Epigenetic Regulation in Psychiatric Disorders
Abstract
Many neurological and most psychiatric disorders are not due to mutations in a single gene; rather, they involve molecular disturbances entailing multiple genes and signals that control their expression. Recent research has demonstrated that complex “epigenetic” mechanisms, which regulate gene activity without altering the DNA code, have long-lasting effects within mature neurons. This review summarizes recent evidence for the existence of sustained epigenetic mechanisms of gene regulation in neurons that have been implicated in the regulation of complex behaviour, including abnormalities in several psychiatric disorders such as depression, drug addiction and schizophrenia.
(Reprinted with permission from Nature Reviews Neuroscience 2007; 8(May):355)
Pathophysiological changes in the brain that are associated with psychiatric disorders or animal models of these conditions include gross differences in the sizes of specific brain regions, alterations in the morphology of subpopulations of neurons, neurochemical changes at the synaptic cleft, alterations in intracellular signalling and changes in the regulation of gene expression. Most psychiatric disorders share important features, including a substantial genetic predisposition (1)) and a contribution from environmental factors. Another common attribute of psychiatric conditions is long-lasting behavioural abnormalities. In most individuals, these illnesses develop gradually and show a chronic, remitting course, often over a lifetime. Likewise, the reversal of symptoms in response to treatment occurs over weeks or months. Psychiatric medications are virtually unique in their requirement for chronic administration to achieve their full clinical effect. Accordingly, an important challenge in psychiatric research has been to identify the molecular basis of stable changes in behaviour that account for both the symptoms of mental illness and their reversal during treatment.
The regulation of gene expression has been proposed as one molecular mechanism that could mediate stable adaptations and maladaptations in the brain(2). Changes in mRNA levels have been documented in specific brain regions both in animal models of psychiatric illness and in human brains, and have been related to altered behaviour. However, it has been difficult to identify the molecular mechanisms that underlie such stable changes in gene expression; virtually all reported changes in transcription factors and other nuclear regulatory proteins in animal models revert to normal within hours or days of chronic perturbation. One exception is the induction of ΔFOSB, a FOS family transcription factor, which accumulates in specific regions of the brain in response to many chronic stimuli (drugs of abuse, stress, antipsychotic drugs and so on), and persists for several weeks after the end of the stimulus (3, 4). But even the induction of ΔFOSB does not persist as long as the behavioural changes. Thus, the search continues for the molecular basis of particularly stable adaptations and maladaptations in the brain.
Recent research has raised the notion that epigenetic mechanisms, which exert lasting control over gene expression without altering the genetic code, could mediate stable changes in brain function. Historically, the field of epigenetics has focused on how cellular traits can be inherited without a change in DNA sequence. Studies of epigenetic mechanisms that underlie heritable transmission have flourished in the fields of developmental and cancer biology, where the continuity of unique patterns of gene expression between parent and daughter cells is crucial. These studies have converged on a set of common enzymatic modifications to chromatin structure that can up- or downregulate gene expression in a manner that is transmissible to daughter cells. These mechanisms also regulate gene expression in neurons, but, as most neurons do not divide, chromatin modifications are instead sustained within individual cells.
This review outlines recent discoveries involving the epigenetic regulation of neurobiological adaptations that are associated with long-lasting behaviours in animal models of psychiatric conditions and in the brains of humans with these disorders. After a brief overview of epigenetic mechanisms, we focus on a small number of conditions, including depression, addiction, schizophrenia and developmental disorders, for which the supporting evidence is best established.
Overview of epigenetic mechanisms
Chromatin is the complex of DNA, histones and nonhistone proteins in the cell nucleus. Remodelling of chromatin is a dynamic process that modulates gene expression. The fundamental unit of chromatin is the nucleosome, which consists of ≈147 base pairs of DNA wrapped around a core histone octamer (≈1.65 turns). Each octamer contains two copies each of the histones H2A, H2B, H3 and H4 (Fig. 1a). The nucleosomal structure of chromatin allows DNA to be tightly packaged into the nucleus by organized folding (5). Intricate chromatin remodelling mechanisms ensure that DNA remains accessible to the transcriptional machinery. These epigenetic mechanisms alter gene activity by modulating DNA-protein interactions without changing the genetic code.
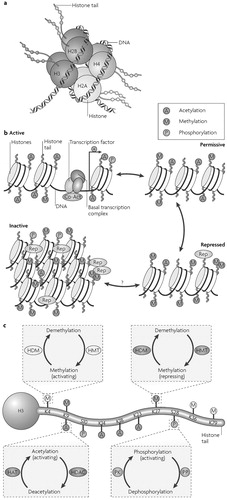
a Picture of a nucleosome showing a DNA strand wrapped around a histone octamer composed of two copies each of the histones H2A, H2B, H3 and H4. The amino (N) termini of the histones face outward from the nucleosome complex. b Chromatin can be conceptualized as existing in two primary structural states: as active, or open, euchromatin (top left) in which histone acetylation (A) is associated with opening the nucleosome to allow binding of the basal transcriptional complex and other activators of transcription; or as inactive, or condensed, heterochromatin where all gene activity is permanently silenced (bottom left). In reality, chromatin exists in a continuum of several functional states (active; permissive (top right); repressed (bottom right); and inactive). Enrichment of histone modifications such as acetylation and methylation (M) at histone N-terminal tails and related binding of transcription factors and co-activators (Co-Act) or repressors (Rep) to chromatin modulates the transcriptional state of the nucleosome. Recent evidence suggests that inactivated chromatin may in some cases be subject to reactivation in adult nerve cells, although this remains uncertain. c Summary of common covalent modifications of H3, which include acetylation, methylation and phosphorylation (P) at several amino acid residues. H3 phosphoacetylation commonly involves phosphorylation of S10 and acetylation of K14. Acetylation is catalysed by histone acetyltransferases (HATs) and reversed by histone deacetylases (HDACs); lysine methylation (which can be either activating or repressing) is catalysed by histone methyltransferases (HMTs) and reversed by histone demethylases (HDMs); and phosphorylation is catalysed by protein kinases (PK) and reversed by protein phosphatases (PP), which have not yet been identified with certainty. K, lysine residue; S, serine residue. Panels a,c modified, with permission, from Nature Rev. Neurosci. REF. (62) © (2005) Macmillan Publishers Ltd.
In simplified terms, chromatin exists in an inactivated, condensed state, heterochromatin, which does not allow transcription of genes, and in an activated, open state, euchromatin, which allows individual genes to be transcribed (Fig. 1b). The opening of chromatin is associated with acetylation of nearby histones, although it remains unclear whether acetylation mediates or reflects chromatin decondensation. In reality, chromatin can exist in many states in between these two extremes (Fig. 1b). Portions of chromatin are highly repressed, owing to DNA and histone methylation and the binding of repressor proteins, and might never be accessible for transcription. Other portions of chromatin are in repressed or permissive states; their basal activity is low owing to histone methylation and perhaps other modifications, but the genes are available for derepression and activation in response to transcription factors and transcriptional co-activators. Chromatin remodelling modulates gene expression with high temporal and spatial resolution by permitting small groups of nucleosomes to become more or less open, which consequently enhances or inhibits access of the transcriptional machinery to specific promoter regions.
Experiments in yeast have yielded detailed information about the molecular mechanisms that control chromatin architecture to alter gene expression. Several general mechanisms have emerged and it is generally believed that their complex interactions determine the appropriate expression of specific genes in eukaryotic cells (5, 6).
By far the best characterized chromatin remodelling mechanism in the brain is the post-translational, covalent modification of histones at distinct amino acid residues on their amino (N)-terminal tails. Such modifications include acetylation, ubiquitylation or SUMOylation at lysine (K) residues, methylation at lysine or arginine (R) residues, phosphorylation at serine (S) or threonine (T) residues, and ADP-ribosylation at glutamate (E) residues (Fig. 1c). Hyperacetylation is generally thought to promote decondensation of chromatin and an increase in gene activity, whereas hypoacetylation marks condensation and decreased activity. It has also been proposed that increased gene activity is best associated not with the level of acetylation, but with the dynamic cycling of acetylation and deacetylation. In contrast to acetylation, histone methylation can correlate with either gene activation or repression, depending on the residue undergoing methylation (7). Phosphorylation of histones is also associated with chromatin inhibition or activation (6). The roles of histone ubiquitylation, SUMOylation and ADP ribosylation are less well understood (8, 9). The diversity of histone modifications supports the “histone code hypothesis”, which posits that the sum of modifications at a particular promoter region defines a specific epigenetic state of gene activation or silencing (10).
The enzymes that mediate these covalent modifications are becoming increasingly understood. Many histone acetyltransferases (HATs), which catalyse acetylation, have been identified. Several transcriptional activators contain intrinsic HAT activity (10, 11). Histone deacetylases (HDACs) catalyse deacetylation; they also associate with several transcriptional repressors to further repress chromatin activity (11) (Box 1). The balance between the opposing activities of HATs and HDACs maintains acetylation on core histones and is thought to be an important determinant of transcription. Methylation at lysine or arginine residues is mediated by histone methyltransferases (HMTs). In general, histone lysine methylation is regarded as a more stable modification than other histone modifications, which seem to be more readily reversible (6, 7), although the recent discovery of histone demethylases (HDMs) indicates that even methylation can be reversed (12).
