Ethical Considerations in Psychiatric Genetics
Abstract
Ethical considerations in psychiatric genetics are highly complex and fluid. This review introduces the reader to the wide range of ethical considerations in this field by examining four characteristics of genetic information. First, genetic information may, to a greater or lesser extent, predict a person's future health. Second, learning about one's genotype may have profound psychosocial consequences. Third, genetic information pertains to a person's biological relatives and thus can affect family members, communities, and population groups. Finally, psychiatric genetics is a rapidly evolving field. None of these characteristics is necessarily “exceptional” or unique to genetics, but they provide a useful framework for teasing apart a complex set of ethical considerations. This article reviews conceptual and empirical data that speak to these four characteristics and then presents a set of conceptual frameworks that can be used to systematically analyze the ethics of psychiatric genetic research and clinical genotyping. Finally, directions for future study are described, including the urgent need to gather data on actual risks and benefits of psychiatric genetic research and clinical applications, so that their utility can be assessed and appropriate ethical safeguards identified.
Psychiatric genetic research is a source of great hope for many individuals with mental illness, their families, and the clinicians who care for them. In 2010, the research enterprise appears poised to begin to fulfill some of those hopes. Molecular genetic variants associated with bipolar disorder, major depression, and schizophrenia have been validated in independent samples (1–5), and researchers working to find genes associated with other common complex inheritance diseases such as diabetes (6) have had clear successes. Many observers believe that psychiatric genetic research is on the same promising path (7, 8).
The long-awaited genomic revolution in psychiatry brings with it, however, a new set of ethical concerns. The field of psychiatric genetics is haunted by memories of the eugenics movement of the early 1900s, which targeted psychiatric patients and others considered “genetically inferior” for forced sterilization and death (9–12). Even strong proponents of genetic research may feel some concern that the modern “genome era” could cause some vulnerable individuals to experience discrimination and distress. Thus, great care and reflection are necessary to facilitate the beneficent aim of psychiatric genetics—reducing human suffering—while providing safeguards against potential harms (13).
Psychiatric genetics is a complex and rapidly changing endeavor, however, and it requires a detailed and dynamic ethics (14). On the most general level, psychiatric genetics describes our profession's current understanding of mental disorders as heritable and biologically mediated rather than solely the result of psychosocial influences. The field also incorporates a long history of quantitative and qualitative psychiatric genetic research, including family, twin, and adoption studies, as well as linkage analyses and association scans. Finally, psychiatric genetics describes an emerging group of clinical applications such as pharmacogenetic, diagnostic, and susceptibility genotyping. Each of these aspects of psychiatric genetics provokes numerous ethical questions—ranging from how the “geneticization” of mental illness will affect its stigma (15) to whether psychiatric genetic research should be conducted among marginalized communities to the morality of prenatal testing for susceptibility to mild and treatable mental disorders (16, 17).
This review aims to introduce the reader to the complexity and fluidity of ethical issues in psychiatric genetics. Rather than attempting a fully comprehensive study, which has been the subject of book-length monographs (13, 18), this article will focus primarily on four broad characteristics of psychiatric genetics that merit ethical attention. First, genetic information may, to a greater or lesser extent, predict a person's future health. Second, learning about one's genotype may have profound psychosocial consequences. Third, genetic information pertains to a person's biological relatives and thus can affect family members, communities, and population groups. Finally, psychiatric genetics is a rapidly evolving field. None of these characteristics is necessarily “exceptional” or unique to genetics (19, 20), but they will provide a useful structure for teasing apart a complex set of ethical issues.
After a review of empirical literature that speaks to these four characteristics of psychiatric genetics, this review will present two conceptual frameworks that can be used to systematically analyze the ethics of psychiatric genetic research and clinical genotyping. Throughout this article, the emphasis will be on ethical considerations arising from today's technology, rather than the morality of less immediate applications such as new diagnostic classification systems or highly speculative applications such as germ-line manipulation through genetic engineering. Because most of the literature in this field has centered on clinical genetic testing and because such tests are now becoming available, genetic testing will be a major focus of the article. Similarly, because the great burden of psychiatric illness is due to common mental illnesses with complex inheritance, such as schizophrenia, bipolar disorder, depression, attention-deficit disorder, and autism, the emphasis here will be on these diseases rather than the rare, Mendelian illnesses such as Huntington's disease (HD) or Rett's disorder. The related topic of human behavioral genetics, which has been well described elsewhere (21, 22), will not be addressed. Finally, because in 2010, the ethics of psychiatric genetics provokes far more questions than answers, this article will conclude by identifying several topics for future research.
GENETICS AS A PREDICTOR OF FUTURE HEALTH
Since the 1980s, molecular genetics researchers have found more than 1,000 mutations associated with highly penetrant genetic disorders such as HD, cystic fibrosis, and muscular dystrophy (7). These triumphs of gene finding have been followed by the availability of clinical genetic testing to detect carrier status and to predict whether at-risk individuals actually develop disease. During the early days of gene-finding research, media reporters, experts, and public figures tended to describe DNA in awe-inspiring, even mystical terms, for example, as the “code of life,” the “book of life,” “central to our core” (20), “uniquely powerful and uniquely personal” (23), “the language in which God created man” (24), and a “future diary” (23) that could foretell the rest of one's life. Such language suggests that there will be tremendous benefits to learning genetic information, and great harm if the privacy of one's genetic “future diary” were violated.
