of danger. (120) Finally, individuals with high trait anxiety or GAD tend to interpret ambiguous events as threatening (e.g., (121)). These findings also are true for anxious children; compared to controls, anxious children expect a larger number of negative outcomes and more negative events to happen to them (e.g., (122)). Studies of lexical decisions that assess “on-line” interpretations at the time participants are exposed to ambiguous stimuli have similarly shown a negative bias in relation to trait anxiety and social anxiety (e.g., (123)), albeit not always (e.g., (124)). No within-study comparisons between anxiety disorders were located.
Appraisal bias in anxiety vs. depression.
Depression also is associated with biased interpretation of ambiguous information (e.g., (125)) although not always (e.g., (126)). One study showed greater startle reflex responding to ambiguous imagery as a function of level of depression, (127) suggesting that the ambiguous imagery was interpreted negatively and thereby potentiated the startle reflex in the same way as an explicitly negative stimulus. However, direct comparisons between anxious and depressed populations are almost nonexistent. In the only study found, interpretative bias was compared between depressed and anxious children and adolescents by assessing perceived probabilities of negative events to self and to others (128). Anxious youths rated events as more probable in relation to others and not to themselves whereas depressed youths showed no bias for self or others. These findings are difficult to interpret since they are at odds with other studies that have evaluated each disorder separately. Clearly, there is need for more comparative research of appraisal bias both across anxiety disorders and between anxiety and depression.
Summary of information processing biases and implications for DSM.
Whereas both anxiety disorders and unipolar depression exhibit attentional biases to threat-relevant stimuli, the biases appear to occur at different stages of processing (Table 6); early, nonconscious stage of processing in anxiety disorders vs. later, more strategy/conceptual-based stage of processing in depression. Memory biases also seem to differ between anxiety and depression. That is, depression is reliably associated with both implicit and explicit memory biases toward negative self-referent information and general autobiographic memory. In contrast, memory biases are unstable, weak phenomena in the anxiety disorders. Conceivably, the information processing features support threat sensitivity in persons with anxiety disorders, whereas they support deeper evaluation and rumination in persons with depression. However, no studies have specifically evaluated the memory features of fear disorders vs. anxious misery disorders. Finally, both anxiety and depression are associated with appraisal biases, to interpret ambiguous information in a negative fashion, albeit more threat-laden in the first case and more negative self-evaluation in the second case.
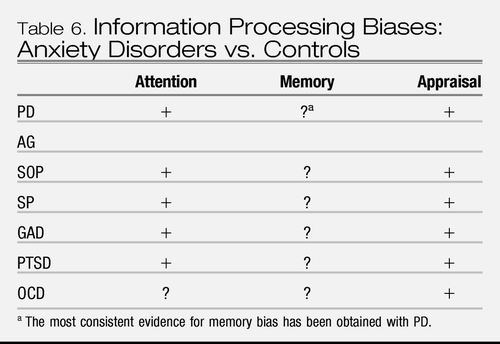 |
Table 6. Information Processing Biases: Anxiety Disorders vs. Controls
Thus, aspects of cognitive biases support the existing nosological distinctions between anxiety and depression. However, very few studies directly compare anxiety and depression and none have compared fear disorders vs. anxious-misery disorders. Thus, the cognitive data either do not support or are insufficient at this time to justify changes to the nosological structure of DSM.
Information processing bias and aversive con-ditioning/stress reactivity.
Conceivably, the non-conscious attentional bias toward threat seen in anxiety disorders contributes to various aspects within Pavlovian conditioning: elevated responding to explicit threat cues, cues that should signal safety (CS−), extinction, and contexts associated with threat. For example, an attentional bias to threat may result in more rapid excitatory fear responding to cues and contexts that signal threat. It may also contribute to sensitization and lack of habituation effects, leading to elevated responding to the CS+ and CS−. Similarly, by overattending to threat relevant stimuli, processing of the features that distinguish the CS− from the CS+ may be impaired, thereby supporting stimulus generalization to the CS−. A correlate of such interference effects may be lack of contingency awareness of the relationships among the CS+, CS− and the US, that again contributes to elevated responding to both types of stimuli. Furthermore, attention to bodily states (interoception) is likely to enhance conditioning, since stronger perceived physiological responses to aversive stimuli generally elicit stronger conditioning (129). In addition, an attentional bias toward threat may increase stress reactivity, in anticipation of stressors and/or in acute response to stressors.
Similarly, threat-biased appraisals may contribute to the acquisition of conditional fear and anxiety responses, and/or contribute to the weakened extinction of such responses. That is, as a result of an interpretive focus that exaggerates rather than reduces threat, anxious individuals may perceive the US as more threatening than nonanxious individuals, thereby supporting both sensitization and associative excitation mechanisms. In addition, anxious individuals may have difficulty reappraising situations in less threatening ways, thereby interrupting inhibitory learning throughout extinction and at extinction recall, such that responding remains more elevated to the CS+ and/or CS− relative to controls. Similar processes could contribute to elevated anticipatory or acute response to stressors in stress reactivity paradigms.
BRAIN IMAGING AND THE NEUROCIRCUITRY OF ANXIETY DISORDERS
Goals
This section reviews pertinent neural circuitry with an emphasis on information gleaned from neuroimaging research. The brain basis of anxiety disorders and the potential neural underpinnings for the clinical, cognitive, and behavioral phenomena reviewed in prior sections are addressed. To the extent that neurobiology has been posed as one of the key validators for DSM-V, it is germane to explore a common circuitry-based pathophysiology that might serve to define the anxiety disorders as a category, as well as features that might distinguish among disorders. While pathophysiology may emanate from dysfunction at the level of molecules, cells, nodes, and/or networks, contemporary imaging has tended to characterize differences at the level of nodes or specific brain regions; we appreciate that while this represents an oversimplification, it provides for a relatively accessible approach. Where appropriate, we will draw upon recent reviews to enable a more concise treatment of these issues here (e.g., (47, 130, 131, 132)).