Fundamental neurodevelopmental processes, such as cell fate specification and neurogenesis, are highly regulated at the level of chromatin remodelling. One of the best-established examples concerns the transcription factor neuron-restrictive silencing factor (NRSF; also known as repressor element 1 silencing transcription factor (REST)). NRSF represses neuronal differentiation by binding to conserved NRS elements (NRSEs) in gene promoters in non-neuronal cells, where it associates with one of several large repressor complexes, including the transcriptional co-repressor mSIN3a/b, nuclear receptor co-repressor 1 (N-CoR1), and coREST/histone deacetylase 2 (HDAC2) (93,94). In this way, NRSF keeps neural-specific genes turned off in non-neuronal cells. More recently, NRSF has been shown to modulate the expression of NRSE-containing genes in mature neurons: inhibition of NRSF leads to neuronal activation and the promotion of neurogenesis (95).
Histone acetylation, SWI/SNF-mediated remodelling and DNA methylation have also been implicated in brain development. HDAC inhibitors induce neural differentiation in embryonic cortical cells. Additionally, histone deacetylation is crucial for the timing of oligodendrocyte differentiation and myelination in the developing corpus callosum: administration of valproate (a non-selective HDAC inhibitor; see Supplementary information S1 (table)) leads to hypomyelination, delayed expression of differentiation markers and prolonged expression of progenitor markers in these cells (96). Abnormal DNA methylation as a result of deficiency in the DNA methyltransferase DNMT1, or the methyl-CpG binding protein MBD1, causes abnormal neuronal function and postnatal death (97) or decreased neurogenesis (98), respectively. Chromatin remodelling may also be involved in the regulation of adult neurogenesis, which occurs in highly restricted brain regions: the subgranular zone of the hippocampus dentate gyrus and the subventricular zone adjacent to the striatum. HDAC inhibitors induce neuronal differentiation, suppress glial differentiation and decrease proliferation of adult hippocampal neural progenitor cells (93).
Several other general mechanisms of chromatin remodelling have been described, although they remain less well characterized in the nervous system. Nucleosome sliding involves the movement of the histone octamer along DNA and thereby allows the transcriptional machinery to transcribe a gene (5). This process is facilitated by the SWI/SNF family of chromatin remodelling complexes, which use ATP-derived energy to disrupt nucleosome structure non-covalently (11). Histone substitution, where canonical histones within a nucleosome are switched with naturally occurring histone variants, is a further example of chromatin remodelling, although its physiological function in the brain is poorly understood (6).
DNA methylation is another important mechanism of gene repression. It occurs by transfer of a methyl group from S-adenosyl methionine (SAM) to cytosine residues at the dinucleotide sequence CpG, and is catalysed by DNA methyltransferases (DNMTs) (6, 7). Although CpG sequences throughout the genome are usually heavily methylated, those at the promoter regions of genes, specifically at CpG clusters or islands, are methylated to a much lesser extent, and the amount of DNA methylation at a promoter correlates with the extent of gene inactivation. The functional significance of DNA methylation is best established in X chromosome inactivation and genetic imprinting; abnormal imprinting can lead to neurodevelopmental diseases (Box 1; Table 1). More recently, DNA methylation has been implicated in the regulation of gene activity in the adult brain under normal and pathological conditions (see below).
Patterns of DNA methylation are intricately linked to patterns of histone modification. Methyl binding domain proteins (MBDs), such as methyl-CpG-binding protein 2 (MeCP2), can be recruited to methylated DNA. MBDs are associated with large protein complexes containing HDACs and HMTs, which further repress gene transcription (7). Thus, DNA methylation and histone methylation and deacetylation are intricately interconnected, each representing epigenetic hallmarks of the silenced promoter.
Signalling pathways in chromatin remodelling
Although chromatin remodelling is best understood for its influence on neural development (Box 1), increasing evidence suggests a role in regulating mature, fully differentiated neurons. During synaptic transmission, neurons respond to neurotransmitters by receptormediated intracellular signal transduction events, which, among other actions, activate or inhibit transcription factors. The regulation of transcriptional activity by transcription factor binding to DNA depends on interactions of the transcription factors with many co-activators or co-repressors and the underlying structure of chromatin. Chromatin remodelling is thus intimately linked to activation or repression of genes by synaptic activity. Such mechanisms regulate the expression of specific sets of neuronal genes that are important for neural activity, survival, morphology and ultimately the integrated regulation of complex behaviour.
We are beginning to understand how intracellular signalling in the brain regulates chromatin remodelling. The best-established mechanism involves the transcription factor cyclic AMP (cAMP)-response element binding protein (CREB). The activation of several signalling pathways involving cAMP, Ca2+ and extracellular signal regulated kinase (ERK) leads to the phosphorylation of CREB at Ser133 (Fig. 2). CREB phosphorylation triggers the recruitment of CREB-binding protein (CBP), a transcriptional co-activator whose intrinsic HAT activity acetylates nearby histones, which loosens the chromatin and allows subsequent transcriptional activation. CBP is important for normal learning and memory, and mutations of CBP cause Rubinstein-Taybi syndrome, a form of mental retardation in humans (see below).
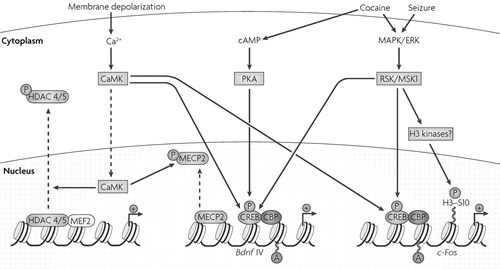
Multiple kinases such as protein kinase A (PKA), calcium/calmodulin-activated protein kinases (CaMKs), ribosomal S6 kinase (RSK) and mitogenand stress-activated protein kinase 1 (MSK1), which are activated by intracellular signalling cascades involving cyclic AMP (cAMP), calcium- and mitogen-activated protein/extracellular signal-regulated protein kinase (MAPK/ERK), respectively, can phosphorylate the transcription factor cAMP-response element binding protein (CREB). This causes it to associate with the histone acetyltransferase CREB-binding protein (CBP). CBP, in turn, acetylates nearby histones and thereby promotes transcriptional activation. This mechanism of regulation has been described for, among other genes, c-Fos and brain-derived neurotrophic factor (Bdnf). Membrane depolarization and calcium influx activate CaMKs. CaMKII-induced phosphorylation of the repressor methyl-CpG-binding protein 2 (MeCP2) is proposed to be important for the release of this repressor from the Bdnf promoter P4 and the subsequent transcriptional activation of the Bdnf gene. CaMKs also phosphorylate class II histone deacetylases (HDACs) 4 and 5 in neurons, which results in their translocation out of the nucleus. At certain promoters, the phosphorylation of HDACs also decreases their affinity to the activator myocyte enhancing factor 2 (MEF2) and promotes further transcriptional activation. Acute cocaine administration, presumably acting through dopamine receptors, activates MSK1 in striatal neurons. This results in the downstream phosphorylation of histone H3 at the c-Fos promoter with concomitant induction of c-Fos transcription. The effect of MSK1 may be indirect.
Several external stimuli can induce rapid changes in histone modifications in the brain, but the intracellular mediators of these signals are poorly defined. For example, cocaine and antipsychotic drugs induce acetylation of histone H4 and phosphoacetylation of histone H3 in the striatum (a brain region that is important for the behavioural effects of these drugs; see below) (13–15). Among the genes that show the most marked histone changes, which can be identified by use of chromatin immunoprecipitation (ChIP) assays, are immediate-early genes, such as c-Fos. c-Fos transcription is induced rapidly in the brain by numerous stimuli, including cocaine, antipsychotic drugs and seizures. These stimuli trigger rapid and transient enrichment of H4 acetylation and H3 phosphoacetylation at the c-Fos promoter in neurons, in association with transcriptional activation of c-Fos. However, the signalling pathways through which these stimuli modify histones are unknown. One study implicated the ERK pathway, in particular mitogen- and stress-activated kinase 1 (MSK1), in cocaine induction of H3 phosphorylation and c-Fos activation in the striatum (13), although it remains unclear whether MSK1 directly phosphorylates H3 or induces its phosphorylation by other kinases. Likewise, the ERK pathway may mediate H3 phosphoacetylation in response to seizures (16). Several other kinases can phosphorylate H3 in non-neuronal cells, but their actions in the brain are unexplored (17). Therefore, although H3 phosphoacetylation has been directly associated with c-Fos induction by acute stimuli, much work is needed to understand the underlying molecular mechanisms involved.
Indeed, one of the main challenges in the field is to elaborate the precise steps through which neural activity and synaptic transmission signal to the nucleus to regulate the enzymes and other proteins that mediate chromatin remodelling. Insight into these pathways has come from studies of non-neural cells. The activation of cellular Ca2+ pathways in muscle leads to the activation of CaMKs (calcium/calmodulin-activated protein kinases), which phosphorylate class II HDACs. This phosphorylation triggers the shuttling of the enzyme out of the nucleus, and results in increased histone acetylation (Fig. 2). This pathway has now been demonstrated in hippocampal cells (18) and cerebellar granule neurons (19), indicating that chromatin signalling mechanisms in different tissues overlap substantially.
