The Clinical Assessment and Treatment of Nicotine Dependence
Abstract
In the United States and around the world, nicotine dependence is a leading cause of preventable death. Smoking cessation results in immediate and enduring health benefits. A wide range of clinical interventions have been shown to facilitate smoking cessation and to have a favorable cost-benefit profile. Practice guidelines and national reports have issued calls to action and have made smoking cessation resources readily available. Yet utilization of smoking cessation interventions and resources is lagging. Health care systems and providers have a shared responsibility to ensure that all smokers are offered appropriate interventions. This article summarizes the epidemiology and pathophysiology of nicotine dependence as a background for reviewing the central components of clinical evidence-based smoking cessation interventions: screening, assessment, advice, assistance, and follow-up. Specific patient populations are discussed, as well as emerging strategies to address nicotine dependence.
An impressive body of evidence demonstrates the effectiveness of evidence-based smoking cessation interventions. Nonetheless, every day a large number of smokers slip through the fingers of health care providers with unaddressed nicotine dependence. Many factors contribute to the disparity between smoking prevalence and smoking intervention. This article will review the clinical assessment and treatment of nicotine dependence in the general population, as well as in special populations such as individuals with mental illness.
EPIDEMIOLOGY
Statistics on the prevalence and destructiveness of tobacco use are often cited, but their significance warrants repetition. Tobacco use remains a global epidemic. Worldwide, tobacco is estimated to kill more than 5 million people each year (1). At current rates, tobacco-related mortality is expected to grow to 8 million by 2030 (2).
In the United States, tobacco use has decreased from a peak of 42.0% of adults in 1965 to a current rate of 20.6% (3). The decrease is largely attributable to a multimodal public health and policy campaign targeting tobacco use cessation. Nevertheless, tobacco use continues to be the leading cause of preventable death, accounting for one in five premature deaths, mostly due to cardiovascular disease, cancer, and pulmonary disease (4). The total economic burden of tobacco use in the United States, including both lost productivity and health care costs, is more than 193 billion dollars (5). Put another way, for every pack of cigarettes smoked, the cost to society is $10.47 (6).
Individuals with low educational attainment, low socioeconomic status, and mental health problems are disproportionately affected by tobacco-related morbidity (3). Among individuals with mental illness, the prevalence of nicotine dependence is two to threefold higher than that for the general population (7). Mental illness is associated with more severe nicotine dependence and a greater burden of smoking-related medical illness (7). Approximately 7% of the U.S. population have both a psychiatric illness and nicotine dependence, yet this population consumes more than 34% of all cigarettes (7).
The Benefits of quitting smoking are immediate and enduring. The risk of acute myocardial infarction falls shortly after discontinuation (8), and cardiovascular risk continues to decrease such that 15 years after quitting smoking the risk of coronary artery disease or stroke is equivalent to that of a nonsmoker (9). Lung function also improves after smoking cessation, leading to improved mucus clearance and decreased risk of infection and cancer. Ten years after smoking cessation the risk of lung cancer is half that of someone who continued smoking (9).
Within any given year, 70% of smokers express the desire to quit, and approximately 40% make an attempt to quit (4). Unassisted, only 2.5%–7% of smokers are successful each year (4, 10, 11). Self-help increases quit rates slightly, but patients who receive a tobacco cessation intervention from a clinician are approximately twice as likely to quit smoking as those who do not (4).
There appears to be a dose-response relationship between quantity of smoking cessation assistance and success in quitting (4). With comprehensive extended treatment, integrating psychological counseling, pharmacological management, and long-term follow-up, as many as half of smokers can achieve long-term abstinence (12). Thus, when implemented systematically, smoking cessation interventions are effective. This article will review the pathophysiology and treatment of nicotine dependence.
PATHOPHYSIOLOGY
There are more than 4000 chemicals in tobacco smoke (13), and more than 250 of these have been demonstrated to be harmful (14). Nicotine, an alkaloid compound with stimulant properties, does not appear to have significant toxicity at the doses present in cigarettes. However, nicotine dependence sustains tobacco smoking, and in that capacity its actions are a critical underpinning of tobacco dependence.
Nicotine readily diffuses into the brain, where it binds to the nicotinic cholinergic receptor, mimicking the actions of acetylcholine (15). Binding of nicotine to the nicotinic cholinergic receptor opens voltage-gated calcium channels by inducing a change in the conformation of α and β subunits (16), resulting in altered brain concentrations of dopamine, serotonin, noradrenaline, γ-aminobutyric acid, glutamate, acetylcholine, and endorphins (17). Nicotinic cholinergic receptors are widespread in the central and peripheral nervous systems; however, nicotine dependence is particularly affected by receptors localized in the ventral tegmental area, which promote release of dopamine in the nucleus accumbens and prefrontal cortex (18).