This deterministic view of genetics formed the backdrop for early conceptual and empirical ethics studies. Investigations of highly penetrant disorders enabled a cataloging of risks and benefits of predictive genetic testing in the research and clinical context (25). These risks and benefits can be categorized as medical (i.e., interventions to reduce the impact of the illness or biological side effects of interventions) and psychosocial (i.e., peace of mind, life planning, psychological distress, social stigmatization, and discrimination) (13, 26–28). Patient-centered safeguards, such as confidentiality protections, careful attention to the informed consent process, and genetic counseling, were put in place to protect individuals against potential harms and to enhance autonomous decision making (13). Thorny ethical issues arising in specific testing contexts were identified and described, such as the person with a known predisposition to HD who refuses to disclose this information to family members (29, 30), parents who seek to have minor children tested for adult-onset conditions with no preventive treatment (31–33), or pregnant couples wishing to obtain prenatal testing and abortion for a mild genetic disability that does not preclude a satisfactory quality of life (16, 17).
Highly penetrant disorders are rare in the overall population, however, and they are not necessarily good models for understanding either the genetics or the “genethics” of the vast burden of human illnesses (26) including the major psychiatric diseases, which have complex inheritance. Complex inheritance disorders, sometimes called multifactorial illnesses, are caused by a combination of at least two and perhaps hundreds of genes and nongenetic factors such as environmental and epigenetic influences (i.e., patterns of DNA methylation and histone modification) (34). It now appears that multiple genes are involved in the etiology of most mental disorders, each gene contributing a small amount of increased risk (7).
Unlike genetic tests for highly penetrant disorders, tests for individual genetic variants associated with most cases of psychiatric disorders have been expected to have low predictive power (an absolute increase or decrease in risk on the order of a few percentage points), and the risk variants have been expected to be common in the population (7). Eventually, it may be possible to test for a combination of susceptibility variants to achieve much greater predictive power (35), although genotyping alone will never provide results with 100% power because of the contributions of environment and epigenetic factors.
The lack of certainty associated with susceptibility genotyping is likely to engender a number of ethical dilemmas. For example, in the future it may be possible to use genotyping in the research context to help identify individuals who are at increased risk of developing mental disorders. Such individuals may then be enrolled into protocols designed to test interventions to prevent or ameliorate disease. The potential benefits of this line of research are tremendous, but they must be balanced against the risks of genetic labeling. For example, a child who is considered to be at elevated risk for schizophrenia may be viewed negatively by others, and the effects of this knowledge on the developing sense of self may be devastating. More well-known risks include the harms associated with any interventions provided to high-risk individuals (36–38). Will it be ethical, for example, to conduct studies in which antipsychotic medications are administered to adolescents whose genotype suggests that they have a 20% risk of schizophrenia, given that the majority of those treated will incur unnecessary biological and psychosocial risks? What if the likelihood of illness is slightly higher or lower, the disorder more or less stigmatized, or the intervention more or less benign?
Regarding the clinical use of susceptibility genotyping, it has been argued that low predictive power will prevent such tests from ever being viable (39). It appears, however, that commercial interests may propel psychiatric susceptibility testing onto the market in advance of empirical evidence of clinical utility. In 2007, for example, a diagnostic genetic test for bipolar disorder based on variants in the GRK3 gene became available from an Internet-based laboratory (40). Results of this test were to provide risk estimates for bipolar disorder of 2% or 3% (versus 1% at baseline). Other psychiatric genetic tests have been proposed as well (41).
The ethical implications of the commercialization of psychiatric genotyping will be discussed later in this article. For now, we will consider difficulties particular to working with probabilistic tests, such as the hurdles that must be overcome to ensure informed consent and to avoid misinterpretation of tests. A growing literature suggests that patients' interpretation of risk estimates is extraordinarily complex (42–46) influenced by cognitive, affective, and cultural factors. We know very little about how individuals will understand and act upon the results of tests that provide subtle information about the risk of highly stigmatized disorders. Furthermore, moral questions arise about what level of absolute risk increase is sufficient for specific applications, such as prenatal testing or clinical susceptibility testing to aid life planning.
Existing empirical evidence sheds some light on one aspect of this issue: how the probabilistic nature of genetic information may affect consumer interest in genetic testing. A number of investigators have surveyed patients and families about their attitudes toward genetic testing for psychiatric illnesses and have generally found keen interest in clinical genotyping (47–59) Early survey instruments tended to present psychiatric genetics in highly deterministic terms, but later work has emphasized the probabilistic nature of genetic testing. If we consider only the studies that specifically mentioned that psychiatric susceptibility testing might not be 100% predictive, the data still demonstrate that patients and families see a substantial benefit to genetic testing for the purposes of life planning and early diagnosis (49, 50, 53, 55, 56, 58). Patients and families appear to be more focused on the perceived benefits of testing and less focused on the risks than clinicians or researchers (56). This disparity may be the result of the layperson's lack of awareness of the risks of genetic testing. It may also be that individuals who have intimate experience in living with mental illness are more attuned to the benefits of information that could guide treatment or future plans.