It is unclear how histone acetylation can be regulated only at specific genes, but this is believed to involve multiprotein complexes. For example, class II HDACs can target specific genes for repression through N-terminal regulatory domains that mediate interactions between these HDACs and certain transcription factors, such as myocyte enhancing factor 2 (MEF2) (Fig. 2). Independent of histone deacetylation, class II HDACs can recruit cyclin-dependent kinase 5 (CDK5) to phosphorylate MEF2 and repress its transcriptional activity (19, 20). However, little is known about these regulatory mechanisms in the brain. Among neural genes, brain-derived neurotrophic factor (Bdnf) is one of the most studied for its regulation by chromatin remodelling (Box 2).
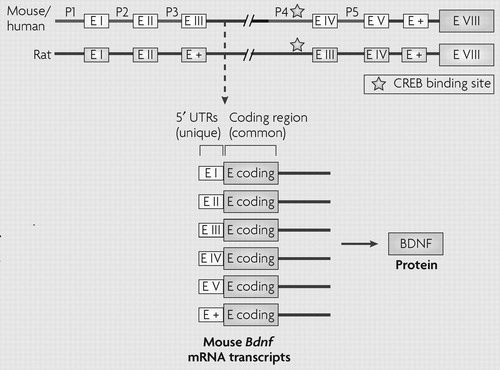
The most recent analysis indicates that the brain-derived neurotrophic factor gene (Bdnf) contains at least nine short 5′ non-coding exons in rodents and humans, each of which can be alternatively spliced to a common coding exon to generate at least nine different transcripts (99). To minimize confusion, the figure depicts the older nomenclature used in most previous studies (14,22,29,100–104), but illustrates the existence of additional non-coding exons (E +) with a common coding exon E VIII as described most recently (99). The use of alternatively spliced mRNA transcripts with distinct promoter regions, all of which encode the same protein, allows temporal and spatial specificity of BDNF expression (29,100).
Indeed, unique chromatin modifications at specific Bdnf promoters drive differential expression of distinct Bdnf splice variants in the context of acute and chronic neuronal stimulation. Acute membrane depolarization increases calcium-dependent transcription of two Bdnf transcripts: exons I and III in rats and the homologous exons I and IV in mice (100,101). Neuronal activity can trigger the phosphorylation of the repressor methyl-CpG-binding protein 2 (MeCP2), which regulates activity-dependent induction of Bdnf expression and provides a possible mechanism for the regulation of BdnfIII after neuronal stimulation (102,103). Phosphorylation of MeCP2 depends on calcium/calmodulin-activated protein kinase II (CaMKII) (FIG. 2). Rapid activitydependent changes in the DNA methylation of specific Bdnf promoters have also been observed in neurons (35,69).
Chromatin remodelling at Bdnf promoters is also altered after in vivo stimulation of neuronal activity, such as after the induction of seizures in rodent models. An acute pilocarpine-induced seizure increases levels of H4 acetylation most prominently at the P2 promoter of Bdnf in the rat hippocampus (104). Acute electroconvulsive seizure similarly increases histone H4 acetylation at Bdnf P2, in correlation with a robust increase in Bdnf transcription (22). In addition to the transient induction of H4 acetylation, seizure activity induces the rapid and transient phosphoacetylation of H3 in hippocampal neurons (16). As discussed in the main text, more sustained changes in H3 acetylation and methylation at the Bdnf promoter have been seen in mouse models of depression and antidepressant treatment (29) (FIG. 3). CREB, cyclic AMP-response element binding protein; E I-V, exons I-V; P, promoter; UTR, untranslated region
The above studies underscore the importance of dynamic chromatin remodelling in the transcriptional response to acute stimuli in neuronal cells. However, much research on psychiatric disorders is focused on neuroadaptations that evolve slowly but can cause lasting changes in brain circuitry. The next sections provide evidence for such stable neuronal regulation at the level of chromatin remodelling as it relates to the pathogenesis and maintenance of complex psychiatric disorders.
Epigenetic mechanisms in depression
Depression is a common, chronic and debilitating disease. Although many patients benefit from antidepressant medication, electroconvulsive seizures (ECS) or psychotherapy, only about half of depressed patients show a complete remission, which underscores the need for more effective agents (21). The mechanisms that precipitate depression, such as stress, are incompletely understood. One mystery of the disease is its longlasting nature and delayed response to antidepressant treatment. This persistence is thought to be mediated by slowly developing but stable adaptations, which might include epigenetic regulation.
To investigate such adaptations, one study examined changes in histone modifications after chronic ECS in the rat hippocampus (22), a brain region implicated in the pathophysiology and treatment of depression (23, 24). Like antidepressant medications, ECS is effective only after repeated administration, indicating that long-term adaptations at the level of gene expression might be involved. Chronic ECS upregulates the expression of Bdnf and Creb in the hippocampus, and such upregulation has been shown to mediate antidepressant activity in animal models (25–27). Chronic ECS produced chromatin remodelling changes that were very different from those seen after acute ECS, and that were detected at distinct Bdnf promoter regions. Chronic ECS increased H3 acetylation (instead of H4 acetylation as seen after acute ECS) at Bdnf promoters 3 and 4, in correlation with increased expression of the corresponding Bdnf transcripts (Box 2).
Chronic social defeat stress, an animal model of depression (28) that mimics many symptoms of human depression, also alters chromatin regulation of Bdnf (29). Prolonged exposure to an aggressor induces lasting changes in mouse behaviour such as social avoidance, which are reversed by chronic (but not acute) treatment with antidepressants (28,29). At a molecular level, chronic defeat stress in mice induces sustained downregulation of the expression of two splice variants of Bdnf, BdnfIII and BdnfIV, in the hippocampus (29). These changes are reversed only after chronic treatment with the antidepressant imipramine. Chronic defeat stress induces robust and long-lasting increases in H3-K27 dimethylation, a repressive modification, specifically at the promoters of the downregulated Bdnf transcripts.
This histone modification was present four weeks after the cessation of defeat stress and was not reversed by antidepressant treatment, indicating that chronic defeat stress imposes a long-lasting marker of repression at these Bdnf promoters (Fig. 3). Rather, chronic imipramine seems to reverse repression of the Bdnf gene by inducing H3 acetylation, as well as H3-K4 methylation—an activating modification (Fig. 1)—at the same promoters. Direct support for this hypothesis comes from the observation that chronic imipramine downregulates Hdac5 expression in the hippocampus, but only in animals previously subjected to chronic social defeat. Moreover, viral-mediated overexpression of Hdac5 in this region prevents imipramine's restoration of Bdnf levels as well as the drug's antidepressant effects (29). Further support for this scheme is the observation that systemic administration of sodium butyrate, a nonspecific HDAC inhibitor (see Supplementary information S1 (table)), acts as an antidepressant in models of depression, including social defeat (29, 30).
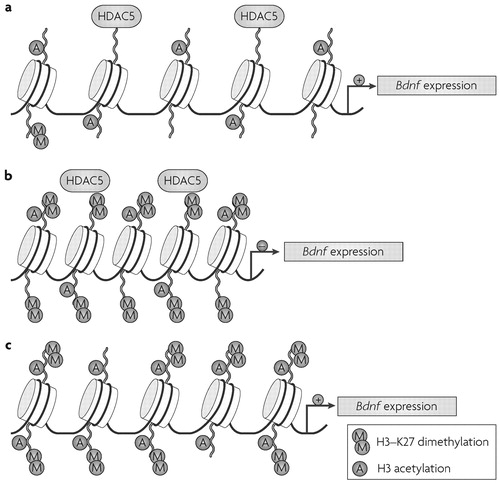
a In the absence of stress, the chromatin state of brain-derived neurotrophic factor (Bdnf) is at a basal level, characterized by moderate levels of histone H3 acetylation and virtually no H3-K27 dimethylation. In this state, histone deacetylase 5 (HDAC5) might repress unnecessary activation of BDNF and maintain a chromatin balance. b Chronic defeat stress induces the specific and prolonged dimethylation of histone H3-K27. This induces a more “closed” chromatin state at Bdnf promoters P3 and P4, and a corresponding repression of Bdnf transcripts III and IV expression. H3 acetylation and HDAC5 regulation are not affected after chronic defeat stress alone, corroborating the idea that the main repressive marker after chronic stress is histone methylation. c Chronic imipramine (antidepressant) treatment after defeat stress downregulates Hdac5 expression and increases H3 acetylation, with little if any change in H3-K27 dimethylation. Imipraminedependent H3 hyperacetylation at the Bdnf promoters P3 and P4 allows partial “reopening” of the repressed chromatin state caused by defeat stress, and results in transcriptional reactivation of the Bdnf gene. K, lysine residue.