Initially, the pleasurable sensations produced by nicotine are positively reinforcing. Prolonged exposure to nicotine leads to neuroadaptation, a process by which the number of binding sites on the nicotinic cholinergic receptor change (19), contributing to physical dependence. Physical dependence is characterized by desensitization to elements of nicotine intoxication (tolerance), sensitization to nicotine-induced incentive salience (craving), and withdrawal after pharmacokinetic elimination (16, 20). As stated in DSM-IV-TR, withdrawal symptoms include depressed mood, insomnia, irritability, anxiety, difficulty concentrating, restlessness, and weight gain or increased appetite.
The repetitive use of nicotine, compelled by positive reinforcement (pursuit of pleasure) and negative reinforcement (avoidance of withdrawal), also becomes a psychologically conditioned behavior (21). Over time, an ever-widening number of associated moods, environments, behaviors, and sensations become conditioned cues that independently trigger the craving to smoke.
Studies of twins suggest that more than 50% of the risk for nicotine dependence is genetically transmitted (22). Genome-wide association and candidate gene studies have implicated genes affecting the nicotine receptor, nicotine metabolism, the dopamine transporter, dopamine metabolism, and opioid receptors (23, 24). The effort to identify specific genetic determinants is complicated by the fact that individuals appear to be predisposed to nicotine dependence by cumulative small gene effects, with both gene-gene and gene-environment interactions.
APPROACH TO THE PATIENT WHO SMOKES
Over the past decade, a wide range of reviews, reports, and practice guidelines addressing smoking cessation have been developed (2, 4, 25–30). The consistent recommendations emerging from these reports comprise a standard of care for the screening and treatment of nicotine dependence (31, 32). Among protocols, the 5 A's has been particularly influential; it stands on a robust evidence-based platform, as well as being simple, comprehensive, and easy to use (Figure 1). For practice settings in which treatment interventions are not feasible, the AAR model (Ask, Advise, and Refer to a national quit-line) has been recommended as an alternative (33). The central components common to clinical smoking cessation interventions are as follows.
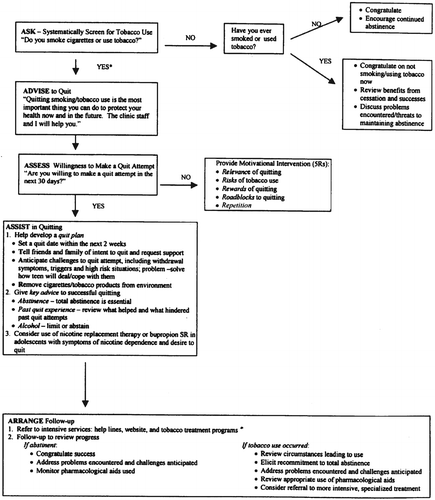
Assessment
The first phase of any clinical smoking intervention is assessment. Assessment involves screening for tobacco use, determining the current level of nicotine dependence, assessing the current motivation to stop smoking, and evaluating patient-specific factors that affect selection of an intervention. Merely having a screening intervention in place is associated with increased odds of smoking cessation (4).
Establishing a diagnosis of nicotine dependence can be done with a clinical interview eliciting DSM-IV-TR dependence criteria. Inquiring about “time to first cigarette” is particularly effective for determining severity of dependence (34): in the morning, when nicotine has been largely eliminated from circulation during sleep, nicotinic cholinergic receptors are in a sensitized state, and highly dependent smokers experience intense craving (35). The Fagerstrom Test for Nicotine Dependence is a brief clinician-administered questionnaire with excellent reliability and validity for measuring nicotine dependence (34). When definitive confirmation is sought, biological indicators such as breath carbon monoxide can detect smoking within the last few hours, and urine cotinine levels can detect smoking within the past 7 days (30, 36).
Assessment of motivation to stop smoking can be done using the “stages of change” model. This model posits that for any volitional lifestyle modification, individuals go through phases of precontemplation, contemplation, preparation, action, and maintenance (37). Movement through these phases need not be linear; however, frequently assessing where on the continuum a patient lies facilitates a determination of how best to encourage and support him or her. For patients who are preparing to stop smoking, a targeted assessment of key areas facilitates individualization of smoking cessation interventions. These areas include experiences with past quit attempts, severity of nicotine dependence, vulnerability to withdrawal symptoms, social resources, cultural orientation, medical comorbidities, weight gain propensity, pregnancy status, potential drug interactions, and stability of any mental illness (4, 30).
Education
Education is a central component of all smoking cessation interventions. Many smokers are misinformed about the health risks of nicotine and the safety and efficacy of smoking cessation interventions. For instance, in a study examining what subjects know about nicotine replacement, it was found that as many as half of smokers believed that nicotine itself was the cause of cancer (38). Such beliefs may undermine adherence with nicotine replacement therapy. Likewise, whereas many smokers view cessation as overcoming the period of acute withdrawal, education about long-term relapse risk may facilitate engagement in an ongoing follow-up plan.
Smokers should understand that withdrawal symptoms peak in the first 1–2 weeks but may persist for months. Even a single puff increases the risk of relapse. In addition to reviewing common symptoms of withdrawal, clinicians should explore patient-specific obstacles to smoking cessation. Weight gain is a frequent concern. Nicotine suppresses appetite and increases metabolism (39) such that on average, smokers gain 3–5 kg within the first 6 months of smoking cessation (4, 40). Both behavioral weight reduction interventions and smoking cessation medications prevent weight gain while being used; however, neither has been shown to generate enduring reductions in weight (41).