Another possibility is that consumers' interest in genetic information is related to an exaggerated sense of the risk of recurrence of mental disorders. Evidence of such an association was provided by Austin et al. (57) in a web-based survey of 116 family members of individuals with mental illnesses who were identified through a consumer web site regarding psychosis. Most respondents incorrectly estimated the risk for psychosis in the sibling of an affected person, with 45% overestimating the risk and 8% underestimating the risk. The investigators found that the overestimation of risk was associated with more positive views toward predictive genetic testing in general and specifically during pregnancy. This finding is consistent with the existing literature on prenatal testing for other disorders, whose uptake appears more dependent upon individuals' perception of risk than actual risk (60). It is also consistent with studies of interest in testing for genetic susceptibility to breast and ovarian cancer (61).
PSYCHOSOCIAL RISKS AND BENEFITS OF GENETIC INFORMATION
The ethical principles of beneficence and nonmaleficence suggest that careful attention must be paid to the psychosocial effects of psychiatric genetics to maximize the personal and social benefits and to minimize the risks. Understanding these risks and benefits is also necessary to fulfill the ethical duty of respect for persons, which requires researchers and clinicians to provide information about the likely results of medical interventions to obtain fully informed consent. Finally, justice requires consideration of the potential social harms that may befall individuals and groups as a result of their participation in clinical and research activities.
In theory, psychosocial effects may be especially strong in the case of psychiatric disorders. Mental illnesses affect emotions, cognitions, and behavior; as such, they may seem more inextricably bound to one's personhood than disorders that are exclusively somatic. Given the pervasive stigma of mental illness, people who are thought to be susceptible to these disorders may be more vulnerable to discrimination and stigmatization. Furthermore, because many psychiatric disorders begin in young adulthood, genetic information about disease susceptibility may be obtained relatively early in life, with unknown results on an individual's developing sense of self and future prospects. The sections below will consider empirical evidence regarding the likely quality and magnitude of three types of psychosocial effects in psychiatric genetics—psychological consequences, insurance and employment discrimination, and social stigmatization.
Psychological consequences
Learning about genetic information related to future health may provoke intense emotions (28) ranging from relief, an enhanced sense of well-being, reduced uncertainty, and improved ability to focus on future planning, at one extreme, to anxiety, embarrassment, depression, and hopelessness, at the other. Negative results on a probabilistic test may result in a false sense of security that one is free of all risk of illness, whereas positive results may cause “genetic hypochondria” (27), if individuals become obsessively watchful for the first manifestations of a disease that may never come.
The reviews of research assessing the psychological impact of genetic testing by Lerman et al. (28) and Wiggins et al. (62) suggest that overall psychological risks may be mild, however, at least for testing that occurs in the context of a research trial. However, specific subsets of individuals appear especially vulnerable to negative psychological consequences of predictive or susceptibility testing for HD or cancer. Pretest symptoms of distress were a better predictor of posttest distress than genetic test results among 160 individuals tested for HD (63). Among 65 persons undergoing genetic testing for cancer susceptibility, participants who underestimated the emotional effects of testing were more likely to report feeling distressed afterward (64). Differences in ability to tolerate uncertainty and ambiguity may also have an important effect on posttest well-being (28).
It is not known whether susceptibility testing for common mental disorders will have special psychological consequences beyond what has been seen with genetic testing for other disorders. Perhaps the most relevant empirical data regarding the psychological effects of susceptibility testing for a common psychiatric disorder have been gathered by an ongoing multisite study called Risk Evaluation and Education for Alzheimer's Disease (REVEAL) (65–68). In this randomized, controlled trial, individuals with a family history of Alzheimer's disease (AD) received genetic counseling regarding their personal AD risk based either on their age, sex, family history, and apolipoprotein E (APOE) genotype (intervention arm) or on their age, sex, and family history alone (control arm).
A preliminary analysis of data for 162 participants found no differences in depression or anxiety among the participants at 6 weeks, 6 months, and 1 year postintervention, and no individuals met the criteria for clinical depression or anxiety diagnoses (65). As a more targeted measure of the psychological sequelae of genotyping, researchers compared survey responses of a subset of 30 women in the genotype group and 36 women in the control group, all of whom were determined to have a 29% lifetime risk of AD. Even though all the women had the same absolute risk of disease, those in the genotype group (none of whom had an APOE e4 risk allele) perceived their risk of AD as lower, had decreased anxiety about developing AD, and had a more positive attitude toward the risk assessment (67).
These data suggest that learning one has a “good” or “bad” genotype may have a more profound psychological impact than learning one's absolute risk of illness. Consistent with this notion was a finding that participants in the intervention arm were better able to recall their genotype than their absolute risk assessment at 1 year after the intervention (65).
Genetic discrimination
For more than a decade, concerns have been mounting about the potential misuse of genetic data to discriminate against individuals in insurance and employment. A survey published in Science in 1996 described the incidence of discrimination among 332 individuals who belonged to genetics support groups (69). Substantial minorities believed that they or their family members had been denied life insurance (25%), health insurance (22%), or a job (13%) because of a genetic disorder. The findings raised concerns that fears of genetic discrimination may cause many individuals to refuse participation in genetic research protocols or to forgo clinical genetic testing, creating barriers to the clinical translation of research (70).