Brain regions associated with anxiety
A constellation of brain regions has been implicated in mediating the normal functions pertinent to anxiety disorders. First, a network involving the amygdala, ventromedial prefrontal cortex (vmPFC), and the hippocampus has been a focal point for contemporary models of anxiety disorders (47, 130). The amygdala plays a critical role in threat assessment, in forming associations regarding danger in the environment (e.g., conditioned fear acquisition; see earlier section), and in mediating response to threat or potential threat via descending projections to regions that mediate autonomic responses (e.g., heart rate, blood pressure, respiration, sweating, etc.) (133). The vmPFC and hippocampus provide top-down governance over the amygdala, capable of inhibiting fear responding; for instance, the vmPFC mediates extinction recall via inhibition of the amygdala response to learned threat cues (see earlier section), and the hippocampus provides information that is permissive of extinction recall by providing information regarding safe vs. dangerous contexts (47) for review, see Reference (134). Importantly, the hippocampus also mediates contextual conditioning (30, 31, 135, 136) (see earlier section). Of note, the extended amygdala, including the BNST, is purported to play an important role in anxiety per se, as opposed to fear (26, 43) (see earlier section). However, given the challenges in resolving the extended amygdala vs. the amygdala proper, it is important to appreciate that most imaging studies to date that report amygdala findings have not sought to make this distinction. Although, the imminence continuum from anxiety to fear as a function of proximity to threat (see initial section) has been substantiated at the neural level as a shift from vmPFC to periaqueductal gray (137).
Second, the orbitofrontal cortex (OFC) is divisible into medial (mOFC) and lateral (IOFC) subterritories (138). To the extent that OFC plays an important role in computing, assigning, or weighing value, in the service of decision making and guiding behavior, the mOFC principally mediates positive valuations (e.g., of reward and safety) whereas the IOFC principally mediates negative valuations (e.g., of punishment) (e.g., (139)). In this context, mOFC (highly overlapping with vmPFC) plays a role in suppression of fear and anxiety, such as through mediating extinction recall (see earlier section), whereas IOFC with extension in ventrolateral PFC more generally, appears to mediate negative cognitions, obsessions and worry (see first section and earlier section).
Third, the insular cortex mediates interoception, and hence plays a critical role in individuals' awareness of and sensitivity to visceral activity (140) (see earlier section). In this context, the insula is implicated in anxiety sensitivity, something which is purported to enhance Pavlovian conditioning, since stronger perceived (as well as actual) physiological responses to aversive stimuli generally elicit stronger conditioning (129).
Fourth, the anterior cingulate cortex (ACC) is functionally heterogeneous, with distinct dorsal (dACC), pregenual (pgACC), and subgenual (sgACC) subdivisions (e.g., (141)). The dACC has been termed the cognitive division, and has been implicated in a host of functions including error detection, conflict monitoring, and attention (see earlier section). The pgACC has been termed the affective division; both the dACC and pgACC play a role in suppressing attention and response to specific input channels. To elaborate, the pgACC mediates the capacity to suppress attention and response to affective stimuli, whereas the dACC is implicated in suppressing attention and response to cognitive stimuli (see earlier sections). Of note, recent findings suggest that a subterritory of dACC may represent the human homologue of prelimbic cortex, in augmenting amygdala responses to conditioned fear cues (142, 143) (see earlier sections). The sgACC has been termed the visceromotor division, and is contiguous with vmPFC and mOFC; this territory has been implicated in depression (144).
Hypothesized links between brain regions and anxiety symptoms/disorders
Building on knowledge of normal functional anatomy, one can pose a variety of hypotheses seeking to link the observed clinical and behavioral phenomena of anxiety disorders and their neural substrates. For instance, exaggerated responsivity or sensitivity of the amygdala could mediate abnormal threat assessment (see earlier section), exaggerated fear responses including exaggerated autonomic output (see earlier section), or abnormalities in learning about danger in the environment (see earlier section). Further, in addition to vulnerabilities to anxiety conferred by intrinsic abnormality in amygdala function, abnormal amygdala responses could be secondary to insufficient vmPFC function, leading to inability to recall extinction information (see earlier section); or, secondary to abnormal hippocampal function, undermining the capacity to distinguish between safe and dangerous contexts (see earlier section). Cognitive manifestations such as worrying and obsessing are likely mediated by excessive activity in IOFC (and related regions). Interestingly, conditions characterized by globally excessive OFC activity may involve both complaints of such cognitive symptoms (mediated by IOFC) and paradoxically relatively reduced amygdala as well as autonomic responsivity (due to suppression by mOFC), perhaps characteristic of GAD (see earlier section).
Elevated insula activity would be expected as a consequence of normal interoceptive function in the face of elevated autonomic responses (such as secondary to exaggerated amygdala responses) in anxiety disorders. Interestingly however, aberrant insula activity, due to intrinsic dysfunction or hypersensitivity, could also be seen as a correlate of anxiety sensitivity (i.e., interoceptive hypersensitivity) (earlier section), in the absence of elevated autonomic measures or elevated amygdala output, as has been observed in relation to carbon dioxide inhalations and PD (e.g., (63)) (earlier section). Aberrant error detection (i.e., false alarms) leading to pathological doubting, as seen in OCD, could be mediated by dACC dysfunction. It is also possible that elevated activity in this region could exacerbate conditioned fear expression as seen with prelimbic cortical stimulation in rodents (142). In contrast, deficiency in pgACC may mediate attention biases to disorder-relevant cues in the anxiety disorders (earlier sections).
The above series of hypotheses hints at the range of possibilities as well as the potential heterogeneity of pathophysiological roots for the anxiety disorders. While numerous brain imaging studies have been performed in an effort to elucidate the brain basis of anxiety disorders, to date the aggregate data provide initial support for heuristic models as opposed to conclusive evidence of distinct pathophysiology by diagnosis. In fact, few of the above hypotheses have been investigated systematically across the anxiety disorders. We propose that it would be illuminating to take such a systematic approach, including comparisons with other conditions beyond the anxiety disorders, to test for the specificity of findings.