Results of studies regarding whether, in the absence of clinician assistance, the provision of self-help information alone reduces smoking have been inconsistent (42). Provision of educational materials is always advisable, but there is a need for the development of more effective self-help materials and strategies.
Counseling
Counseling for smoking cessation should always occur within a supportive framework. The core features of any counseling approach include
| 1. | establishing a therapeutic alliance and treatment frame, | ||||
| 2. | increasing motivation, | ||||
| 3. | overcoming barriers, | ||||
| 4. | eliciting patient preferences about treatment, | ||||
| 5. | determining timing and quit date, | ||||
| 6. | deciding method for smoking cessation, | ||||
| 7. | problem solving, and | ||||
| 8. | monitoring and follow-up (30). | ||||
A number of distinct psychotherapies have been manualized and studied for smoking cessation. These include contingency management, relaxation techniques, aversive therapy, cue exposure, problem solving, skills training, motivation enhancement, supportive psychotherapy, and interventions to increase social support in the smoker's environment (4). Studies of these approaches have rarely compared them with each other, making it difficult to infer superiority of any treatment over another. By and large, the therapies can be grouped into three overlapping classifications. Behavioral approaches focus on changing behaviors through techniques such as conditioning, desensitization, behavior modification, and reinforcement. Cognitive approaches attempt to modify dysfunctional thoughts and beliefs that underlie maladaptive behaviors, as well as using skill acquisition to deal with triggers and cravings. Supportive approaches emphasize patient-centered goal setting, empathy, establishment of intrinsic motivation, and problem solving. In practice, the above-stated counseling approaches may have substantial areas of overlap.
In the meta-analysis conducted for the U.S. Public Health Service (PHS) report, four therapy techniques provided significant increases in abstinence compared with untreated control conditions: practical counseling, intratreatment social support, extratreatment social support, and aversive smoking procedures (29). The latter two treatments were dropped from PHS recommendations after analysis of subsequent Cochrane reviews (4). The APA guideline recommends behavioral therapies as first-line treatment for smoking cessation because of the quantity of studies supporting their effectiveness (30).
Cognitive behavior therapies may be particularly helpful in treating nicotine dependence among patients with co-occurring disorders (30). Counseling can and should be integrated with medication management, because there are synergistic benefits (4, 43). Group therapy doubles quit rates and is substantially more effective than self-help alone, although results of studies have been mixed with respect to how it compares with individual therapy (44).
Telephone counseling, a widely available but underused resource, has also been shown to be effective. The U.S. national quit-line (1-800-QUIT NOW) uses a “proactive” model for intervention, whereby calls to smokers are initiated by cessation counselors based on a prearranged schedule (45). A recent meta-analysis of 22 randomized controlled trials investigating computer-based programs found that such programs increased quit rates, but that effects dissipated 1 year out of treatment (46).
Biological interventions
Three classes of medication are considered first-line for smoking cessation: nicotine replacement therapies (NRTs), of which there are five U.S. Food and Drug Administration (FDA)-approved agents, the atypical antidepressant bupropion, and the partial agonist varenicline. Each of these agents has been shown to be effective in multiple systematic meta-analyses (4, 47–52). Whereas the optimal duration of treatment with smoking cessation pharmacotherapies has not been determined, most medications are used for periods ranging from 6 weeks to 6 months, and studies generally reveal a dose-response relationship between duration of treatment and long-term abstinence (54–56). Table 1 summarizes the PHS meta-analyses on 6-month quit rates with various cessation medications compared with placebo.
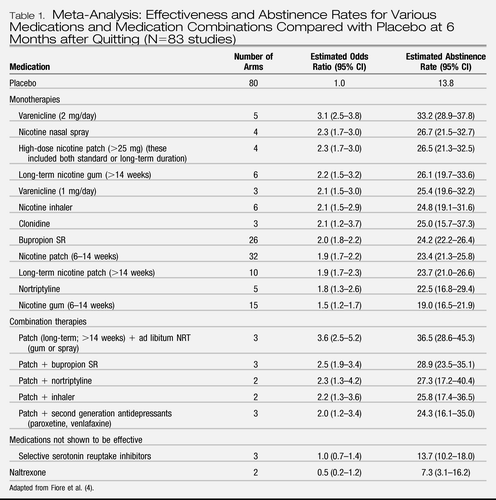 |
Table 1. Meta-Analysis: Effectiveness and Abstinence Rates for Various Medications and Medication Combinations Compared with Placebo at 6 Months after Quitting (N=83 studies)
NRTs function by alleviating nicotine withdrawal symptoms, as well as by interfering with the behavioral ritual of smoking. Short-acting agents, such as gum, inhaler, spray, or lozenge, simulate the periodic burst of nicotine associated with smoking. The nicotine patch produces much more constant nicotine levels, and its ease of administration facilitates high adherence. The combination of the patch with a short-acting agent (to attenuate breakthrough craving) is more effective than the patch alone (56). NRTs should be initiated on the quit date and titrated upward to alleviate subjective craving, as higher doses have been associated with lower relapse (57).