There is some evidence that persons with genetic propensities to psychiatric disorders are especially likely to become targets of discriminatory practices. In a survey of 62 workers by Roberts et al. (71), respondents felt that personal genetic information showing a moderate risk of disease would be more likely to cause employment discrimination if the disease were mental rather than physical. In a questionnaire survey of 45 individuals with bipolar disorder and their spouses, Trippitelli et al. (50) found that they most often mentioned insurance discrimination as a risk associated with genetic testing for that illness. Finally, a disturbing case series published in 2001 documented employment discrimination against three young men in Hong Kong who had applied for civil service jobs and were rejected or fired on the basis of a first-degree family history of schizophrenia (72). However, a 2004 review of the work of 50 researchers studying genetic influences on substance abuse and on psychiatric disorders found no adverse effects such as misuse of data or loss of confidentiality (unpublished data of J. F. Cubells et al. 2004).
As a response to the perceived injustice of discrimination based on genotype, most states have enacted some form of antidiscrimination legislation, and a federal law, the Genetic Information Nondiscrimination Act, was enacted in 2008 (73). Such legislation appears to have strong support among the psychiatric community. In a survey of a probability sample of U.S. psychiatrists (N=45) by Hoop et al. (59), 100% of the sample expressed the belief that genetic test results should be confidential, and 96%–98% agreed that results should not be used in insurance or employment decisions. Similarly, in the survey of Laegsgaard and Mors (58) of 397 Danish psychiatric patients, 164 relatives, and 100 medical or psychology students, majorities of all three groups endorsed statements that genetic test results should be kept confidential and not shared with employers or insurance companies.
Relevant to this discussion are the concepts of “moral hazard” and “asymmetric information”—that is, the notion that individuals may be more likely to purchase insurance if they know they have a genetic predisposition to illness (74). In theory, denying the same information to insurance providers would place insurers at a financial disadvantage because they could not adjust premiums to account for different levels of genetic risk. Furthermore, without the ability to make such adjustments, insurers could not charge lower premiums for individuals who have beneficial genotypes. Ironically, this inability to adjust premiums could unfairly burden people who have a strong family history of illness but for whom genetic testing indicates reduced personal risk (75).
Empirical data on this issue are limited, though the REVEAL study (described in the previous section) assessed insurance-purchasing behaviors after participants were informed of their risk of illness. Participants who learned they had one or two copies of the risk allele for Alzheimer's disease were 5.76 times more likely then those who were not told their risk status to report having changed long-term care insurance coverage at 1 year after the intervention. There were no other differences, however, in behaviors regarding life insurance or other forms of health insurance (76).
Stigma and the “geneticization” of mental illness
Many patients, families, and clinicians have expressed hope that the geneticization of mental illness will decrease its social stigma by demonstrating that these disorders are biologically mediated and that individuals should not be blamed for their illnesses. These ideas are demonstrated in public health campaigns suggesting that psychiatric disorders are “illness[es] like any other[s]” and in advocacy groups' definitions of mental disorders as “brain diseases” (77). Some investigators, however, have suggested that despite potentially salutary effects on blaming, the geneticization of psychiatric disorders will worsen stigma by making the diagnostic label of mental illness “stickier” (15). That is, the diagnosis will appear more credible if it is based on a biological test rather than a clinical interview and more permanent if the illness arises from one's DNA. Patients may also be saddled with an additional label of “genetic deviance” in addition to being categorized as mentally ill (78).
Attempts to investigate these issues empirically have produced conflicting results (15, 77). A vignette survey of 126 New Zealand undergraduates demonstrated that students who were given a biological and genetic explanation for schizophrenia described a person with schizophrenia as more dangerous and unpredictable than did students who were given a psychosocial explanation for the illness (79). An exploratory study of patients with bipolar disorder and their family members in Australia found that most believed that the genetic etiology of bipolar disorder would decrease stigma by absolving individuals of responsibility for the illness (55).
One of the most well-designed and generalizable studies on this topic was Phelan's (8) national telephone survey of Americans' stigmatizing beliefs, attitudes, and behaviors. A random sample of 426 U.S. adults was asked to respond to a vignette about a person hospitalized for schizophrenia or depression whose illness was described as completely, partially, or not at all due to genetic factors. Participants were then asked a series of questions about how they would feel and behave toward such a person. Analysis of the responses indicated that the genetic explanation for illness led to a significant reduction in respondents' desire to see the person punished for a violent act committed as a result of the illness. Interestingly, the genetic explanation also increased respondents' desire for social distance from the ill person's sibling. The findings thus indicate that the geneticization of mental illness may decrease some aspects of stigma (punitive attitudes toward ill persons) and increase others (associative stigma toward family members).
EFFECTS ON RELATIVES, COMMUNITIES, AND POPULATIONS
By its nature, genetic information has implications and consequences for third parties: biological relatives. This phenomenon is not unique to genetics; for example, information about infectious diseases also applies to a patient's contacts (19, 20). As with infectious diseases, the impact of genetic information on third parties can present an ethical dilemma between the need to protect an individual's confidentiality and the third party's “right to know” information that may bear on his or her health. This conflict was the central question in a New Jersey court case brought by a woman with an autosomal dominant form of colon cancer, whose father had died of the same disease. The father's physician had not informed the patient or his family that the illness was heritable and could be prevented with colectomy. The New Jersey Superior Court ruled in favor of the plaintiff, finding that a physician had a “duty to warn” those at risk of avoidable harm due to genetic conditions (81).
The flip side of this situation is the family member who does not wish to know his or her genotype, but who will be deprived of the “right not to know” if a relative is tested. This conflict may occur in certain unusual clinical situations, for example, when an adult grandchild of a person with a highly penetrant genetic disorder wishes to have predictive testing, but the grandchild's parent does not. Such ethical dilemmas are not likely to become commonplace, however, in psychiatric practice. The complex inheritance of mental disorders means that susceptibility testing for these illnesses will provide less directly pertinent information about risks to relatives than testing for Mendelian disorders with clear autosomal dominant, recessive, or X-linked patterns of inheritance.