Here we present three examples for illustrative purposes. The first example pertains to amygdala function. Using a range of symptom provocation and cognitive paradigms, investigators have probed amygdala responses to generic (e.g., emotional faces12) and disorder-specific stimuli (Table 7). In this context, there is evidence that exaggerated response to disorder-specific stimuli is a shared feature of those anxiety disorders tested to date, whereas amygdala responses to generic threat-related stimuli may distinguish among the anxiety disorders (e.g., (145)). Consistent with the psychophysiology data from stress reactivity studies (see earlier section), there is preliminary evidence for PTSD and PD to be associated with elevated responses to generic stressors to a greater degree than SOP or SP. Of note, MD is also characterized by exaggerated amygdala responses to emotional faces (146) weighing against this being a unique feature for the anxiety disorders. However, there may be other aspects of amygdala function that distinguish between anxiety disorders and depression. For instance, with regard to laterality, there is some suggestion that anxiety disorders may be preferentially characterized by right-lateralized amygdala findings, (147) whereas depression is characterized by left-sided amygdala findings; this may reflect lateralized aspects of normal amygdala function in humans (148). Further, elevated resting/baseline metabolism within the amygdala has been consistently found in depression, but not in anxiety disorders (149).
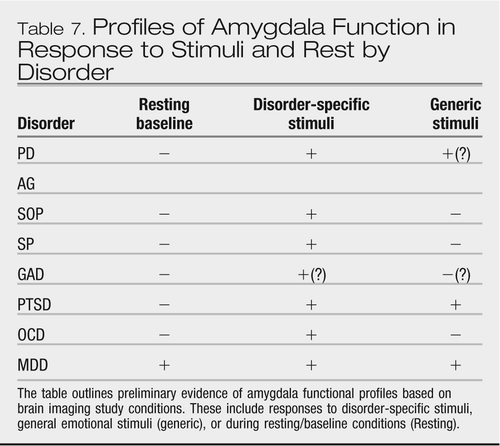 |
Table 7. Profiles of Amygdala Function in Response to Stimuli and Rest by Disorder
Second, using a range of symptom provocation and cognitive paradigms, reduced mOFC function has been consistently found in association with anxiety disorders (131). Interestingly, increased activation within IOFC has been less consistent across anxiety disorders, but may be a hallmark of OCD, and GAD and other disorders characterized by cognitive anxiety (i.e., worry, obsessions, etc.).
Third, again, based upon data from a range of experimental paradigms, elevated insular signal may be a common finding across anxiety disorders (132).
Limitations
Finally, it must be noted that there are limited data with regard to normal function as well as pathophysiology of anxiety disorders across development. There are some initial intriguing findings suggesting exaggerated amygdala response as a potential neural correlate of the behaviorally inhibited phenotype that engenders increased risk for developing anxiety disorders (e.g., (150)), as well as evidence for elevated amygdala and insular responses in individuals with anxiety-prone traits. (151) Moreover, early pioneering studies of anxiety disorders in children implicate potential amygdala dysfunction (e.g., (152, 153)). But more research in this area is especially sorely needed.
Summary
In summary, advances in neuroimaging and translational research of the past ≈20 years have helped to focus the field on neural circuitry implicated in the pathophysiology of anxiety disorders as well as relevant normal functions. While this research has yielded useful heuristic models, much work remains in order to definitively establish the brain basis of anxiety disorders. At this point, measures of amygdala, vmPFC/ OFC, and insula function stand as leading candidates for usefully defining anxiety disorders in future.
CONCLUSION
The goal of this review has been to evaluate commonalities across anxiety disorders in features of responding in self-report estimation/prediction and in on-line responding to aversive stimuli across behavioral, psychophysiological, cognitive domains as well as functional systems at the neural level. We addressed whether a category of fear disorders (i.e., PD, PTSD, SOP, and SP) is distinctly identifiable from anxious-misery disorders (i.e., GAD and depression), and more broadly whether anxiety disorders are distinct from depression.
In terms of symptom self-report, the anxiety disorders are characterized by prototypical fear, com-prised of escape behaviors, physiological arousal, and thoughts of imminent threat, and prototypical anxiety, comprised of avoidant behaviors, tension, and thoughts of future threat. These symptoms are related to, but also distinct from, symptoms of depression. There is some indication that self-reported symptoms of physiological arousal, as a measure of the construct of fear, relate more positively to PD and more negatively to GAD than other anxiety disorders, thereby supporting a distinction between fear and anxious misery disorders. On the other hand, lack of positive affect appears to be strongly associated with both depression and SOP, which is at odds with the proposed subdivision of disorders. However, limitations to self-report methodologies render these findings preliminary and in need of further development.
Together, the bodies of research on Pavlovian conditioning and stress reactivity indicate that, compared to controls, individuals with anxiety disorders show a sensitivity to threat that is expressed in terms of both fear and anxiety responding. More specifically, anxiety disorders are associated with (1) elevated fear responding to cues that signal threat (CS+), (2) elevated fear responding to cues that signal no threat (CS−) when presented in the context of threat, and to cues that formerly signaled threat (i.e., extinction trials), (3) elevated contextual anxiety in contexts and waiting periods (baselines) in which sufficiently aversive stimuli are anticipated, (4) equivalent acute responses to generic stressors, and (5) elevated responses to disorder-specific (personally relevant) stressors. However, these findings have not been fully evaluated across all anxiety disorders and/or are not entirely consistent across all the anxiety disorders that have been tested; only preliminary data suggest greater baseline anticipatory and acute fear responding to disorder-specific stressors in SOP and SP relative to PTSD and PD, and greater baseline anticipatory responding to generic stressors in PTSD and PD.
With respect to our question of “what is an anxiety disorder” these findings imply that anxiety disorders are characterized by elevated sensitivity to threat. However, the nuances of that sensitivity, and whether it discriminates anxiety disorders from depression or fear disorders from anxious misery disorders is undetermined. For these reasons, data from studies of Pavlovian conditioning and stress reactivity do not justify revisions to the DSM nosology for anxiety disorders. Also for these reasons, there is a call for a great deal more research to address the gaps listed above.