There are few contraindications to use of NRTs. Pregnancy is discussed below. Light smokers (<10 cigarettes/day) and adolescents may benefit from reductions in the dosing of NRTs, although overall, the evidence base for NRTs in this population is less strong, and an individualized risk-benefit analysis must be undertaken (4, 58). Given the mild stimulant characteristics of nicotine, patients with unstable cardiac disease should also be carefully evaluated before initiation (59). Most recently, electronic cigarettes have been developed to better mimic the pharmacokinetics of smoking; however, this method of nicotine administration has not been well studied (60), and the FDA has issued warnings pertaining to safety and quality control (61).
Sustained release bupropion, a dopamine and norepinephrine reuptake inhibitor, has been shown to double the likelihood of smoking cessation (50). Bupropion can be used in combination with an NRT, as the two appear to have additive benefits (62). Bupropion should be started 7 days before the quit date to enable stabilization of serum levels. Given the effectiveness of bupropion for depression, it is a reasonable choice for patients with nicotine dependence in the setting of depression. The dose-dependent effect of bupropion on seizure risk poses a relative contraindication among patients with conditions that lower seizure threshold.
Varenicline, a partial nicotinic cholinergic receptor agonist, is thought to work by blocking the reinforcing effects from smoking, while stimulating sufficient release of dopamine to reduce craving and withdrawal. Varenicline has consistently demonstrated efficacy in clinical trials, both in comparison with placebo and with the first-line agents nicotine patch and bupropion (48). Notably, postmarketing surveillance data has revealed a correlation between varenicline and neuropsychiatric decompensation (63). Although this correlation has not yet been demonstrated in randomized controlled trials, a black box warning has been placed, and it is prudent to ensure that psychiatric disorders are stabilized before initiation of varenicline and to monitor patients closely throughout treatment. As with bupropion, varenicline titration should be started 1 week before the quit date such that serum levels are sufficient before nicotine withdrawal develops. As with all smoking cessation pharmacotherapies, dose modification may be indicated to achieve an appropriate balance between tolerability and reduction in nicotine craving.
The tricyclic antidepressant nortriptyline appears to have efficacy similar to that of NRTs; however, the side effect profile of nortriptyline and its toxicity in overdose relegate it to second-line status (4). Other antidepressants, such as the selective serotonin reuptake inhibitors, have not been found to increase smoking cessation (50). Clonidine, a centrally acting α2 receptor agonist, has shown some efficacy compared with placebo. However, its relatively low efficacy and frequent side effects (dry mouth and sedation) limit its role in the treatment of smoking, primarily for individuals in whom multiple other pharmacotherapies have failed (49).
Complementary interventions
A number of complementary alternative therapies have been used for smoking cessation, including acupuncture, acupressure, relaxation, hypnotherapy, yoga therapy, exercise therapy, laser therapy, and electrostimulation. There is a dearth of methodologically robust research on these interventions. Acupuncture is probably the best studied, and thus far meta-analyses have not revealed persisting benefits compared with placebo (sham acupuncture) (64, 65). However, neither have existing studies shown any harm. Patients should be informed about the state of the evidence, but not discouraged from pursuing alternative therapies for smoking cessation (66). Those who perceive or experience benefits from alternative therapies should receive encouragement and support (4).
SPECIAL POPULATIONS
Mental illness
Smokers with mental illness want to quit at the same rate as that of smokers in the general population (67), and although cessation rates are in fact lower than those for the general population, smokers with mental illness can successfully quit (68). Given the potential impact of smoking cessation interventions, the observation that psychiatrists often neglect to discuss smoking with their patients is alarming (69). The smoking cessation interventions discussed in this review represent a standard of care that should be adopted by all physicians (31). Effective smoking interventions among smokers with mental illness must account for common obstacles, including impairments in cognitive function, a higher prevalence of smoking among peers and supported housing environments, and reliance on nicotine to self-medicate psychiatric symptoms (70). Smoking cessation has been associated with exacerbations in underlying psychiatric disorders (71). However, this association is strongest among patients with unstable psychiatric disorders, and how this acute risk should be balanced against the chronic risk of untreated smoking has not been fully resolved because patients selected for treatment studies tend to be psychiatrically stable patients, among whom the incidence of psychiatric decompensation is low (30).
Psychiatric status should be monitored closely throughout quit attempts. Particular attention should be paid to pharmacokinetic interactions with other medications, as well as the potential for either nicotine withdrawal or smoking cessation agents to exacerbate psychiatric symptomatology. Both alone and in combination with medications, counseling is an effective smoking cessation intervention (72). Modified cognitive behavior therapy protocols addressing specific needs among patients with mood disorders and psychotic disorders may be particularly advantageous (73).