More far-reaching ethical issues arise because genetic information may also be used to characterize larger groups of related persons, including communities and populations. There are public health benefits to conducting molecular genetic research on complex inheritance diseases using samples from ethnically and geographically diverse populations, as well as scientific benefits to using samples derived from genetically isolated populations (82). Such groups include the Hutterites, Old Order Amish, Ashkenazi Jews, Pima Native Americans, and isolated subpopulations in Colombia, Costa Rica, Finland, Guatemala, Iceland, Japan, and Sardinia.
Molecular research that compares frequencies of genetic variants associated with stigmatized disorders among subpopulations may unintentionally feed racist beliefs if results are misinterpreted to mean that a given group is especially prone to the disease (82). Growing concerns about group stigmatization based on genetic research have led some minorities to contemplate “opting out of genetic research until it's clear we're not going to use science to validate prejudices” (83). Indeed, in April 2010 Arizona's Havasupai Native American tribe won a $700,000 settlement with Arizona State University because university researchers had used the tribal members' DNA to study ancestry and mental illnesses such as schizophrenia. Although research participants had signed consent forms, many believed the study would be limited to diabetes and found the other research topics stigmatizing or culturally objectionable. As part of the settlement, blood samples were returned to the tribe (84).
The possibility of population-based stigma and discrimination represents a significant burden on groups being studied, which must be balanced by benefits to the same community from the very same research (14). To some bioethicists, the emerging importance of this issue has suggested a need to reconceptualize the dominant ethical considerations in genetic research from autonomy-based ethics to a more communitarian ethics that focuses on the risks and benefits of research for communities and populations rather than for individual participants (14).
GENETICS AND THE ETHICS OF EMERGING TECHNOLOGIES
Psychiatric genetics, as a body of scientific knowledge and a set of clinical activities, is far from being fully mature. There is scientific consensus that the common mental disorders are heritable and that a number of genetic variants are likely to be involved in most illnesses (7), but there is no firm consensus about which specific genes are associated with which mental illnesses, with the exception of APOE and AD (34). In the clinical setting, genetic testing for susceptibility and for drug metabolism and response is just beginning to be applied in psychiatry, but we have few or no data to guide us about their medical and psychosocial outcomes. In the future, it should be possible to make carefully reasoned judgments about the utility and morality of specific types of psychiatric genetic activities, but until then we will also grapple with ethical issues particular to an innovative and evolving technology.
Methodologies for psychiatric genetic research have changed dramatically in recent years, the result of sequencing the human genome and completing the International HapMap project to characterize human variation across the genome (85) along with technological advances that enable rapid and relatively inexpensive genotyping. Genetic protocols are becoming grander in scale (e.g., studies of genetic variation among populations of entire countries), in longitudinal sweep (protocols in which DNA is banked for future study), and in the complexity of information that they gather (genotypic data that are linked with phenotypic data, including complex and continually updated medical records). The rapid development of the “genome era” of medical research has ushered in new ethical concerns (14, 86–88). Today's investigators and research participants are unlikely to anticipate all future research uses of genetic material in stored samples, for example, and there is as yet no firm consensus about whether and how informed consent should be obtained for future projects (89–95). There is also a lack of agreement concerning measures to protect confidentiality of study data while allowing multiple investigators access to genotypic information linked to medical records or other phenotypic data (86–89). Institutional review boards may therefore lack consistent methods for evaluating informed consent forms, confidentiality protections, and other protocol elements (96).
Another set of ethical issues concerns whether and how new applications should be introduced clinically. It will require considerable research funding and many years to gather detailed information about the psychosocial and medical outcomes of genetic counseling and testing for specific psychiatric disorders. Even our understanding of the full implications of testing for a single genetic variant may be incomplete for many years. Because of the phenomenon of genetic pleiotropy (i.e., when a single gene has multiple biological effects), a test for a genetic variant that is associated with one condition today may in the future be found to yield information about susceptibility to another condition (97). For example, the APOE genotype is associated not only with the risk of AD but also with the risk of cardiovascular disease (98). Another example is the serotonin transporter gene, which is associated with response to selective serotonin reuptake inhibitors (99). Pharmacogenetic testing for variants of this gene is now clinically available as an aid in selecting an antidepressant treatment (100). The serotonin transporter gene has also been associated, however, with a large number of psychiatric syndromes. In the future, information that had been sought solely for pharmacogenetic purposes may yield unwanted and unanticipated information about the risk of other conditions (97).
A third ethical issue that derives from the newness of the psychiatric genome era is that it is unclear who should bear ethical and legal responsibility for its clinical applications. On one hand, most psychiatrists are not trained in genetic counseling and testing (101–103) and do not seem prepared to provide these services (54, 101, 102). On the other hand, the current number of certified genetic counselors and fellowship-trained clinical geneticists is likely to be too small to meet the projected need for future counseling and testing (104). Furthermore, the genetics professionals may not feel adequately trained to work with psychiatric patients (105). Increased education and training in psychiatric genetics appears necessary for both groups. A multidisciplinary approach to clinical care, involving both psychiatrists and genetic counselors, has also been suggested (103).