The mechanism by which chronic antidepressant treatment upregulates H3-K4 methylation in vivo is unclear, but a recent in vitro study may provide some insight. Demethylation of histone H3-K4 is catalysed by BHC110/LSD1, an enzyme that has a close structural homology to monoamine oxidases. Monoamine oxidase inhibitors, which are a class of antidepressants, can increase global levels of H3-K4 methylation and cause transcriptional derepression of specific genes in vitro (31). The importance of HMT and HDM inhibition for the action of antidepressants remains unknown, but this can now be tested because distinct HMTs and HDMs act on H3-K4 versus H3-K27. Together, these studies implicate chromatin remodelling in the formation of stable neuronal adaptations (molecular scars) in the hippocampus that might underlie some of the longlasting changes in behaviour which are induced by chronic stress and reversed by chronic antidepressants. The studies also raise the possibility that specific HDAC, HMT and HDM inhibitors might be useful as antidepressant treatments (Fig. 3).
Stressful events early in life might also leave lasting epigenetic marks on the organism. In rats, some mothers naturally display high levels of nurturing behaviours, such as licking, grooming and arched-back nursing (high-LG-ABN), whereas others have low levels of such behaviours (low-LG-ABN) (32). Offspring of highLG-ABN mothers are less anxious, have attenuated corticosterone responses to stress and display increased expression of glucocorticoid receptor (GR) mRNA and protein in the hippocampus when compared to pups of low-LG-ABN mothers (33). The upregulation of GR mRNA specifically involves the alternatively spliced variant GR17. The promoter that drives expression of this variant is brain specific, and contains a binding consensus sequence for nerve growth factor inducible factor A (NGFI-A), the expression of which is upregulated in the pups of high-LG-ABN mothers. A study that compared the methylation status at individual CpG sites within the NGFI-A consensus sequence region of the GR17 promoter between the offspring of high- and low-LG-ABN mothers found that low-LG-ABN pups have increased methylation at these sites (34). This difference in methylation emerged in the first week of life and persisted into adulthood. As adults, the offspring of mothers with low levels of nurturing behaviour were at a molecular disadvantage—their methylated GR17 promoter prevented binding of the transcriptional enhancer NGFI-A, effectively disrupting the normal transcriptional regulation of the GR gene. Although this epigenetic methylation was long-lasting, it could be reversed by infusion of the class I and II HDAC inhibitor trichostatin A (TSA) (see Supplementary information S1 (table)), which increased levels of H3 acetylation, cytosine demethylation and the binding of NGFI-A at the GR17 promoter in the low-LG-ABN-raised offspring. Interestingly, cross-fostering also reversed the differences in methylation at that site (34).
DNA methylation has traditionally been viewed as an irreversible epigenetic marker; however, this study indicates that patterns of DNA methylation might be reversible even in adult neurons. Enzymes that mediate DNA demethylation in the brain have yet to be identified. Another important concept illustrated by the reversal of DNA methylation by an HDAC inhibitor is the interaction between histone modifications and DNA methylation. It has been proposed that demethylation might occur through activation of second messenger signals that lead to the recruitment of HATs, which, through increased histone acetylation, would allow DNA demethylases greater access to the GR promoter (33). Other examples of rapidly reversible changes in DNA methylation in the adult brain have been reported in learning and memory (35) (see below).
Together, these studies indicate that several epigenetic modifications, including lasting changes in histone acetylation, histone methylation and DNA methylation, are important in animal models of stress, depression and antidepressant treatment. However, this work has so far focused only on the Bdnf and GR genes, and only on the hippocampus. Work is now needed to investigate other brain regions that have been implicated in depression and its treatment (24), and to investigate the involvement of additional genes in mediating the long-term effects of stress and antidepressant treatments on gene transcription.
Epigenetic mechanisms in addiction
Drugs of abuse control human behaviour by hijacking the brain's natural reward centres, including the mesolimbic dopamine system. This circuit includes dopaminergic neurons of the ventral tegmental area (VTA), which project directly to the nucleus accumbens (NAc)—the ventral portion of the striatum. During the past few decades, much has been learned about the brain's reward pathway and how drugs of abuse usurp its functions (36,37), but it is unclear why addictive behaviours persist long after drug abstinence, underlying high rates of relapse. Many studies have identified drug-induced changes in mRNA levels in the VTA, NAc and other relevant brain regions (38–43). Some of these changes in gene expression persist even after months of abstinence (44). The longevity of the changes has driven research into chromatin remodelling as the molecular basis of sustained, even life-long, alterations in gene expression in brain reward regions (45).
An acute dose of cocaine induces the expression of c-Fos and FosB in the striatum, and this induction is associated with transient increases in H4 acetylation within 30 min of drug injection (13, 14). CBP, with its intrinsic HAT activity, is an important mediator of cocaine-induced acetylation of histones at the FosB gene, and probably serves this function for other genes as well (46). Acute cocaine administration also induces H3 phosphoacetylation at the c-Fos gene promoter only (14), and this effect requires the protein kinase MSK1, as mentioned above (13).
Chronic cocaine treatment and self-administration activate and repress many distinct genes compared with acute treatment. FosB, for example, is induced by both acute and chronic cocaine exposure, but acute exposure produces H4 acetylation whereas chronic treatment causes H3 acetylation (14). Genes that are selectively induced in the chronic state, such as Cdk5 and Bdnf (44,47), also seem to be acetylated selectively on H3. Interestingly, cocaine induction of histone acetylation at the Bdnf promoter builds during a week of withdrawal (14), and precedes the progressive increase seen in BDNF protein and mRNA levels in this region (44). A similar switch from H4 to H3 acetylation is seen in the hippocampus after acute versus chronic ECS (22), indicating that H3 acetylation may represent a common, chromatin-mediated mark of persistently or repeatedly activated genes. Little is known about the catalytic selectivity of HATs and HDACs for the specific acetylated residues on H3 and H4. It would be interesting to determine whether this switch from H4 to H3 acetylation is mediated by the recruitment of distinct HATs or HDACs to regulated genes after acute versus chronic treatment.
Cocaine regulation of histone acetylation at the Cdk5 and Bdnf genes highlights the importance of exploring genome-wide chromatin structure—by use of ChIP on chip (48, 49) or SACO (50) assays, for example—to identify many other genes, the dysregulation of which at the chromatin level contribute to cocaine addiction. Genome-wide epigenetic approaches have yielded exciting results in the fields of developmental and cancer biology (48, 49), and efforts to carry out similar studies in addiction are now underway. Preliminary evidence from our laboratory has identified several hundred genes that are significantly hyper- or hypoacetylated after chronic cocaine treatment (51–53), and future studies will focus on the role of these chromatin changes in the pathogenesis and maintenance of addiction.
As mentioned earlier, the transcription factor ΔFOSB is implicated in the transition to an addicted state (3,4), and accounts for >25% of all changes in steady state mRNA levels induced by cocaine in the NAc (41). ChIP assays have shown that the induction of one of these mRNAs, Cdk5, by cocaine represents a direct, activating effect of ΔFOSB on the Cdk5 gene (14) (Fig. 4). By contrast, Bdnf is not a direct target of ΔFOSB, indicating that a different mechanism is involved in the induction of this gene. Induction of Cdk5, in turn, partly mediates the effects of chronic cocaine treatment on dendritic remodelling in the NAc (54). These findings support a model in which the accumulation of ΔFOSB interacts with chromatin remodelling factors at specific promoters to regulate genes that are important for the development and maintenance of addiction (Fig. 4).
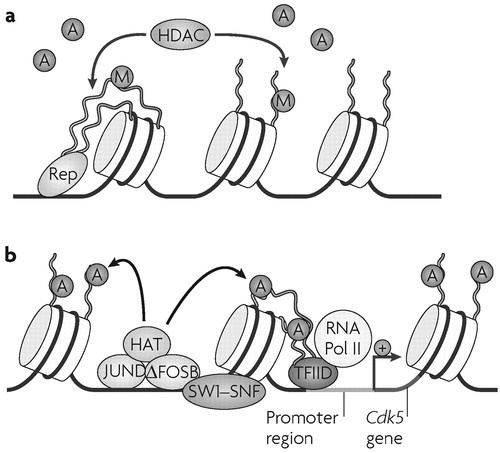
a Repressed state of chromatin, where a site-specific repressor (Rep) recruits a histone deacetylase (HDAC) complex, which removes acetyl groups (A) from histone amino-terminal tails. Gene inactivation may also involve other modifications, such as methylation (M) of histone tails. b Active state of chromatin around a cocaine-activated gene (for example, cyclin-dependent kinase 5 (Cdk5)), where a cocaine-induced transcriptional activator (for example, an activator protein 1 dimer composed of ΔFOSB-JUND) recruits a histone acetyltransferase (HAT) and a chromatin remodelling complex (mating switching and sucrose nonfermenting complex, SWI/SNF), which induce acetylation (and perhaps demethylation and other modifications) of histone tails and repositioning of nucleosomes. These actions facilitate the binding of general transcription factors and the basal transcriptional apparatus (for example, transcription factor IID (TFIID) and RNA polymerase II (PolII)) to the promoter. Modified, with permission, from REF. (45) © (2005) Society for Neuroscience.