Inpatients
The recognition that there is no safe level of exposure to environmental tobacco smoke (27) has prompted the adoption of smoke-free policies across many medical campuses (74, 75). Hospital campus smoking bans protect nonsmoking patients and personnel, prevent environmental degradation related to environmental tobacco residue, and provide vital opportunities for enduring smoking cessation intervention. Clinical benefits of inpatient smoking cessation interventions may be sustained provided that follow-up continues at least 1 month postdischarge (76).
Eliminating smoking from inpatient psychiatry units has at times met resistance because of clinical concerns and civil rights assertions (77); however, a wide range of stakeholders, including the National Alliance on Mental Illness, now support and encourage smoke-free environments in treatment and communal settings (75). Staff surveys from inpatient psychiatric units suggest that smoking bans tend to be preceded by skepticism but followed by support (70). Extensive preparation is warranted, although even after abrupt bans, significant correlation between nicotine withdrawal and psychiatric symptoms is seldom detected (78). As in nonpsychiatric inpatient settings, successful transitions are facilitated by consistent screening of all patients for nicotine dependence, availability of efficient protocols for implementing pharmacotherapy to attenuate withdrawal, staff training related to smoking and mental illness, consistent enforcement of smoke free policies, clear leadership, and cohesive team work (79). The National Association of State Mental Health Program Directors has published a best practice tool kit proving guidance and resources for implementation of systems-based smoking cessation initiatives (80).
Pregnancy
Maternal smoking during pregnancy causes dose-dependent effects on fetal growth, birth defects, premature labor, and pregnancy complications (81, 82). Smoking during pregnancy is the single biggest factor affecting birth weight in developed countries (83) and has been linked with long-term adverse cognitive and behavioral outcomes (84). Both nicotine and tobacco smoke have a pathogenic role. All women should be screened for tobacco use, and pregnant women should also be screened for exposure to environmental tobacco smoke.
Pregnant smokers should receive counseling and close monitoring, with reassessment of tobacco use at every clinical visit. Although none of the FDA-approved smoking medications are safe in pregnancy or breast feeding, the relative risk of smoking is sufficiently great that if counseling is inadequate, pharmacotherapy may be considered. Among the FDA-approved pharmacotherapies for smoking cessation, varenicline and bupropion are pregnancy class C drugs, with no studies demonstrating safety in pregnant women. NRTs are class D, as nicotine has been shown to cause toxicity (85). Use of as-needed dosing of immediate release NRTs may result in decreased total nicotine exposure compared with that with the patch (30).
Adolescents
Nicotine dependence is a disease with childhood onset. Nine of 10 adult smokers had their first cigarette before age 18, and most were daily smokers by that time. In contrast with adults, underaged smokers tend to smoke less heavily, and psychosocial factors such as image and peer group tend to have greater influence over smoking patterns. Like adults, most adolescent smokers report that they want to stop smoking but are unable to do so (86).
Given the comparative lack of randomized controlled trials, guidelines for smoking cessation among youth tend to be driven by expert opinion rather than empirical data. Pediatricians are encouraged to use brief, office-based counseling interventions based on the 5A's PHS protocol, to prescribe smoking cessation medications if counseling is inadequate, and to offer ongoing follow-up (87). Community-wide prevention efforts are particularly effective among youth, including increasing price of cigarettes, enforcing minors' access laws, school-based prevention programs, and mass media campaigns (86).
FUTURE DIRECTIONS
Obtaining a substantial reduction in smoking-related morbidity and mortality will require continued efforts in the realms of primary prevention, legislative regulation, pharmaceutical development, strategic health system initiatives, and patient care. New policy and health initiatives are necessary to increase availability of effective smoking cessation interventions and to achieve greater penetration in segments of the population in which smoking remains highly prevalent. The growth of smoke-free medical campuses, the adoption of systematic tobacco screening, and the availability of evidence-based treatment protocols promises to provide a strong foundation for effective clinical intervention.
A series of investigations has already begun to identify genetic markers as predictors of medication response (23). The nicotine vaccine promises to immunize recipients against the reinforcing effects of nicotine by impairing its diffusion across the blood-brain barrier (88). Genetic polymorphisms also point the way toward novel targets for pharmaceutical development (22). In addition, patients with mental illness are often excluded from treatment studies, and more research is needed to improve clinical interventions within this population (73).
Recognition of the persisting toxic effects of environmental tobacco smoke has made protecting nonsmokers a new public health frontier (27). The concept of third-hand smoke (89) has been developed to educate the public about the fact that toxic residues remain in the environment long after dissipation of first- and second-hand tobacco smoke (90). We are likely to see increasing regulation of environmental tobacco smoke in public spaces (91).
CONCLUSION
In the United States and around the world, nicotine dependence continues to be a leading cause of preventable mortality. Most smokers want to quit. For those not ready to make a quit attempt, counseling strategies can increase intrinsic motivation. A number of smoking cessation interventions have been shown to be clinically effective, cost-effective, and feasible in the busy office practice. Combining treatment interventions improves outcomes, as does ongoing monitoring and follow-up. Relapse is common, but with multiple quit attempts and clinical assistance, smokers can successfully quit.