It is possible that the emerging phenomenon of direct-to-consumer (DTC) genetic testing via Internet laboratories may move psychiatric testing out of the clinic entirely (106–108). DTC genetic testing is now available for a variety of purposes—paternity testing, ancestry tracing, pharmacogenetic testing, and even whole-genome single nucleotide polymorphism genotyping to “help you read and understand your DNA.” (109)
DTC genetic testing is legal in many states, and there is little federal oversight, although professional groups such as the American Society for Human Genetics have raised many concerns (110). The rapid—and virtually unregulated—commercialization of genetic testing is worrisome, evoking memories of the naive treatment of fluoroscopy in the last century, when shoe stores across the United States offered “X-ray shoe fitters” as novelty devices that exposed countless individuals to significant levels of radiation (111, 112). There are few published data specifically regarding the ethical issues of DTC genetic testing in psychiatry, with the exception of the questionnaire survey of a small random sample of U.S. psychiatrists of Hoop et al. (59), in which 100% agreed that laws and regulations should require DTC advertising for genetic testing to be truthful and to describe risks as well as benefits, and 96% agreed that there should be restrictions on the DTC sale of psychiatric genetic testing kits.
Nevertheless, DTC genetic testing may offer some benefits, particularly for stigmatized illnesses, because individuals seeking testing may place a very high premium on privacy. The precedent of HIV testing may be relevant in this context. An over-the-counter kit for HIV testing is currently available, and in-home HIV testing has been proposed as a way to make such testing more anonymous, thereby decreasing its stigma, increasing the empowerment of health care consumers, and increasing the use of HIV tests among young people (113). An important difference between HIV testing and susceptibility testing for psychiatric illness, however, is that HIV-testing kits have high positive and negative predictive power, indicating less need to be concerned about the misinterpretation of test results. Another difference is the public health importance of knowing one's HIV status, enabling people to benefit from available treatment and to take steps to avoid infecting others; there are no similar benefits to psychiatric genetic testing at this time.
Finally, new and innovative technologies tend to be costly, which raises the ethical issue of distributive justice and the equitable distribution of social benefits and burdens. If clinical genotyping proves efficacious, it is unclear what the impact will be on existing health care disparities in the United States (114).
DISCUSSION
Analyzing ethical considerations in psychiatric genetic research and testing
As we have seen, psychiatric genetics provokes a multiplicity of ethical considerations deriving from the complex relationship between genetic information and a person's future mental health, the potential psychosocial impact of genetic information and effects on third parties, and the newness of molecular genetics technology in psychiatry and the uncertainty surrounding its future applications. Although many questions about the impact of these factors remain, the factors themselves can be incorporated into useful frameworks for analyzing the ethics of psychiatric genetic research protocols and clinical activities.
Ethically relevant domains in medical and psychiatric research have been described by several ethicists. Emanuel et al. (115) identified seven features of ethical clinical research: value to society, scientific validity, favorable risk-benefit ratio, fairness in subject selection, informed consent, demonstration of respect for enrolled subjects, and independent review. Roberts (116) developed a framework for analyzing the ethical acceptability of psychiatric research protocols, identifying nine key features: scientific merit and design issues; expertise, commitment, and integrity issues; risks and benefits; confidentiality; participant selection and recruitment; informed consent and decisional capacity; and incentives and other issues. A modification of Roberts's framework (Table 1) (116) that includes ethical considerations particular to genetics provides a useful model for analyzing psychiatric genetic research protocols, particularly gene-finding studies.
 |
Table 1. Ten Domains for Assessing the Ethical Acceptability of Psychiatric Genetic Research Protocols
A strategy for categorizing ethical considerations in clinical genotyping was proposed by Burke et al. (26) in 2001 (Table 2). Using current clinical practice as a descriptive guide, these authors categorized ethical considerations on the basis of the predictive power of a genetic test and the availability and acceptability of medical treatments for the condition (26). According to this framework, we would expect that the first susceptibility tests for most common mental illnesses would be categorized as low in predictive power and as lacking acceptable medical interventions, because of a dearth of evidence regarding the utility of preventive interventions. For such tests, according to the framework of Burke et al, the dominant ethical consideration would be nonmaleficence—that is, to do no harm by withholding the test until it could be established that an individual would have a favorable risk/benefit ratio.
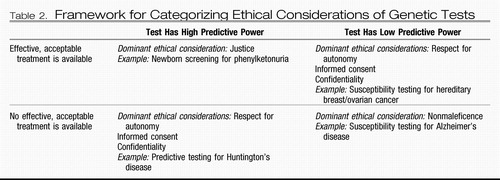 |
Table 2. Framework for Categorizing Ethical Considerations of Genetic Tests
Other experts (20) have suggested that such a system should incorporate not only the medical benefits, but also the possibility of psychosocial risks—which seems particularly apt in the case of psychiatric genetics. A more detailed framework would encompass all these elements plus factor in the newness of the test, the testing circumstance (i.e., academic setting, private clinic, DTC), and the test's purpose (i.e., susceptibility, diagnostic, pharmacogenetic, prenatal, newborn screening). Thus, tests would be categorized according to their predictive power, purposes, availability of acceptable interventions, level of stigma of the condition, likely psychological impact of the condition, possibility of negative effects on third parties, availability of safeguards, and newness of the test (Table 3). This cluster of factors is dependent upon the test, the point in time, the disorder, the testing circumstances, and the characteristics of the individual being tested, the last of which may affect the predictive power of the test, the likelihood that third parties will be affected, and the vulnerability to psychosocial harms.
 |
Table 3. Seven Domains for Assessing the Ethical Acceptability of Clinical Genetic Testing
According to this method of analysis, a newly developed DTC susceptibility genetic test that is poorly predictive and for a condition that lacks intervention and is highly stigmatized should raise many more red flags than an established, highly predictive diagnostic test offered by well-trained clinicians for a condition that is less stigmatized and for which preventive treatment is available. Some might say that the former test should be withheld entirely on the principle of nonmaleficence, whereas others might believe it overly paternalistic to do so if informed consent procedures could be established to ensure that individuals being tested fully understood and accepted the implications.