Further studies are needed to identify the enzymes that mediate these cocaine-induced chromatin remodelling events. Histone and DNA modifying complexes are obvious targets. A recent report found that chronic cocaine treatment increases MeCP2 and MBD1 in the adult brain (55), both of which bind to methylated DNA and aid in transcriptional repression. Several proteins that have been implicated behaviourally in drug addiction are now being rediscovered as histone modifying enzymes. For example, CDK5 phosphorylates an HDAC-interacting protein, which then alters chromatin structure (56). Another example is the transcriptional regulator named nucleus accumbens 1 (NAC1), which is highly induced by cocaine in the NAc and interacts with HDAC3 and HDAC4 (57). Possible effects of CDK5 and NAC1 on chromatin structure in animal models of addiction remain unexplored, but these studies might provide mechanistic insight into how drugs of abuse can direct chromatin-modifying enzymes to specific genes. Further work is also needed to examine cocaine regulation of other chromatin-modifying enzymes, and their specific involvement in shaping the addicted state.
The manipulation of chromatin remodelling enzymes by pharmacological inhibition, viral-mediated gene transfer and genetic mutations in mice have shown that cocaine-induced changes in chromatin structure are behaviourally relevant. HDAC inhibitors increase histone acetylation and potentiate cocaine-induced locomotor activity and cocaine reward, as measured by conditioned place preference (14, 53). Conversely, viral-mediated overexpression of Hdac4 or Hdac5 in the NAc had the opposite effect. Consistent with these findings, mice deficient in CBP show reduced cocaine-induced locomotor activity (46). Together, these findings indicate that the ability of cocaine to increase histone acetylation is important for the behavioural effects of the drug, and set the stage for more in-depth analysis of HDAC inhibitors and related pharmacological and genetic tools in paradigms of cocaine self-administration and relapse.
There are also a few reports of chromatin changes after chronic ethanol administration (58–60). An early study used circular dichroism to show that chronic ethanol induced a more open structure to chromatin, consistent with a switch from heterochromatin to euchromatin (58). Subsequently, changes in histone acetylation and DNA methylation have been reported in alcoholic patients (59, 60).
Epigenetic mechanisms in other disorders
Learning and memory.
The formation of long-term memories, by analogy to pathophysiological mechanisms of depression and addiction, is thought to entail lasting changes in gene expression, and there is growing evidence that histone modifications and DNA methylation may be involved (35, 61, 62). Mice deficient in CBP exhibit memory deficits in several hippocampus-dependent memory tests, including spatial mazes, contextual fear conditioning and novel object recognition, and administration of an HDAC inhibitor can restore normal long-term memory formation in the mutants (62–64), and even enhance it in normal animals (62, 65). Contextual fear conditioning and/or activation of the ERK pathway, which is thought to contribute to memory formation, increase levels of H3 phosphoacetylation in the CA1 area of the hippocampus (62, 66). H4 acetylation, another common marker of chromatin activation, was not regulated in these learning and memory models (65), substantiating the hypothesis that H3 acetylation might mark chronic and stable events, whereas H4 acetylation might signify more acute and dynamic changes. In addition, there is early evidence that the chromatin remodelling complexes polycomb and trithorax, which contain HMT activity, contribute to the epigenetic regulation of contextual fear conditioning (67). Synaptic plasticity is believed to contribute to the formation of long-term memories and also seems to have an epigenetic component: in Aplysia, H4 acetylation around the C/EBP (CAATT-enhancing binding protein) promoter is altered after long-term potentiation (LTP) and long-term depression (68), and HDAC inhibitors promote LTP in mammalian neurons (65).
Recent findings have also implicated changes in DNA methylation in learning and memory. In contrast to the more traditional view that DNA methylation is a highly stable modification, they suggest that DNA methylation may be subject to rapid and dynamic regulation in the nervous system. Contextual fear conditioning induced DNA methyltransferase 3A (Dnmt3A) and Dnmt3B expression in CA1 of the hippocampus, and administration of the DNMT inhibitors zebularine and 5-aza-2′-deoxycytidine blocked the induction of both contextual fear conditioning (69) and hippocampal LTP (35). Fear conditioning also caused rapid methylation and silencing of the protein phosphatase 1 (PP1) gene promoter (69), a gene known to be important for LTP and memory formation. Interestingly, fear conditioning also induced demethylation of the reelin promoter, indicating that both DNA methylation and demethylation are highly regulated. Some of these effects may be mediated by protein kinase C (PKC), the activation of which in vitro decreased reelin promoter methylation and increased Dnmt3A expression (35). PKC also induced H3 acetylation, which seemed to depend on DNMT activity; this provides another example of the intimate interplay between histone modifications and DNA methylation in chromatin remodelling. However, one question raised by these studies is the mechanism by which zebularine and 5-aza-2′-deoxycytidine lead to demethylation of genes in adult neurons. Thus, these drugs are generally thought to act through incorporation into DNA during mitosis, thereby preventing methylation of the new DNA strand. Further work is needed to understand their actions in postmitotic, mature neurons.
Several developmental conditions, including common forms of mental retardation, can be attributed, at least in part, to disruption in the brain's epigenetic machinery (62, 70) (Table 1).
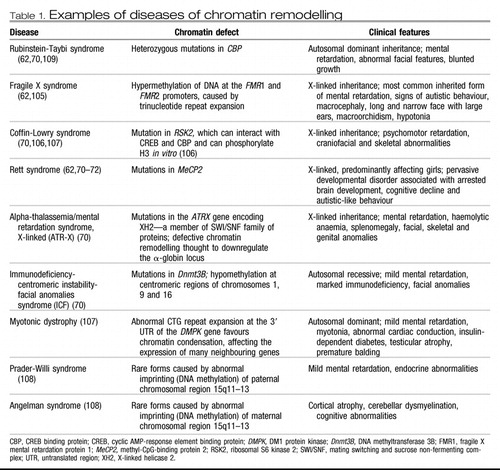 |
Table 1. Examples of diseases of chromatin remodelling
Rett syndrome.
Rett syndrome is an X-linked, pervasive developmental disorder that is associated with arrested brain development and cognitive decline. It is considered an autism spectrum disorder, given the prominence of social and language abnormalities in many patients, and predominantly affects girls. Patients develop normally until 6–18 months of age, but thereafter begin to lose acquired language and motor skills and develop neurological and psychiatric symptoms, including stereotyped motor movements, seizures, autonomic instability, breathing irregularities, severe cognitive decline and autistic-like behaviour (71).
Rett syndrome is an epigenetic disorder, because it is caused by mutations in the gene that encodes the transcriptional repressor MeCP2 (72). Several studies have begun to define the influence of MeCP2 in the CNS and the pathophysiology of Rett syndrome at behavioural, functional and molecular levels (73–80). Mice that lack Mecp2 recapitulate many aspects of the neurological (73,79) and behavioural (76, 80) phenotypes of Rett syndrome. Moreover, transgenic overexpression of Mecp2 in mutant mice can rescue the Rett-like phenotype (73). Learning, memory and synaptic transmission are also impaired in one mouse model of the disease (77). Electrophysiology studies in MeCP2-deficient mice have shown that MeCP2 is important for synaptic function (74, 78). MeCP2 deficiency causes a global reduction in cortical synaptic excitation with concomitant increased inhibition (but without any changes in the intrinsic electrophysiological properties of individual neurons), resulting in an overall shift towards synaptic inhibition in cortical neurons (74). There is also a regulatory relationship between the presynaptic function of MeCP2 and its ability to silence transcription (78). First, cultured hippocampal neurons from Mecp2-knockout mice show a decreased frequency of spontaneous excitatory postsynaptic potentials and increased short-term synaptic depression, substantiating the previous findings that MeCP2 deficiency causes synaptic inhibition. Furthermore, treatment of wild-type hippocampal cultures with the HDAC inhibitor TSA effectively reduced transcriptional silencing, and recapitulated the synaptic changes seen in MeCP2−/− cultures. Importantly, these effects of TSA could be blocked by actinomycin D, an inhibitor of DNA synthesis (78). These results suggest that a deficiency in MeCP2 leads to overactive gene transcription, which in turn causes a selective impairment in presynaptic excitatory function. An encouraging recent finding is that reversal of an MeCP2 deficit in mice, even after Rett-like symptoms appear, can reverse many of these symptoms (81), which parallels the ability to reverse synaptic deficiencies caused by MeCP2 mutation in fully differentiated neurons (78). These findings suggest the possibility of effectively treating Rett syndrome in humans even after it has become symptomatic.
The molecular features of the pathogenesis of Rett syndrome are also under study. In MeCP2-deficient mice, levels of BDNF are reportedy elevated (102) or reduced (75); however, deletion of Bdnf causes earlier onset of Rett-like symptoms and Bdnf overexpression can rescue some behavioural and electrophysiological defects (75). Bdnf is a presumed target of MeCP2 transcriptional repression and is likely to be one of many such targets that are involved in Rett syndrome (75, 102, 103).
Schizophrenia.