1 Ezzati M, Lopez AD: Estimates of global mortality attributable to smoking in 2000. Lancet 2003; 362:847–852Crossref, Google Scholar
2 World Health Organization Report on the Global Tobacco Epidemic: Implementing Smoke-Free Environments. Geneva, World Health Organization, 2009Google Scholar
3 Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1227–1232Google Scholar
4 Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME: Treating Tobacco Use and Dependence: 2008. Update. Clinical Practice Guideline. Rockville, MD, U.S. Department of Health and Human Services, Public Health Service, 2008Google Scholar
5 Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004. MMWR Morb Mortal Wkly Rep, 2008; 57:1226–1228Google Scholar
6 Economic facts about U.S. tobacco production and use. Centers for Disease Control and Prevention. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/economics/econ_facts/index.htm#costsGoogle Scholar
7 Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA: Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 2004; 61:1107–1115Crossref, Google Scholar
8 Mahmud A, Feely J: Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension 2003; 41:183–187Crossref, Google Scholar
9 The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Bethesda, MD, U.S. Public Health Service, Office on Smoking and Health, 1990Google Scholar
10 Cohen S, Lichtenstein E, Prochaska JO, Rossi JS, Gritz ER, Carr CR, Orleans CT, Schoenbach VJ, Biener L, Abrams D: Debunking myths about self-quitting. Evidence from 10 prospective studies of persons who attempt to quit smoking by themselves. Am Psychol 1989; 44:1355–1365Crossref, Google Scholar
11 Hughes JR: Motivating and helping smokers to stop smoking. J Gen Intern Med 2003; 18:1053–1057Crossref, Google Scholar
12 Hall SM, Humfleet GL, Reus VI, Muñoz RF, Cullen J: Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry 2004; 161:2100–2107Crossref, Google Scholar
13 Fowles J, Dybing E: Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control 2003; 12:424–430Crossref, Google Scholar
14 National Toxicology Program: Report on Carcinogens, 11th ed. Bethesda, MD, U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program, 2005Google Scholar
15 Balfour DJ, Fagerström KO: Pharmacology of nicotine and its therapeutic use in smoking cessation and neurodegenerative disorders. Pharmacol Ther 1996; 72:51–81Crossref, Google Scholar
16 Benowitz NL: Nicotine addiction. N Engl J Med 2010; 362:2295–3303Crossref, Google Scholar
17 Benowitz NL: Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther 2008; 83:531–541Crossref, Google Scholar
18 Laviolette SR, van der Kooy D: The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 2004; 5:55–65Crossref, Google Scholar
19 Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ: Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther 1999; 289:1545–1552Google Scholar
20 Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG: Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 2006; 63:907–915Crossref, Google Scholar
21 Le Foll B, Goldberg SR: Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol 2009; 192:335–367Crossref, Google Scholar
22 Lessov-Schlaggar CN, Pergadia ML, Khroyan TV, Swan GE: Genetics of nicotine dependence and pharmacotherapy. Biochem Pharmacol 2008; 75:178–195Crossref, Google Scholar
23 Berrettini WH, Lerman CE: Pharmacotherapy and pharmacogenetics of nicotine dependence. Am J Psychiatry 2005; 162:1441–1451Crossref, Google Scholar
24 Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, Shields PG, Kaufmann V, Redden D, Benowitz N, Berrettini WH: The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J 2005; 4:184–192Crossref, Google Scholar
25 Morales-Suárez-Varela MM, Bille C, Christensen K, Olsen J: Smoking habits, nicotine use, and congenital malformations. Obstet Gynecol 2006; 107:51–57Crossref, Google Scholar
26 Reducing Tobacco Use: A Report of the Surgeon General—Executive Summary. Bethesda, MD, U.S. Public Health Service, Office on Smoking and Health, 2000Google Scholar
27 The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General—Executive Summary. Bethesda, MD, U.S. Department of Health and Human Services, Office on Smoking and Health, 2006Google Scholar
28 Best Practices for Comprehensive Tobacco Control Programs. Bethesda, MD, U.S. Department of Health and Human Services, Office on Smoking and Health, 2007Google Scholar
29 Fiore MC, Bailey WC, Cohen SJ, et al.: Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD, U.S. Department of Health and Human Services, 2000Google Scholar
30 Kleber HD, et al.: Treatment of patients with substance use disorders, 2nd ed. American Psychiatric Association. Am J Psychiatry 2007; 164(4 suppl):5–123Google Scholar
31 Hopkins DP, Husten CG, Fielding JE, Rosenquist JN, Westphal LL: Evidence reviews and recommendations on interventions to reduce tobacco use and exposure to environmental tobacco smoke: a summary of selected guidelines. Am J Prev Med 2001; 20(2 suppl):67–87Crossref, Google Scholar