Data from measurement of cognitive biases indicates that the anxiety disorders are characterized by a preconscious attentional bias toward personally relevant threat stimuli, and a bias to interpret ambiguous information in a threat-relevant manner. Comparisons across subtypes of anxiety disorders are lacking and thus it is not known whether features of cognitive bias differentiate fear disorders from anxious-misery disorders. However, the cognitive bias data support the nosological distinction between anxiety and depression, with the latter characterized by a slower attentional bias to threat and a stronger memory bias for negative information relative to anxiety. Still, very few studies directly compare cognitive biases in anxiety and depression, such that the data are insufficient at this time to have direct relevance to changes to the nosology of DSM.
Data from neuroimaging studies indicate elevated amygdala responses to disorder-specific threat cues as a common characteristic across anxiety disorders. In contrast, amygdala responses to general threat cues may distinguish among anxiety disorders. However, the various profiles of amygdala response remain to be extended across development, to be well replicated, and to be sufficiently well studied in comparison groups to ascertain their specificity to anxiety vs. mood disorders. Similarly, while there is some indication that anxiety disorders are characterized by elevated insular, lateral OFC, and dorsal ACC responses as well as deficient responses within pgACC, vmPFC, and hippocampus, these profiles also remain to be thoroughly elaborated and replicated. Nevertheless, this implicated circuitry provides a heuristic model for the next step of research, whereby cognitive, behavioral, and physiological findings can be linked to mediating neuroanatomy and brain pathophysiology.
In summary, our review has helped to sharpen our knowledge of the features that characterize anxiety disorders. These features represent an elevated sensitivity to threat, as observed across symptom reporting, behavioral, cognitive and physiological responding, and underlying neural systems. However, the degree to which these features are differentiated between subtypes of anxiety disorders, or discriminate anxiety disorders from depression remain open to further investigation. One of the major barriers to such investigation is the high rate of co-morbidity within and across anxiety disorders and depression, and the associated overlap in diagnostic symptom criteria in some cases. Advances will most likely require dimensional approaches that evaluate both the shared as well as the unique contributions of fear, anxiety, and depression to the various facets of threat sensitivity.
1 Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry 1999;56:921–926.Crossref, Google Scholar
2 Vollegerg WA, Iedema J, Biji RV, et al. The structure and stability of common mental disorders: the NEMESIS study. Arch Gen Psychiatry 2001;58:597–603.Crossref, Google Scholar
3 Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J Abnorm Psychol 2005;114:522–536.Crossref, Google Scholar
4 Andrews G, Charney DS, Sirovatka PJ, Regier DA, eds. Stress Induced and Fear Circuitry Disorders: Refining the Research Agenda for DSM-V. Arlington, VA: American Psychiatric Association; 2009.Google Scholar
5 Barlow DH. Anxiety and its Disorders: The Nature and Treatment of Anxiety and Panic. 2nd ed. New York: Guilford Press; 2002.Google Scholar
6 Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, eds. Evolution and Learning. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988:185–212.Google Scholar
7 Lang PJ. Fear reduction and fear behavior: problems in treating a construct. In: Schlien J, ed. Research in Psychotherapy. Volume III. Washington, DC: American Psychiatric Press; 1968:90–103.Crossref, Google Scholar
8 Zinbarg RE. Concordance and synchrony in measures of anxiety and panic reconsidered: a hierarchical model of anxiety and panic. Behav Ther 1998;29:301–323.Crossref, Google Scholar
9 Joiner TE, Steer RA, Beck AT, et al. Physiological hyperarousal: construct validity of a central aspect of the tripartite model of depression and anxiety. J Abnorm Psychol 1999;108:290–298.Crossref, Google Scholar
10 Ree MJ, MacLeod C, French D, Locke V. The State-Trait Inventory for Cognitive and Somatic Anxiety: Development and Validation.
11 Grös DF, Antony MM, Simms LJ, McCabe RE. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): Comparison to the State-Trait Anxiety Inventory (STAI). Psychol Assess 2007;19:369–381.Crossref, Google Scholar
12 Chorpita BF, Albano AM, Barlow DH. The structure of negative emotions in a clinical sample of children and adolescents. J Abnorm Psychol 1998;107:74–85.Crossref, Google Scholar
13 Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol 1991;100:316–336.Crossref, Google Scholar
14 Watson D, Clark LA, Weber K, et al. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol 1995;104: 15–25.Crossref, Google Scholar
15 Joiner TE, Catanzaro SJ, Laurent J. Tripartite structure of positive and negative affect, depression, and anxiety in child and adolescent psychiatric inpatients. J Abnorm Psychol 1996;105:401–409.Crossref, Google Scholar
16 Chorpita BF. The tripartite model and dimensions of anxiety and depression: an examination of structure in a large school sample. J Abnorm Child Psychol 2002;30:177–190.Crossref, Google Scholar
17 Cook JM, Orvaschel H, Simco E, et al. A test of the tripartite model of depression and anxiety in older adult psychiatric outpatients. Psychol Aging 2004;19:444–451.Crossref, Google Scholar
18 Ollendick TH, Seligman LD, Goza AB, et al. Anxiety and depression in children and adolescents: a factor-analytic examination of the tripartite model. J Child Fam Stud 2003;12: 157–170.Crossref, Google Scholar
19 Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol 1998;107:179–192.Crossref, Google Scholar