There is mounting evidence that epigenetic mechanisms are involved in the pathogenesis of schizophrenia (82, 83). Much of this work has focused on epigenetic alterations at the reelin promoter. Reelin is a glycoprotein that is expressed during development and in adult GABA (γ-aminobutyric acid)-containing neurons, and is important for proper neural positioning during brain development. Post-mortem studies of patients with schizophrenia reveal significant down-regulation of reelin expression in several brain regions that is not associated with neuronal loss (84). The reelin promoter contains a large CpG island, indicating that DNA methylation might be important for regulating its expression (85). Repeated methionine administration causes hypermethylation of the promoter and results in downregulation of reelin transcription in the heterozygous Reln+/− mouse model of schizophrenia (82). Methionine treatment also induced MeCP2 binding to the reelin promoter (86). By contrast, treatment with the methylation inhibitor 5-aza-2′-deoxycytidine upregulated reelin expression in vitro (85). Interestingly, a role for methylation in the pathogenesis of schizophrenia was suggested decades ago by clinical studies, in which treatment with the methylating agent SAM elicited psychotic episodes in some patients with schizophrenia (87).
Histone remodelling might also contribute to schizophrenia. The antipsychotic drugs haloperidol and raclopride (both dopamine receptor D2 antagonists) rapidly induce phosphoacetylation of H3 in the mouse striatum, specifically at the c-Fos gene promoter (15). Treatment with valproate (an anticonvulsant and mood stabilizer) that, among other actions, inhibits HDACs, increased reelin expression in vitro and in vivo (82, 85), and this effect was accompanied by decreased methylation of the reelin promoter. Furthermore, valproate attenuated schizophrenia-like behavioural abnormalities in a methionine-induced epigenetic mouse model of the illness. Other HDAC inhibitors produced similar results (88). Although the mechanisms by which HDAC inhibitors reduce DNA methylation are unknown, it is thought that hyperacetylation can regulate the accessibility of DNMT1 to promoter regions or that it might induce DNA demethylase activity (84, 89, 90).
One hypothesis that has been proposed for how epigenetic malfunction may contribute to schizophrenia is that hypermethylation (regulated by DNMT1 and other chromatin-related complexes) downregulates multiple genes (such as reelin) in GABA-containing neurons, causing dysfunction of GABA-mediated neuronal circuitry. Higher brain function would be impaired because synchronization with other neuronal networks would be disrupted as a consequence. Agents that can reactivate gene expression, such as inhibitors of DNMT1 or HDACs, might therefore provide improved pharmacological treatment for schizophrenia (84). The administration of valproate in conjunction with antipsychotic medication has been shown to accelerate the onset of the antipsychotic effects in patients with schizophrenia (91). However, valproate has many actions in addition to its HDAC inhibitor activity, and so direct evidence for this hypothesis remains lacking.
Future directions
There is much evidence that epigenetic regulation is involved in neurogenesis, neuronal plasticity and learning and memory, and in disorders such as depression, addiction, schizophrenia and cognitive dysfunction. Changes in histone modifications and DNA methylation have been found both globally and at the promoters of genes that have been implicated in these phenomena. For example, chromatin remodelling at the Bdnf promoter is associated with neuronal activity, seizures, chronic stress, cocaine addiction and Rett syndrome; at the reelin promoter with a mouse model of schizophrenia; and at the Cdk5 promoter with drugs of abuse. However, chromatin remodelling is likely to affect many more genes, and it is important to investigate this in both animal models of psychiatric conditions and post-mortem human brain tissue. Coordinated gene expression arrays and ChIP on chip arrays are likely to be helpful in elucidating the promoter gene targets for histone modifications as well as the in vivo binding sites of transcriptional activators or repressors in relevant brain regions. Such studies will provide a global picture of epigenetic regulation, which is currently lacking. Genome-wide epigenetic approaches have yielded exciting results in the fields of developmental and cancer biology (48–50), and efforts to carry out similar studies in addiction and depression are ongoing. Many genes show abnormal levels of histone acetylation or methylation after chronic cocaine administration or stress, and future studies will focus on the influence of these global changes in chromatin on the pathogenesis of addiction and depression, and on identifying the transcription factors that mediate this regulation. It will be necessary to use a system-based biological approach to interpret the data from such studies (92).
The lack of specific inhibitors for many epigenetic regulators impedes progress in understanding the mechanisms by which chromatin remodelling contributes to psychiatric disorders. As just one example, all available HDAC inhibitors act broadly and inhibit all Class I and Class II HDACs (see Supplementary information S1 (table)). Moreover, certain HDACs (Class III HDACs, termed sirtuins, as well as HDAC6, a Class IIb HDAC) can deacetylate proteins other than histones (for example, tubulin, MEF2, the tumour suppressor p53 and the DNA repair protein Ku70), and these non-histone actions complicate interpretation of the inhibitors' biological effects. Inhibitors that are specific for particular HDACs or other chromatin modifiers would allow us to identify the specific players in the epigenetic regulation of psychiatric phenomena. Such specific inhibitors would also have the potential of being used as new treatment agents for these disorders. In addition, it will be important to generate mouse models that lack particular chromatin-related genes in order to study their molecular and behavioural influences.
Lasting changes in chromatin modifications in animal models often occur only after chronic behavioural manipulations, which try to mimic the long-lasting behavioural changes associated with psychiatric disorders. A better understanding of the mechanisms by which such stable changes are brought about would not only advance our knowledge of the basic neurobiology of these illnesses, but might also provide new therapeutic avenues for disorders such as depression, addiction, schizophrenia and neurodevelopmental illnesses.
DATABASES
The following terms in this article are linked online to: Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
ΔFOSB | Bdnf | CBP | c-Fos | CREB | FosB | GR |MeCP2 |
OMIM:http://www.ncbi.nim.nih.gov/entrez/query.fcgi?db=OMIM
Rett syndrome | Rubinstein–Taybi syndrome |schizophrenia
1 Kendler, K. S. Twin studies of psychiatric illness: an update. Arch. Gen. Psychiatry 58, 1005– 1014 ( 2001).Crossref, Google Scholar
2 Hyman, S. E. & Nestler, E. J. The Molecular Foundations of Psychiatry ( American Psychiatric, Washington, D. C., 1993).Google Scholar
3 McClung, C. A. et al. ΔFosB: a molecular switch for long-term adaptation in the brain. Brain Res. Mol. Brain Res. 132, 146– 154 ( 2004).Crossref, Google Scholar
4 Nestler, E. J., Barrot, M. & Self, D. W. ΔFosB: a sustained molecular switch for addiction. Proc. Natl Acad. Sci. USA 98, 11042– 11046 ( 2001).Crossref, Google Scholar
5 Felsenfeld, G. & Groudine, M. Controlling the double helix. Nature 421, 448– 453 ( 2003).Crossref, Google Scholar
6 Hake, S. B., Xiao, A. & Allis, C. D. Linking the epigenetic “language” of covalent histone modifications to cancer. Br. J. Cancer 90, 761– 769 ( 2004).Crossref, Google Scholar
7 Lachner, M. & Jenuwein, T. The many faces of histone lysine methylation. Curr. Opin. Cell Biol. 14, 286– 298 ( 2002).Crossref, Google Scholar
8 Gill, G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 18, 2046– 2059 ( 2004).Crossref, Google Scholar
9 Hassa, P. O., Haenni, S. S., Elser, M. & Hottiger, M. O. Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol. Mol. Biol. Rev. 70, 789– 829 ( 2006).Crossref, Google Scholar
10 Jenuwein, T. & Allis, C. D. Translating the histone code. Science 293, 1074– 1080 ( 2001).Crossref, Google Scholar
11 Narlikar, G. J., Fan, H. Y. & Kingston, R. E. Cooperation between complexes that regulate chromatin structure and transcription. Cell 108, 475– 487 ( 2002).Crossref, Google Scholar
12 Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941– 953 ( 2004).Crossref, Google Scholar
13 Brami-Cherrier, K. et al. Parsing molecular and behavioral effects of cocaine in mitogen- and stressactivated protein kinase-1-deficient mice. J. Neurosci. 25, 11444– 11454 ( 2005). Explores the signal transduction cascades, and their effect on downstream chromatin remodelling and associated gene expression, in striatal neurons in response to cocaine. It shows that cocaine causes induction of H4 acetylation, H3 phosphorylation and CREB phosphorylation through MSK1.Crossref, Google Scholar
14 Kumar, A. et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48, 303– 314 ( 2005). Establishes an important role for chromatin remodelling in the reward responses to cocaine. Also, using chromatin immunoprecipitation assays, it shows that cocaine induces distinct histone modifications and in vivo binding of the transcription factor ΔFOSB at specific gene promoters in the striatum.Crossref, Google Scholar