32 Torrijos RM, Glantz SA: The US Public Health Service “treating tobacco use and dependence clinical practice guidelines” as a legal standard of care. Tob Control 2006; 15:447–451Crossref, Google Scholar
33 Schroeder SA: What to do with a patient who smokes. JAMA 2005; 294:482–487Crossref, Google Scholar
34 Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO: The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86:1119–1127Crossref, Google Scholar
35 Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, Colby S, Conti D, Giovino GA, Hatsukami D, Hyland A, Krishnan-Sarin S, Niaura R, Perkins KA, Toll BA: Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res 2007; 9(suppl 4):S555–S570Crossref, Google Scholar
36 Benowitz NL: The use of biologic fluid samples in assessing tobacco smoke consumption. NIDA Res Monogr 1983; 48:6–26Google Scholar
37 Prochaska JO, DiClemente CC, Norcross JC: In search of how people change. Applications to addictive behaviors. Am Psychol 1992; 47:1102–1114Crossref, Google Scholar
38 Bansal MA, Cummings KM, Hyland A, Giovino GA: Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res 2004; 6(suppl 3):S303–S310Google Scholar
39 Filozof C, Fernández Pinilla MC, Fernández-Cruz A: Smoking cessation and weight gain. Obes Rev 2004; 5:95–103Crossref, Google Scholar
40 O'Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R: Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol 1998; 148:821–830Crossref, Google Scholar
41 Spring B, Howe D, Berendsen M, McFadden HG, Hitchcock K, Rademaker AW, Hitsman B: Behavioral intervention to promote smoking cessation and prevent weight gain: a systematic review and meta-analysis. Addiction 2009; 104:1472–1486Crossref, Google Scholar
42 Lancaster T, Stead LF: Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2005; 3:CD001118Google Scholar
43 Reus VI, Smith BJ: Multimodal techniques for smoking cessation: a review of their efficacy and utilisation and clinical practice guidelines. Int J Clin Pract 2008; 62:1753–1768Crossref, Google Scholar
44 Stead LF, Lancaster T: Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2005; 2:CD001007Google Scholar
45 Stead LF, Perera R, Lancaster T: Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2006; 3:CD002850Google Scholar
46 Myung SK, McDonnell DD, Kazinets G, Seo HG, Moskowitz JM: Effects of Web- and computer-based smoking cessation programs: meta-analysis of randomized controlled trials. Arch Intern Med 2009; 169:929–937Crossref, Google Scholar
47 Eisenberg MJ, Filion KB, Yavin D, Bélisle P, Mottillo S, Joseph L, Gervais A, O'Loughlin J, Paradis G, Rinfret S, Pilote L: Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ 2008; 179:135–144Crossref, Google Scholar
48 Cahill K, Stead LF, Lancaster T: Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2008; 3:CD006103Google Scholar
49 Gourlay SG, Stead LF, Benowitz NL: Clonidine for smoking cessation. Cochrane Database Syst Rev 2004; 3:CD000058Google Scholar
50 Hughes J, Stead L, Lancaster T: Antidepressants for smoking cessation. Cochrane Database Syst Rev 2004; 4:CD000031Google Scholar
51 Silagy C, Lancaster T, Stead L, Mant D, Fowler G: Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2004; 4:CD000146Google Scholar
52 Stead LF, Perera R, Bullen C, Mant D, Lancaster T: Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2008; 1:CD000146Google Scholar
53 Doggrell SA: Which is the best primary medication for long-term smoking cessation–nicotine replacement therapy, bupropion or varenicline? Expert Opin Pharmacother 2007; 8:2903–2915Crossref, Google Scholar
54 Etter JF, Stapleton JA: Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 2006; 15:280–285Crossref, Google Scholar
55 Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, Lerman CA: Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med 2010; 152:144–151Crossref, Google Scholar
56 Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB: A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry 2009; 66:1253–1262Crossref, Google Scholar
57 Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, Rijcken B, Sawe U: Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation. European Respiratory Society. Eur Respir J 1999; 13:238–246Crossref, Google Scholar
58 Grimshaw GM, Stanton A: Tobacco cessation interventions for young people. Cochrane Database Syst Rev 2006; 4:CD003289Google Scholar
59 Ford CL, Zlabek JA: Nicotine replacement therapy and cardiovascular disease. Mayo Clin Proc 2005; 80:652–656Crossref, Google Scholar
60 Etter JF: Electronic cigarettes: a survey of users. BMC Public Health 2010; 10:231Crossref, Google Scholar
61 U.S. Food and Drug Administration: FDA acts against 5 electronic cigarette distributors, 2010. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm225224.htmGoogle Scholar
62 Ebbert JO, Hays JT, Hurt RD: Combination pharmacotherapy for stopping smoking: what advantages does it offer? Drugs 2010; 70:643–650Crossref, Google Scholar