20 Hale WW, Raaijmakers Q, Muris P, Meeus W. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED) in the general adolescent population. J Am Acad Child Adolesc Psychiatry 2005;44:283–290.Crossref, Google Scholar
21 Zinbarg RE, Barlow DH, Liebowitz M, et al. The DSM-IV field trial for mixed anxiety-depression. Am J Psychiatry 1994;151: 1153–1162.Crossref, Google Scholar
22 Chorpita BF, Plummer CM, Moffitt CE. Relations of tripartite dimensions of emotion to childhood anxiety and mood disorders. J Abnorm Child Psychol 2000;28:299–310.Crossref, Google Scholar
23 Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorder: its not what you thought it was. Am Psychol 2006;61:10–26.Crossref, Google Scholar
24 Olsson A, Phelps EA. Learned fear of “unseen” faces after pavlovian, observational, and instructed fear. Psychol Sci 2004; 15:822–828.Crossref, Google Scholar
25 Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. 2nd ed. Oxford: Oxford University Press; 2000.Google Scholar
26 Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 2003;463:199–216.Crossref, Google Scholar
27 Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science 1992;256:675–677.Crossref, Google Scholar
28 Moses SN, Winocur G, Ryan JD, Moscovitch M. Environmental complexity affects contextual fear conditioning following hippocampal lesions in rats. Hippocampus 2007;17:333–337.Crossref, Google Scholar
29 Vansteenwegen D, Iberico C, Vervliet B, et al. Contextual fear induced by unpredictability in a human fear conditioning preparation is related to the chronic expectation of a threatening US. Biol Psychiatry 2008;77:39–46.Crossref, Google Scholar
30 Grillon C, Baas JMP, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biol Psychiatry 2006;60:752–759.Crossref, Google Scholar
31 LaBar KS, Phelps EA. Arousal-mediated memory consolidation: role of the medial temporal lobe in humans. Psychol Sci 1998;9: 490–493.Crossref, Google Scholar
32 Alvarez RB, Biggs A, Chen G, et al. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci 2008;28:6211–6219.Crossref, Google Scholar
33 Marschner A, Kalisch R, Vervliet B, et al. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci 2008;28:9030–9036.Crossref, Google Scholar
34 Lissek S, Powers AS, McClure EB, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther 2005;43:1391–1424.Crossref, Google Scholar
35 Blechert J, Michael T, Vriends N, et al. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther 2007;45:2019–2033.Crossref, Google Scholar
36 Orr SP, Milad MR, Metzger LJ, Lasko, et al. Effects of beta blockade, PTSD diagnosis, and explicit threat on the extinction and retention of an aversively conditioned response. Biol Psychol 2006;73:262–271.Crossref, Google Scholar
37 Michael T, Blechert J, Vriends N, et al. Fear conditioning in PD: enhanced resistance to extinction. J Abnorm Psychol 2007;116: 612–617.Crossref, Google Scholar
38 Craske MG, Kircanski K, Zelikowsky M, et al. Optimizing inhibitory learning during exposure therapy. Behav Res Ther 2008;46:5–27.Crossref, Google Scholar
39 Waters AM, Henry J, Neumann DL. Aversive palovian conditioning in childhood anxiety disorders: impaired response inhibition and resistance to extinction. J Abnorm Psychol 2009;118:311–321.Crossref, Google Scholar
40 Lau JY, Lissek S, Nelson EE, et al. Fear conditioning in adolescents with anxiety disorders: results from a novel experi-mental paradigm. J Am Acad Child Adolesc Psychiatry 2008;47: 94–102.Crossref, Google Scholar
41 Liberman LC, Lipp OV, Spence SH, March S. Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther 2006;44:1491–1502.Crossref, Google Scholar
42 Rescorla RA, Wagner AR. A theory of Pavlovian condi-tioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, eds. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972:64–99.Google Scholar
43 Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, ed. The Amygdala. Oxford, UK: Oxford University Press; 2000: 213–288.Google Scholar
44 Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioural extinction. Biol Psychiatry 2002;52: 976–986.Crossref, Google Scholar
45 Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 2006;60: 337–343.Crossref, Google Scholar
46 Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci 2004;7:1144–1152.Crossref, Google Scholar
47 Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroima-ging research-past, present, and future. Biol Psychiatry 2006;60: 376–382.Crossref, Google Scholar
48 Bremner JD, Vermetten E, Schmahl C, et al. Positron emission tomographic imaging of neural correlates of fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med 2005; 35:791–806.Crossref, Google Scholar
49 Pliszka SR, Hatch JP, Borcherding SH, Rogeness GA. Classical conditioning in children with attention deficit hyperactivity disorder (ADHD) and anxiety disorders: a test of Quay's model. J Abnorm Child Psychol 1993;21:411–423.Crossref, Google Scholar
50 Veit R, Flor H, Erb M, et al. Brain circuits involved in emotional learning in antisocial behavior and SOP in humans. Neurosci Lett 2002;328:233–236.Crossref, Google Scholar
51 Grillon C, Morgan III CA. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol 1999;108:134–142.Crossref, Google Scholar
52 Grillon C, Morgan III CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorders. Biol Psychiatry 1998;44:1027–1036.Crossref, Google Scholar
53 Carson MA, Metzger LJ, Lasko NB, et al. Physiologic reactivity to startling tones in female Vietnam nurse veterans with PTSD. J Trauma Stress 2007;20:657–666.Crossref, Google Scholar
54 Grillon C, Lissek S, Rabin S, et al. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of PD. Am J Psychiatry 2008;165: 898–904.Crossref, Google Scholar
55 Melzig CA, Weike AI, Zimmermann J, Hammn AO. Startle reflex modulation and autonomic responding during anxious apprehension in PD patients. Psychophysiology 2007;44: 846–854.Crossref, Google Scholar
56 Grillon C, Warner V, Hille J, et al. Families at high and low risk for depression: a three-generation startle study. Biol Psychiatry 2005;57:953–960.Crossref, Google Scholar