15 Li, J. et al. Dopamine D2-like antagonists induce chromatin remodeling in striatal neurons through cyclic AMP-protein kinase A and NMDA receptor signaling. J. Neurochem. 90, 1117– 1131 ( 2004). Demonstrates that acute administration of antipsychotic drugs to rodents increases global levels of histone acetylation in the striatum and provides evidence for the signal transduction mechanisms that mediate this effect.Google Scholar
16 Crosio, C., Heitz, E., Allis, C. D., Borrelli, E. & SassoneCorsi, P. Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J. Cell Sci. 116, 4905– 4914 ( 2003).Crossref, Google Scholar
17 Bode, A. M. & Dong, Z. Inducible covalent posttranslational modification of histone H3. Sci. STKE 2005, re4 ( 2005).Google Scholar
18 Chawla, S., Vanhoutte, P., Arnold, F. J., Huang, C. L. & Bading, H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J. Neurochem. 85, 151– 159 ( 2003).Crossref, Google Scholar
19 Linseman, D. A. et al. Inactivation of the myocyte enhancer factor-2 repressor histone deacetylase-5 by endogenous Ca2+//calmodulin-dependent kinase II promotes depolarization-mediated cerebellar granule neuron survival. J. Biol. Chem. 278, 41472– 41481 ( 2003).Crossref, Google Scholar
20 Gregoire, S. et al. Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation. J. Biol. Chem. 281, 4423– 4433 ( 2006).Crossref, Google Scholar
21 Berton, O. & Nestler, E. J. New approaches to antidepressant drug discovery: beyond monoamines. Nature Rev. Neurosci. 7, 137– 151 ( 2006).Crossref, Google Scholar
22 Tsankova, N. M., Kumar, A. & Nestler, E. J. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J. Neurosci. 24, 5603– 5610 ( 2004). Outlines a standardized approach to performing chromatin immunoprecipitation assays in rodent brain tissue. It also provides a detailed analysis of several transient and lasting changes in histone modifications after acute and chronic seizure, in correlation with changes in gene expression at the specific gene promoters.Crossref, Google Scholar
23 Duman, R. S. Depression: a case of neuronal life and death? Biol. Psychiatry 56, 140– 145 ( 2004).Crossref, Google Scholar
24 Nestler, E. J. et al. Neurobiology of depression. Neuron 34, 13– 25 ( 2002).Crossref, Google Scholar
25 Duman, R. S. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 5, 11– 25 ( 2004).Crossref, Google Scholar
26 Monteggia, L. M. et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl Acad. Sci. USA 101, 10827– 10832 ( 2004).Crossref, Google Scholar
27 Monteggia, L. M. et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry 61, 187– 197 ( 2006).Crossref, Google Scholar
28 Berton, O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864– 868 ( 2006). The authors use chronic social defeat stress as an animal model of depression and demonstrate a crucial role for the neurotrophic factor BDNF in the mesolimbic dopamine pathway in mediating some of the deleterious molecular and behavioural sequelae of this stress paradigm.Crossref, Google Scholar
29 Tsankova, N. M. et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature Neurosci. 9, 519– 525 ( 2006). The first study to examine the involvement of chromatin remodelling in an animal model of depression. It reveals robust and lasting in vivo changes in histone modifications, and a role for HDAC5 in chronic social defeat stress and in antidepressant efficacy.Crossref, Google Scholar
30 Schroeder, F. A., Lin, C. L., Crusio, W. E. & Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse ( in the press).Google Scholar
31 Lee, M. G., Wynder, C., Schmidt, D. M., McCafferty, D. G. & Shiekhattar, R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem. Biol. 13, 563– 567 ( 2006).Crossref, Google Scholar
32 Champagne, F. A., Francis, D. D., Mar, A. & Meaney, M. J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 79, 359– 371 ( 2003).Crossref, Google Scholar
33 Meaney, M. J. & Szyf, M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 28, 456– 463 ( 2005).Crossref, Google Scholar
34 Weaver, I. C. et al. Epigenetic programming by maternal behavior. Nature Neurosci. 7, 847– 854 ( 2004). This important study provides highly novel evidence that the epigenetic state of GR in the hippocampus of rodent offspring, in particular its level of DNA methylation, can be modulated by maternal nurturing behavior in a lasting but reversible manner.Crossref, Google Scholar
35 Levenson, J. M. et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J. Biol. Chem. 281, 15763– 15773 ( 2006). Along with reference 69, this study implicates rapid and reversible changes in DNA methylation in synaptic plasticity in the rodent hippocampus, and in the formation of long-term memory. The notion that DNA methylation is subject to dynamic regulation in the adult brain is highly novel and has important implications for our understanding of the epigenetic control of brain function.Crossref, Google Scholar
36 Everitt, B. J. & Robbins, T. W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neurosci. 8, 1481– 1489 ( 2005).Crossref, Google Scholar
37 Hyman, S. E., Malenka, R. C. & Nestler, E. J. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 29, 565– 598 ( 2006).Crossref, Google Scholar
38 Freeman, W. M. et al. Cocaine-responsive gene expression changes in rat hippocampus. Neuroscience 108, 371– 380 ( 2001).Crossref, Google Scholar
39 Freeman, W. M. et al. Changes in rat frontal cortex gene expression following chronic cocaine. Brain Res. Mol. Brain Res. 104, 11– 20 ( 2002).Crossref, Google Scholar
40 Kreek, M. J., Bart, G., Lilly, C., LaForge, K. S. & Nielsen, D. A. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol. Rev. 57, 1– 26 ( 2005).Crossref, Google Scholar
41 McClung, C. A. & Nestler, E. J. Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nature Neurosci. 6, 1208– 1215 ( 2003).Crossref, Google Scholar
42 McClung, C. A. et al. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J. Neurosci. 25, 6005– 6015 ( 2005).Crossref, Google Scholar
43 Yao, W. D. et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron 41, 625– 638 ( 2004).Crossref, Google Scholar
44 Grimm, J. W. et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 23, 742– 747 ( 2003).Crossref, Google Scholar
45 Colvis, C. M. et al. Epigenetic mechanisms and gene networks in the nervous system. J. Neurosci. 25, 10379– 10389 ( 2005).Crossref, Google Scholar
46 Levine, A. A. et al. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc. Natl Acad. Sci. USA 102, 19186– 19191 ( 2005). Characterizes the influence of chromatin remodelling on cocaine action in the brain. In particular, it shows that recruitment of CBP to the FosB promoter and the resulting H4 acetylation are essential for normal levels of FosB expression, for accumulation of the transcription factor ΔFOSB, and for normal sensitivity to cocaine.Crossref, Google Scholar
47 Bibb, J. A. et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410, 376– 380 ( 2001).Crossref, Google Scholar
48 Lee, M. P. Genome-wide analysis of epigenetics in cancer. Ann. NY Acad. Sci. 983, 101– 109 ( 2003).Crossref, Google Scholar
49 Lee, T. I. et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301– 313 ( 2006).Crossref, Google Scholar
50 Impey, S. et al. Defining the CREB regulon: a genomewide analysis of transcription factor regulatory regions. Cell 119, 1041– 1054 ( 2004). Introduces a novel method of genome-wide analysis of transcription factor binding sites, termed SACO, which combines chromatin immunoprecipitation with long serial analysis of gene expression by direct sequencing rather than by the use of microarray chips.Crossref, Google Scholar
51 Kumar, A. et al. Global maps of histone acetylation and gene regulatory networks in the nucleus accumbens after chronic cocaine using chip on chip. Soc. Neurosci. Abstr. 451. 4 ( 2005).Google Scholar
52 Kumar, A. et al. Transcriptional and post-transcriptional regulation of gene expression in nucleus accumbens associated with chronic stress-induced neuroadaptations in mouse. Soc. Neurosci. Abstr. 191. 23 ( 2006).Google Scholar
53 Renthal, W. et al. Epigenetic control of cocaine reward by class II histone deacetylases. Soc. Neurosci. Abstr. 294. 27 ( 2006).Google Scholar
54 Norrholm, S. D. et al. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience 116, 19– 22 ( 2003).Crossref, Google Scholar
55 Cassel, S. et al. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol. Pharmacol. 70, 487– 492 ( 2006).Crossref, Google Scholar
56 Li, Z. et al. Cdk5/p35 phosphorylates mSds3 and regulates mSds3-mediated repression of transcription. J. Biol. Chem. 279, 54438– 54444 ( 2004).Crossref, Google Scholar
57 Korutla, L., Wang, P. J. & Mackler, S. A. The POZ/BTB protein NAC1 interacts with two different histone deacetylases in neuronal-like cultures. J. Neurochem. 94, 786– 793 ( 2005).Crossref, Google Scholar
58 Mahadev, K. & Vemuri, M. C. Effect of ethanol on chromatin and nonhistone nuclear proteins in rat brain. Neurochem. Res. 23, 1179– 1184 ( 1998).Crossref, Google Scholar
59 Bonsch, D., Lenz, B., Kornhuber, J. & Bleich, S. DNA hypermethylation of the alpha synuclein promoter in patients with alcoholism. Neuroreport 16, 167– 170 ( 2005).Crossref, Google Scholar