63 McIntyre RS: Varenicline and suicidality: a new era in medication safety surveillance. Expert Opin Drug Saf 2008; 7:511–514Crossref, Google Scholar
64 Abbot NC, Stead LF, White AR, Barnes J, Ernst E: Hypnotherapy for smoking cessation. Cochrane Database Syst Rev 2000; 2:CD001008Google Scholar
65 White AR, Rampes H, Campbell JL: Acupuncture and related interventions for smoking cessation. Cochrane Database Syst Rev 2006; 1:CD000009Google Scholar
66 Villano LM, White AR: Alternative therapies for tobacco dependence. Med Clin North Am 2004; 88:1607–1621Crossref, Google Scholar
67 Prochaska JJ, Rossi JS, Redding CA, Rosen AB, Tsoh JY, Humfleet GL, Eisendrath SJ, Meisner MR, Hall SM: Depressed smokers and stage of change: implications for treatment interventions. Drug Alcohol Depend 2004; 76:143–151Crossref, Google Scholar
68 Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH: Smoking and mental illness: a population-based prevalence study. JAMA 2000; 284:2606–2610Crossref, Google Scholar
69 Thorndike AN, Stafford RS, Rigotti NA: US physicians' treatment of smoking in outpatients with psychiatric diagnoses. Nicotine Tob Res 2001; 3:85–91Crossref, Google Scholar
70 Moss TG, Weinberger AH, Vessicchio JC, Mancuso V, Cushing SJ, Pett M, Kitchen K, Selby P, George TP: A tobacco reconceptualization in psychiatry: toward the development of tobacco-free psychiatric facilities. Am J Addict 2010; 19:293–311Google Scholar
71 Glassman AH: Cigarette smoking: implications for psychiatric illness. Am J Psychiatry 1993; 150:546–553Crossref, Google Scholar
72 Tsoi DT, Porwal M, Webster AC: Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev 2010; 6:CD007253Crossref, Google Scholar
73 Hitsman B, Moss TG, Montoya ID, George TP: Treatment of tobacco dependence in mental health and addictive disorders. Can J Psychiatry 2009; 54:368–378Crossref, Google Scholar
74 Williams SC, Hafner JM, Morton DJ, Holm AL, Milberger SM, Koss RG, Loeb JM: The adoption of smoke-free hospital campuses in the United States. Tob Control 2009; 18:451–458Crossref, Google Scholar
75 National Alliance on Mental Illness: Public Policy: Quality Monitoring, Accountability, and Accreditation, 2010. http://www.nami.org/Template.cfm?Section=NAMI_Policy_Platform&Template=/ContentManagement/ContentDisplay.cfm&ContentID=38252Google Scholar
76 Rigotti NA, Munafo MR, Stead LF: Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med 2008; 168:1950–1960Crossref, Google Scholar
77 Pandya A: President, National Alliance on Mental Illness, 2010Google Scholar
78 Smith CM, Pristach CA, Cartagena M: Obligatory cessation of smoking by psychiatric inpatients. Psychiatr Serv 1999; 50:91–94Crossref, Google Scholar
79 Lawn S, Campion J: Factors associated with success of smoke-free initiatives in Australian psychiatric inpatient units. Psychiatr Serv 2010; 61:300–305Crossref, Google Scholar
80 Tobacco-Free Living in Psychiatric Settings: A Best-Practices Toolkit Promoting Wellness and Recovery. Alexandria, VA, National Association of State Mental Health Program Directors, 2007Google Scholar
81 DiFranza JR, Aligne CA, Weitzman M: Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics 2004; 113:1007–1015Google Scholar
82 MacArthur C, Knox EG: Smoking in pregnancy: effects of stopping at different stages. Br J Obstet Gynaecol 1988; 95:551–555Crossref, Google Scholar
83 Kramer MS: Intrauterine growth and gestational duration determinants. Pediatrics 1987; 80:502–511Google Scholar
84 Ernst M, Moolchan ET, Robinson ML: Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry 2001; 40:630–641Crossref, Google Scholar
85 Ginzel KH, Maritz GS, Marks DF, Neuberger M, Pauly JR, Polito JR, Schulte-Hermann R, Slotkin TA: Critical review: nicotine for the fetus, the infant and the adolescent? J Health Psychol 2007; 12:215–224Crossref, Google Scholar
86 Preventing tobacco use among young people. A report of the Surgeon General. Executive summary. MMWR Recomm Rep 1994; 43(RR-4):1–10Google Scholar
87 Pbert L, Moolchan ET, Muramoto M, Winickoff JP, Curry S, Lando H, Ossip-Klein D, Prokhorov AV, DiFranza J, Klein JD: The state of office-based interventions for youth tobacco use. Pediatrics 2003; 111:e650–e660Crossref, Google Scholar
88 Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, Horwith G, Pentel PR: Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther 2005; 78:456–467Crossref, Google Scholar
89 Winickoff JP, Friebely J, Tanski SE, Sherrod C, Matt GE, Hovell MF, McMillen RC: Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics 2009; 123:e74–e79Crossref, Google Scholar
90 Matt GE, Quintana PJ, Hovell MF, Bernert JT, Song S, Novianti N, Juarez T, Floro J, Gehrman C, Garcia M, Larson S: Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control 2004; 13:29–37Crossref, Google Scholar
91 Winickoff JP, Gottlieb M, Mello MM: Regulation of smoking in public housing. N Engl J Med 2010; 362:2319–2325Crossref, Google Scholar