57 Craske MG, Rose RD, Lang A, et al. Computer-assisted delivery of cognitive behavioral therapy for anxiety disorders in primary-care settings. Depress Anxiety 2009;26:235–242.Crossref, Google Scholar
58 Grillon C, Ameli R, Goddard A, et al. Baseline and fear-potentiated startle in PD patients. Biol Psychiatry 1994;35: 431–439.Crossref, Google Scholar
59 Morgan CA, Grillon C, Southwick SM, et al. Yohimbine facilitated acoustic startle in combat veterans with post-traumatic stress disorder. Psychopharmacology 1995;117:466–471.Crossref, Google Scholar
60 Blechert J, Michael T, Grossman P, et al. Autonomic and respiratory characteristics of posttraumatic stress disorder and PD. Psychosom Med 2007;69:935–943.Crossref, Google Scholar
61 Roth WT, Margraf J, Ehlers A, et al. Stress test reactivity in PD. Arch Gen Psychiatry 1992;49:301–310.Crossref, Google Scholar
62 Gorman JM, Kent J, Martinez J, et al. Physiological changes during carbon dioxide inhalation in patients with PD, major depression, and premenstrual dysphoric disorder. Arch Gen Psychiatry 2001;58:125–131.Crossref, Google Scholar
63 Gorman JM, Martinez J, Coplan JD, et al. The effect of successful treatment on the emotional and physiological response to carbon dioxide. Biol Psychiatry 2004;56:862–867.Crossref, Google Scholar
64 Pine DS, Klein RG, Coplan JD, et al. Differential carbon dioxide sensitivity in childhood anxiety disorders and nonill comparison group. Arch Gen Psychiatry 2000;57:960–967.Crossref, Google Scholar
65 Perna G, Casolari A, Bussi R, et al. Comparison of 35% carbon dioxide reactivity between PD and eating disorder. Psychiatry Res 2004;125:277–283.Crossref, Google Scholar
66 van Beek N, Griez E. Reactivity to a 35% CO-sub-2 challenge in health first-degree relative of patients with panic disorder. Biol Psychiatry 2000;47:830–835.Crossref, Google Scholar
67 Coryell W, Dindo L, Fyer A, Pine DS. Onset of spontaneous panic attacks: a prospective study of risk factors. Psychosom Med 2006;68:754–757.Crossref, Google Scholar
68 Battaglia M, Pesenti-Gritti P, Spatola CA. A twin study of the common vulnerability between heightened sensitivity to hyper-capnia and panic disorder. Am J Med Genet B Neuropsychiatr Genet 2008;147B:586–593.Crossref, Google Scholar
69 Miller MW, Litz BT. Emotional-processing in posttraumatic stress disorder II: startle reflex modulation during picture processing. J Abnorm Psychol 2004;113:451–463.Crossref, Google Scholar
70 Bryant RA, Harvey AG, Gordon E, Barry RJ. Eye movement and electrodermal responses to threat stimuli in post-traumatic stress disorder. Int J Psychophysiol 1995;20:209–213.Crossref, Google Scholar
71 Pitman RK, Orr SP, Forgue DF, et al. Psychophysiologic responses to combat imagery of Vietnam veterans with posttrau-matic stress disorder versus other anxiety disorders. J Abnorm Psychol 1990;99:49–54.Crossref, Google Scholar
72 Levin AP, Saoud JB, Strauman T, et al. Responses of “generalized” and “discrete” social phobics during public speaking. J Anxiety Disord 1993;7:207–221.Crossref, Google Scholar
73 Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biol Psychiatry 2000;47:85–95.Crossref, Google Scholar
74 Alpers GW, Abelson JL, Wilhelm FH, Roth WT. Salivary cortisol response during exposure treatment in driving phobics. Psychosom Med 2003;65:679–687.Crossref, Google Scholar
75 Hamm AO, Cuthbert BN, Globisch J, Vaitl D. Fear and the startle reflex: blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology 1997;34:97–107.Crossref, Google Scholar
76 Hoehn-Saric R, McLeod DR, Zimmerli WD. Somatic manifestations in women with GAD: psychophysiological responses to psychological stress. Arch Gen Psychiatry 1989;46: 1113–1119.Crossref, Google Scholar
77 Thayer JF, Friedman BH, Borkovec TD. Autonomic character-istics of GAD and worry. Biol Psychiatry 1996;39:255–266.Crossref, Google Scholar
78 Thayer JF, Friedman BH, Borkovec TD, et al. Phasic heart period reactions to cued threat and nonthreat stimuli in generalized anxiety disorder. Psychophysiology 2000;37:361–368.Crossref, Google Scholar
79 Hoehn-Saric R, McLeod DR, Hipsley P. Is hyperarousal essential to OCD? Diminished physiologic flexibility, but not hyperarousal, characterizes patients with OCD. Arch Gen Psychiatry 1995;52:688–693.Crossref, Google Scholar
80 Boulougouris JC, Rabavilas AD, Stefanis C. Psychophysiological responses in obsessive-compulsive patients. Behav Res Ther 1977;15:221–230.Crossref, Google Scholar
81 Cuthbert BN, Lang PJ, Strauss C, et al. The psychophysiology of anxiety disorder: fear memory imagery. Psychophysiology 2003;40:407–422.Crossref, Google Scholar
82 Monk C, Kovelenko P, Ellman LM, et al. Enhanced stress reactivity in pediatric anxiety disorders: implications for future cardiovascular health. Int J Neuropsychopharmacol 2001;4: 199–206.Crossref, Google Scholar
83 van Lang ND, Tulen JH, Kallen VL, et al. Autonomic reactivity in clinically referred children attention-deficit/hyperactivity disorder versus anxiety disorder. Eur Child Adolesc Psychiatry 2007;16:71–78.Crossref, Google Scholar
84 Pine DS, Klein RG, Roberson-Nay R, et al. Response to 5% carbon dioxide in children and adolescents: relationship to PD in parents and anxiety disorders in subjects. Arch Gen Psychiatry 2005;62:73–80.Crossref, Google Scholar
85 Dorn LD, Campo JC, Thato S, et al. Psychological comorbidity and stress reactivity in children and adolescents with recurrent abdominal pain and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:66–75.Crossref, Google Scholar
86 Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther 2002;40:1403–1414.Crossref, Google Scholar
87 Mogg K, Mathews A, Eysenck M. Attentional bias to threat in clinical anxiety states. Cogn Emot 1992;6:149–159.Crossref, Google Scholar
88 Maidenberg E, Chen E, Craske MG, et al. Specificity of attentional bias in PD and SOP. J Anxiety Disord 1996;10:529–541.Crossref, Google Scholar
89 Chen YP, Ehlers A, Clark DM, Mansell W. Patients with generalized SOP direct their attention away from faces. Behav Res Ther 2002;40:677–687.Crossref, Google Scholar