The ethics of clinical innovation can also provide some guidance regarding the wise use of the first applications of psychiatric genetics. A medical practice falls into the category of “clinical innovation” if its intention is primarily to help individual patients; it is considered “clinical research” if it is conducted systematically to gather generalizable knowledge. Roberts and colleagues (117) have proposed that clinical innovation in psychiatry is ethically justifiable if the following conditions are met: 1) scientific evidence suggests that the innovation is uniquely beneficial; 2) there is a clinical need for the innovative treatment; 3) the possible risks of the intervention do not exceed the risks of the individual's current condition; 4) all standard efforts have been tried and failed; and 5) appropriate safeguards (such as informed consent, monitoring for adverse effects, and reevaluating the scientific evidence) are implemented. If all five conditions are met, the practice may be justified on the basis of the principles of beneficence, nonmaleficence, and respect for persons.
In application of this model to psychiatric genetic testing, it is possible that some innovative uses of pharmacogenetic and diagnostic testing could meet all five criteria relatively quickly if appropriate safeguards are established. Of prime importance are confidentiality protections and informed consent processes that address, among other considerations, the possibility of secondary information arising in the future due to genetic pleiotropy. The innovative use of clinical susceptibility testing may be more difficult to justify ethically: in the absence of substantial new knowledge about outcomes, it may be difficult to prove either that there are unique benefits (beyond genetic risk assessment based on family history) or that the possible risks of susceptibility testing do not exceed the risks of forgoing testing.
Directions for future research
As we have seen, most of the published data that are directly pertinent to psychiatric genetics ethics concern clinical, rather than research, ethics, and most of these studies consist of questionnaire surveys of patients, families, and clinicians regarding interest in genetic testing. Most surveys polled small convenience samples, which limits the generalizability of their results. Some early studies assessed opinions about scenarios that now appear moot, such as 100% predictive genetic testing for a common psychiatric disorder or in utero gene therapy to prevent such an illness. Meanwhile, attitudes toward the most feasible form of psychiatric genetic testing—pharmacogenetic—have only rarely been assessed. Investigators in this field have obviously been chasing a moving target over the past two decades.
Despite the limitations of existing studies, their findings are consistent. The clear message is that psychiatric patients and family members are keenly interested in the clinical applications of psychiatric genetics, a message requiring that we work carefully to identify and assess the benefits, as well as the risks, of these new technologies.
The REVEAL study (65) of susceptibility testing for Alzheimer's disease demonstrates that ethically important considerations can be assessed using the gold standard of protocol designs, the randomized controlled trial. The REVEAL study also suggests that the psychological risks of susceptibility testing may be lower, and the benefits higher, than previously expected, at least for highly educated individuals who have a family history of illness and in the context of a comprehensive program that includes pre- and posttest genetic counseling and careful attention to informed consent. As new clinical applications of psychiatric genetics become feasible, it should be possible to design prospective evaluations of the outcomes of psychiatric genetic counseling and testing, following the model of the REVEAL study. Such investigations could complement traditional “empirical ethics research” methods, such as focus groups, key informant interviews, and questionnaire surveys of stakeholders.
Protocols designed to gather empirical data regarding the following questions are urgently needed to ground future conceptual ethical work, provide a scientific foundation for ethical and legal guidelines, and ensure that the clinical translation of psychiatric genetic research engenders the public trust:
| •. | What are the actual risks and benefits of clinical psychiatric genotyping? How do those risks and benefits vary, if at all, for different psychiatric disorders, for different testing scenarios (i.e., pharmacogenetic, diagnostic, susceptibility), for different populations (i.e., prenatal testing; newborn screening; testing children, adoptees, decisionally incapable adults), and in different testing circumstances (i.e., private clinic, academic center, direct-to-consumer)? | ||||
| •. | What are the actual risks and benefits of clinical psychiatric genetic counseling for various indications and populations? | ||||
| •. | How can informed consent be best achieved, given the probabilistic nature of test results and the possibility of secondary information arising in the future? | ||||
| •. | What safeguards, such as confidentiality protections and pre- and posttest counseling, are necessary to protect individuals from psychosocial harms? | ||||
| •. | What is the appropriate role of psychiatrists and other clinicians in providing genetic counseling and testing? | ||||
As we have seen, the empirical evidence base on ethically relevant considerations in psychiatric genetics is almost exclusively focused on clinical, rather than research, ethics. The following questions (among others) need to be addressed regarding the ethics of psychiatric genetic research:
| •. | How can research benefits be maximized and risks minimized for individuals, populations, and communities that participate in genetic studies of psychiatric disorders, given the social stigma of these conditions? | ||||
| •. | Should research participants be informed of the results of genotyping? If so, under what circumstances, and with what safeguards? | ||||
| •. | How is fully informed consent best achieved for psychiatric genetic studies involving the storage of DNA for future genotyping? | ||||
| •. | What confidentiality protections are necessary to protect research data that may involve individuals' genotypes and also their entire medical records, including psychiatric histories? | ||||
| •. | What safeguards are required for psychiatric genetic research involving children and adults without decisional capacity, as well as other vulnerable populations? | ||||
CONCLUSION
This article has attempted to highlight the diversity and fluidity of ethical concerns in psychiatric genetics and to identify areas in most urgent need of research. It is clear that the study of genetics/ethics, like the study of genetics itself, has moved further and further away from a reductionist stance and is now engaged in the process of uncovering complexity. The wide variety of psychiatric genetics activities described here cannot be squeezed into a “one size fits all” ethics.