60 Kim, J. S. & Shukla, S. D. Acute in vivo effect of ethanol (binge drinking) on histone H3 modifications in rat tissues. Alcohol Alcohol. 41, 126– 132 ( 2006).Crossref, Google Scholar
61 Bailey, C. H., Kandel, E. R. & Si, K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron 44, 49– 57 ( 2004).Crossref, Google Scholar
62 Levenson, J. M. & Sweatt, J. D. Epigenetic mechanisms in memory formation. Nature Rev. Neurosci. 6, 108– 118 ( 2005).Crossref, Google Scholar
63 Alarcon, J. M. et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42, 947– 959 ( 2004).Crossref, Google Scholar
64 Korzus, E., Rosenfeld, M. G. & Mayford, M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42, 961– 972 ( 2004).Crossref, Google Scholar
65 Levenson, J. M. et al. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 279, 40545– 40559 ( 2004).Crossref, Google Scholar
66 Chwang, W. B., O'Riordan, K. J., Levenson, J. M. & Sweatt, J. D. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn. Mem. 13, 322– 328 ( 2006).Crossref, Google Scholar
67 Kim, Y. et al. Epigenetic regulation of brain function by Polycomb and Trithorax complexes. Soc. Neurosci. Abstr. 750. 5 ( 2006).Google Scholar
68 Guan, Z. et al. Integration of long-term-memoryrelated synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell 111, 483– 493 ( 2002).Crossref, Google Scholar
69 Miller, C. A. & Sweatt, J. D. Covalent modification of DNA regulates memory formation. Neuron 53, 857– 869 ( 2007). Demonstrates rapid changes in the methylation of several memory-related genes (for example, Bdnf, PP1 and reelin) in the hippocampus during contextual fear conditioning. The study, along with reference 35, provides one of the best indications so far that DNA methylation may be rapidly induced and reversed in the adult brain.Crossref, Google Scholar
70 Ausio, J., Levin, D. B., De Amorim, G. V., Bakker, S. & Macleod, P. M. Syndromes of disordered chromatin remodeling. Clin. Genet. 64, 83– 95 ( 2003).Crossref, Google Scholar
71 Zoghbi, H. Y. MeCP2 dysfunction in humans and mice. J. Child Neurol. 20, 736– 740 ( 2005).Crossref, Google Scholar
72 Amir, R. E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet. 23, 185– 188 ( 1999).Crossref, Google Scholar
73 Luikenhuis, S., Giacometti, E., Beard, C. F. & Jaenisch, R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc. Natl Acad. Sci. USA 101, 6033– 6038 ( 2004).Crossref, Google Scholar
74 Dani, V. S. et al. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc. Natl Acad. Sci. USA 102, 12560– 12565 ( 2005).Crossref, Google Scholar
75 Chang, Q., Khare, G., Dani, V., Nelson, S. & Jaenisch, R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 49, 341– 348 ( 2006).Crossref, Google Scholar
76 Gemelli, T. et al. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol. Psychiatry 59, 468– 476 ( 2006).Crossref, Google Scholar
77 Moretti, P. et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 26, 319– 327 ( 2006).Crossref, Google Scholar
78 Nelson, E. D., Kavalali, E. T. & Monteggia, L. M. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr. Biol. 16, 710– 716 ( 2006). Characterizes abnormalities in presynaptic excitatory transmission in cultured hippocampal neurons from mice lacking MeCP2. It shows that such abnormalities are not developmental in nature, but rather can be induced in adult neurons by deletion of Mecp2 and, conversely, that the consequences of early gene deletion can be corrected by inhibitors of transcription.Google Scholar
79 Chen, R. Z., Akbarian, S., Tudor, M. & Jaenisch, R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genet. 27, 327– 331 ( 2001).Crossref, Google Scholar
80 McGill, B. E. et al. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl Acad. Sci. USA 103, 18267– 18272 ( 2006).Crossref, Google Scholar
81 Guy, J., Gan, J., Selfridge, J., Cobb, S. & Bird, A. Reversal of neurological defects in a mouse model of Rett syndrome. Science 315, 1143– 1147 ( 2007). Shows that Rett-like symptoms induced in mice as a result of loss of function mutations in MeCP2 can be largely reversed upon correction of the MeCP2 deficit. This supports the notion that it may be possible to treat Rett syndrome in humans even after it has become symptomatic.Crossref, Google Scholar
82 Tremolizzo, L. et al. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc. Natl Acad. Sci. USA 99, 17095– 17100 ( 2002).Crossref, Google Scholar
83 Tamminga, C. A. & Holcomb, H. H. Phenotype of schizophrenia: a review and formulation. Mol. Psychiatry 10, 27– 39 ( 2005).Crossref, Google Scholar
84 Grayson, D. R. et al. The human reelin gene: transcription factors (+), repressors (−) and the methylation switch (+/−) in schizophrenia. Pharmacol. Ther. 111, 272– 286 ( 2006).Crossref, Google Scholar
85 Chen, Y., Sharma, R. P., Costa, R. H., Costa, E. & Grayson, D. R. On the epigenetic regulation of the human reelin promoter. Nucleic Acids Res. 30, 2930– 2939 ( 2002).Crossref, Google Scholar
86 Dong, E. et al. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc. Natl Acad. Sci. USA 102, 12578– 12583 ( 2005). Provides evidence to support their hypothesis that epigenetic abnormalities, specifically, altered methylation of the reelin and Gad67 gene promoters, contribute to the pathophysiology of schizophrenia in a mouse model.Crossref, Google Scholar
87 Antun, F. T. et al. The effects of L-methionine (without MAOI) in schizophrenia. J. Psychiatr. Res. 8, 63– 71 ( 1971).Crossref, Google Scholar
88 Tremolizzo, L. et al. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol. Psychiatry 57, 500– 509 ( 2005).Crossref, Google Scholar
89 Cervoni, N. & Szyf, M. Demethylase activity is directed by histone acetylation. J. Biol. Chem. 276, 40778– 84077 ( 2001).Crossref, Google Scholar
90 Cervoni, N., Detich, N., Seo, S. B., Chakravarti, D. & Szyf, M. The oncoprotein Set/TAF-1β, an inhibitor of histone acetyltransferase, inhibits active demethylation of DNA, integrating DNA methylation and transcriptional silencing. J. Biol. Chem. 277, 25026– 25031 ( 2002).Crossref, Google Scholar
91 Casey, D. E. et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology 28, 182– 192 ( 2003).Crossref, Google Scholar
92 Tsankov, A. M. et al. Communication between levels of transcriptional control improves robustness and adaptivity. Mol. Syst. Biol. 2, 65 ( 2006). Provides a system-level view of how transcription factors, chromatin regulators, RNA processing and nuclear transport proteins affect gene expression, revealing an elegant architecture for transcriptional control that improves the resilience and responsiveness of the eukaryotic cell.Crossref, Google Scholar
93 Hsieh, J. & Gage, F. H. Chromatin remodeling in neural development and plasticity. Curr. Opin. Cell Biol. 17, 664– 671 ( 2005).Crossref, Google Scholar
94 Ballas, N. & Mandel, G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr. Opin. Neurobiol. 15, 500– 506 ( 2005).Crossref, Google Scholar
95 Kuwabara, T., Hsieh, J., Nakashima, K., Taira, K. & Gage, F. H. A small modulatory dsRNA specifies the fate of adult neural stem cells. Cell 116, 779– 793 ( 2004).Crossref, Google Scholar
96 Marin-Husstege, M., Muggironi, M., Liu, A. & Casaccia-Bonnefil, P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J. Neurosci. 22, 10333– 10345 ( 2002).Crossref, Google Scholar
97 Fan, G. et al. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J. Neurosci. 21, 788– 797 ( 2001).Crossref, Google Scholar
98 Zhao, X. et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc. Natl Acad. Sci. USA 100, 6777– 6782 ( 2003).Crossref, Google Scholar
99 Liu, Q. R. et al. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 1067, 1– 12 ( 2006).Crossref, Google Scholar
100 Timmusk, T. et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron 10, 475– 489 ( 1993).Crossref, Google Scholar
101 Tao, X., Finkbeiner, S., Arnold, D. B., Shaywitz, A. J. & Greenberg, M. E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709– 726 ( 1998).Crossref, Google Scholar
102 Chen, W. G. et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302, 885– 889 ( 2003).Crossref, Google Scholar
103 Zhou, Z. et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 52, 255– 269 ( 2006).Crossref, Google Scholar
104 Huang, Y., Doherty, J. J. & Dingledine, R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J. Neurosci. 22, 8422– 8428 ( 2002). The first paper to demonstrate regulation of histone acetylation in the adult rat brain. The authors characterize rapid changes in acetylation at the Bdnf and Glur2 promoters in response to chemically induced seizures.Crossref, Google Scholar
105 Lim, J. H., Booker, A. B. & Fallon, J. R. Regulating fragile X gene transcription in the brain and beyond. J. Cell Physiol. 205, 170– 175 ( 2005).Crossref, Google Scholar
106 Merienne, K., Pannetier, S., Harel-Bellan, A. & Sassone-Corsi, P. Mitogen-regulated RSK2-CBP interaction controls their kinase and acetylase activities. Mol. Cell Biol. 21, 7089– 7096 ( 2001).Crossref, Google Scholar
107 Weeber, E. J., Levenson, J. M. & Sweatt, J. D. Molecular genetics of human cognition. Mol. Interv. 2, 376– 91, 339 ( 2002).Crossref, Google Scholar
108 Davies, W., Isles, A. R. & Wilkinson, L. S. Imprinted gene expression in the brain. Neurosci. Biobehav. Rev. 29, 421– 430 ( 2005).Crossref, Google Scholar
109 Vo, N. & Goodman, R. H. CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 276, 13505– 13508 ( 2001).Crossref, Google Scholar