90 Ohman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. J Exp Psychol 2001;130:466–478.Crossref, Google Scholar
91 Bryant RA, Harvey AG. Attentional bias in posttraumatic stress disorder. J Trauma Stress 1997;10:635–644.Google Scholar
92 Moritz S, von Muhlenen A. Investigation of an attentional bias for fear-related material in obsessive-compulsive checkers. Depress Anxiety 2008;25:225–229.Crossref, Google Scholar
93 van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Disorder-specific neuroanatomical correlates of attentional bias in OCD, PD, and hypochondriasis. Arch Gen Psychiatry 2005;62:922–933.Crossref, Google Scholar
94 Yiend J, Mathews A. Anxiety and attention to threatening pictures. The Q J Exp Psychol A 2001;54A:665–681.Crossref, Google Scholar
95 Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in GAD and depressive disorder. J Abnorm Psychol 2000;109:695–704.Crossref, Google Scholar
96 Ehlers A, Breuer P. Increased cardiac awareness in panic disorder. J Abnorm Psychol 1992;101:371–382.Crossref, Google Scholar
97 Ehlers A, Breuer P. How good are patients with panic disorder at perceiving their heartbeats? Biol Psychol 1996;42:165–182.Crossref, Google Scholar
98 Ehlers A, Breuer P, Dohn D, Feigenbaum W. Heartbeat perception and panic disorder. Possible explanations for dis-crepant findings. Behav Res Ther 1995;33:69–76.Crossref, Google Scholar
99 Zoellner LA, Craske MG. Interoceptive accuracys and panic. Behav Res Ther 1999;37:1141–1158.Crossref, Google Scholar
100 Antony MM, Brown TA, Craske MG, et al. Accuracy of heart beat perception in panic disorder, social phobia, and non-anxious subjects. J Anxiety Disord 1995;9:355–371.Crossref, Google Scholar
101 Rapee RM. Detection of somatic sensations in panic disorder. Behav Res Ther 1994;32:825–831.Crossref, Google Scholar
102 Kampman M, Keijsers GPJ, Verbraak MJPM, et al. The emotional Stroop: a comparison of panic disorder patients, obsessive-compulsive patients, and normal controls, in two experiments. J Anxiety Disord 2002;4:425–441.Crossref, Google Scholar
103 Dalgleish T, Moradi AR, Taghavi MR, et al. An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychol Med 2001;31:541–547.Crossref, Google Scholar
104 Waters AM, Lipp OV, Spence SH. Attentional bias toward fear-related stimuli: an investigation with nonselected children and adults and children with anxiety disorders. J Exp Child Psychol 2004;89:320–337.Crossref, Google Scholar
105 Craske MG, Waters AM. Panic disorder, phobias, and generalized anxiety disorder. Annu Rev Clin Psychol 2005;1: 197–225.Crossref, Google Scholar
106 Kindt M, Bierman D, Brosschot JF. Cognitive bias in spider fear and control children: assessment of emotional interference by a .pb19 card format and a single-trial format of the Stroop task. J Exp Child Psychol 1997;66:163–179.Crossref, Google Scholar
107 Carter CS, Maddock RJ, Magliozzi J. Patterns of abnormal processing of emotional information in PD and major depres-sion. Psychopathology 1992;25:65–70.Crossref, Google Scholar
108 Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol 2004;113:127–135.Crossref, Google Scholar
109 Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol 2005;1:167–195.Crossref, Google Scholar
110 Nugent K, Mineka S. The effect of high and low trait anxiety on implicit and explicit memory tasks. Cogn Emot 1994;8: 147–163.Crossref, Google Scholar
111 Watts FN, Trezise L, Sharrock R. Processing of phobic stimuli. Br J Clin Psychol 1986;25:253–259.Crossref, Google Scholar
112 Williams JMG, Teasdale JD, Segal ZV, Soulsby J. Mindfulness-based cognitive therapy reduces overgeneral autobiographical memory in formerly depressed patients. J Abnorm Psychol 2000;109:150–155.Crossref, Google Scholar
113 Derry PA, Kuiper NA. Schematic processing and self-reference in clinical depression. J Abnorm Psychol 1981;90:286–297.Crossref, Google Scholar
114 Zupan BA, Hammen C, Jaenicke C. The effects of current mood and prior depressive history on self-schematic processing in children. J Exp Child Psychol 1987;43:149–158.Crossref, Google Scholar
115 Gencoz T, Voelz ZR, Gencoz F, et al. Specificity of information processing styles to depressive symptoms in youth psychiatric inpatients. J Abnorm Child Psychol 2001;29:255–262.Crossref, Google Scholar
116 Harvey A, Watkins E, Mansell W, Shafran R. Cognitive Behavioural Processes Across Psychological Disorders: A Transdiagnostic Approach to Research and Treatment. Oxford, UK: Oxford University Press; 2004.Google Scholar
117 Kimble MW, Kaufman ML, Leonard LL, et al. Sentence completion test in combat veterans with and without PTSD: preliminary findings. Psychiatry Res 2002;113:303–307.Crossref, Google Scholar
118 Wells A, Clark DM. SOP: a cognitive approach. In: Davey GCI, ed. Phobias—A Handbook of Theory, Research and Treatment. New York: Wiley; 1997:3–26.Google Scholar
119 Tomarken AJ, Mineka S, Cook M. Fear-relevant seletive associations and covariation bias. J Abnorm Psychol 1989;98: 381–394.Crossref, Google Scholar
120 Jones MK, Menzies RG. The cognitive mediation of obsessive-compulsive handwashing. Behav Res Ther 1997;35:843–850.Crossref, Google Scholar
121 Eysenck MW, Mogg K, May J, et al. Bias in interpretation of ambiguous sentences related to threat in anxiety. J Abnorm Psychol 1991;100:144–150.Crossref, Google Scholar
122 Suarez L, Bell-Dolan D. The relationship of child worry to cognitive biases: threat interpretation and likelihood of event occurrence. Behav Ther 2001;32:425–442.Crossref, Google Scholar
123 Yoon KL, Zinbarg RE. Interpreting neutral faces as threatening is a default mode for socially anxious individuals. J Abnorm Psychol 2008;117:680–685.Crossref, Google Scholar
124 Hirsch CR, Mathews A. Impaired positive inferential bias in SOP. J Abnorm Psychol 2000;109:705–712.Crossref, Google Scholar
125 Nunn JD, Matthews A, Trower P. Selective processing of concern-related information in depression. Br J Clin Psychol 1997;36:489–503.Crossref, Google Scholar
126 Lawson C, MacLeod C. Depression and the interpretation of ambiguity. Behav Res Ther 1999;37:463–474.Crossref, Google Scholar
127 Lawson C, MacLeod C, Hammond G. Interpretation revealed in the blink of an eye: depressive bias in the resolution of ambiguity. J Abnorm Psychol 2002;111:321–328.Crossref, Google Scholar
128 Dalgleish T, Taghavi R, Neshat-Doost H, et al. Information processing in clinically depressed and anxious children and adolescents. J Child Psychol Psychiatry 1997;38:535–541.Crossref, Google Scholar
129 Davey G, ed. Cognitive Processes and Pavlovian Conditioning in Humans. Chichester: Wiley; 1987.Google Scholar
130 Rauch SL, Drevets WC. Neuroimaging and neuroanatomy of stress-induced and fear circuitry disorders. In: Andrews G, Charney DS, eds. Stress-Induced and Fear Circuitry Disorders Arlington, VA: American Psychiatric Association; 2009.Google Scholar
131 Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci 2007;1121:546–561.Crossref, Google Scholar
132 Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry 2006;60:383–387.Crossref, Google Scholar
133 Aggleton JP. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss; 1992.Google Scholar
134 Milad MR, Wright CI, Orr SP, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 2007;62:446–454.Crossref, Google Scholar
135 Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christman C, Schad LR, Flor H. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygda-la, and prefrontal cortex. Eur J Neurosci 2009;29:823–832.Crossref, Google Scholar
136 Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus 2001;11:8–17.Crossref, Google Scholar
137 Mobbs D, Petrovic P, Marchant JL, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 2007;317:1079–1083.Crossref, Google Scholar
138 Zald DH, Rauch SL, eds. The Orbitofrontal Cortex. Oxford: Oxford University Press; 2006.Google Scholar
139 Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005;6: 691–702.Crossref, Google Scholar
140 Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting interoceptive awareness. Nat Neurosci 2004;7:189–195.Crossref, Google Scholar
141 Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulated cortex. Trends Cognit Sci 2000;4:215–222.Crossref, Google Scholar
142 Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 2007;27:840–844.Crossref, Google Scholar
143 Milad MR, Quirk GJ, Pitman RK, et al. A role for the human dorsal anterior cingulated cortex in fear expression. Biol Psychiatry 2007;62:1191–1194.Crossref, Google Scholar
144 Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005;45:651–660.Crossref, Google Scholar
145 Blair K, Shaywitz J, Smith BW, et al. Response to emotional expressions in generalized SOP and GAD: evidence for separate disorders. Am J Psychiatry 2008;165:1193–1202.Crossref, Google Scholar
146 Sheline YI, Barch DM, Donnelly JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 2001;50:651–658.Crossref, Google Scholar
147 Fredrikson M, Furmark T. Amygdaloid regional cerebral blood flow and subjective fear during symptom provocation in anxiety disorders. Ann N Y Acad Sci 2003;985:341–347.Crossref, Google Scholar
148 Wright CI, Fischer H, Whalen PJ, et al. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport 2001;12:379–383.Crossref, Google Scholar
149 Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci 2003;985:420–444.Crossref, Google Scholar
150 Pérez-Edgar K, Roberson-Nay R, Hardin MG, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage 2007;35:1538–1546.Crossref, Google Scholar
151 Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 2007;164:318–327. .pb20Crossref, Google Scholar
152 Thomas KM, Drevets WC, Dahl RE, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry 2001;58:1057–1063.Crossref, Google Scholar
153 Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with GAD. Arch Gen Psychiatry 2008;65:568–576.Crossref, Google Scholar
154 Pennebaker JW. Physical symptoms associated with blood pressure. Psychophysiology 1982;19:201–210.Crossref, Google Scholar
155 Margraf J, Taylor CB, Ehlers A, et al. Panic attacks in the natural environment. J Nerv Ment Dis 1987;175:558–565.Crossref, Google Scholar
156 Taylor CB, Sheikh J, Agras WS, et al. Ambulatory heart rate changes in patients with panic attacks. Am J Psychiatry 1986;143:478–482.Crossref, Google Scholar
157 Gerlach AL, Wilhelm FH, Gruber K, Roth WT. Blushing and physiological arousability in social phobia. J Abnorm Psychol 2001;110:247–258.Crossref, Google Scholar
158 Holroyd KA, Westbrook T, Wolf M, Bradhorn E. Performance, cognition, and physiological responding in test anxiety. J Abnorm Psychol 1978;87:442–451.Crossref, Google Scholar
159 Roberts T-A, Pennebaker JW. Gender differences in perceiving internal state: toward a his-and-hers model of perceptual cue use. In: Zanna M, ed. Advances in Experimental Social Psychology, Volume 27. San Diego: Academic Press; 1991:143–175.Google Scholar
160 Rapee RM, Lim L. Discrepancy between self- and observer ratings of performance in social phobics. J Abnorm Psychol 1992;101:728–731.Crossref, Google Scholar
161 Stopa L, Clark DM. Cognitive processes in social phobia. Behav Res Ther 1993;31:255–267.Crossref, Google Scholar
162 Tran GQ, Chambless DL. Psychopathology of social phobia: effects of subtype and of avoidant personality disorder. J Anxiety Disord 1995;9:489–501.Crossref, Google Scholar
163 Rachman S, Lopatka C. Match and mismatch in the prediction of fear: I. Behav Res Ther 1986;24:387–393.Crossref, Google Scholar
164 Taylor S, Rachman SJ. Stimulus estimation and the over-prediction of fear. Br J Clin Psychiatry 1994;33:173–181.Crossref, Google Scholar
165 Seligman MEP. Phobias and preparedness. Behav Ther 1971;2:307–320.Crossref, Google Scholar
166 Lovibond PF, Davis NR, O'Flaherty AS. Protection from extinction in human fear conditioning. Behav Res Ther 2000;38:967–983.Crossref, Google Scholar
167 Perna G, Bussi R, Allevi L, Bellodi L. Sensitivity to 35% carbon dioxide in patients with generalized anxiety disorder. J Clin Psychiatry 1999;60:379–384.Crossref, Google Scholar