During the past few decades, psychiatric genetics (like many emerging technologies) (118) has tended to provoke passionate debate and polarized views. Genetics is often described as either a boon or a scourge. These perspectives may become more entrenched if the early commercialization of psychiatric genetic testing becomes more widespread and especially if it results in demonstrable harm. It is to be hoped that that will not occur and that geneticists, social scientists, bioethicists, and clinicians will be challenged, instead, to look for new ways to collaborate and to learn more, in turn, about the true risks and the benefits of the genome era in psychiatry. Respectful dialogue among individuals with varied perspectives is no doubt the best way to begin to understand the complex interplay of biology, medicine, psychology, sociology, and ethics in psychiatric genetics.
1 Detera-Wadleigh SD, McMahon FJ: G72/G30 in schizophrenia and bipolar disorder: review and meta-analysis. Biol Psychiatry 2006; 60: 106– 114Crossref, Google Scholar
2 asky-Su JA, Faraone SV, Glatt SJ, Tsuang MT: Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet B Neuropsychiatr Genet 2005; 133B: 110– 115Crossref, Google Scholar
3 Li D, Collier DA, He L: Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet 2006; 15: 1995– 2002Crossref, Google Scholar
4 Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN: Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet 2003; 33: 177– 182Crossref, Google Scholar
5 Munafò MR, Thiselton DL, Clark TG, Flint J: Association of the NRG1 gene and schizophrenia: a meta-analysis. Mol Psychiatry 2006; 11: 539– 546Crossref, Google Scholar
6 Owen KR, McCarthy MI: Genetics of type 2 diabetes. Curr Opin Genet Dev 2007; 17: 239– 244Crossref, Google Scholar
7 Insel TR, Collins FS: Psychiatry in the genomics era. Am J Psychiatry 2003; 160: 616– 620Crossref, Google Scholar
8 Schulze TG, McMahon FJ: Genetic linkage and association studies in bipolar affective disorder: a time for optimism. Am J Med Genet C Semin Med Genet 2003; 123C: 36– 47Crossref, Google Scholar
9 Propping P: The biography of psychiatric genetics: from early achievements to historical burden, from an anxious society to critical geneticists. Am J Med Genet B Neuropsychiatr Genet 2005; 136B: 2– 7Crossref, Google Scholar
10 Selden S: Transforming Better Babies into Fitter Families: archival resources and the history of American eugenics movement, 1908–1930. Proc Am Philos Soc 2005; 149: 199– 225Google Scholar
11 Gejman PV, Weilbaecher A: History of the eugenic movement. Isr J Psychiatry Relat Sci 2002; 39: 217– 231Google Scholar
12 Gottesman II, Bertelsen A: Legacy of German psychiatric genetics: hindsight is always 20/20. Am J Med Genet 1996; 67: 317– 322Crossref, Google Scholar
13 Nuffield Council on Bioethics: Mental Disorders and Genetics: The Ethical Context. London, Nuffield Council on Bioethics, 1998, p 117Google Scholar
14 Knoppers BM, Chadwick R: Human genetic research: emerging trends in ethics. Nat Rev Genet 2005; 6: 75– 79Crossref, Google Scholar
15 Phelan JC: Genetic bases of mental illness—a cure for stigma? Trends Neurosci 2002; 25: 430– 431Crossref, Google Scholar
16 Mahowald MB: Prenatal testing for selection against disabilities. Camb Q Healthc Ethics 2007; 16: 457– 462; discussion 468–482Crossref, Google Scholar
17 Chipman P: The moral implications of prenatal genetic testing. Penn Bioeth J 2006; 2( 2): 13– 16Google Scholar
18 Sram RJ, Bulyzhenkov V, Prilipko L: Ethical Issues of Molecular Genetics in Psychiatry. New York, Springer-Verlag, 1991, p 177Google Scholar
19 Ross LF: Genetic exceptionalism vs. paradigm shift: lessons from HIV. J Law Med Ethics 2001; 29: 141– 148Crossref, Google Scholar
20 Green MJ, Botkin JR: “Genetic exceptionalism” in medicine: clarifying the differences between genetic and nongenetic tests. Ann Intern Med 2003; 138: 571– 575Crossref, Google Scholar
21 Farmer A, McGuffin P: Ethics and psychiatric genetics, in Psychiatric Ethics, 3rd ed. Edited by Bloch S, Chodoff P, Green SA. New York, Oxford University Press, 1999 pp 479– 494Google Scholar
22 Nuffield Council on Bioethics: Genetics and Human Behavior: the Ethical Context, 2002. http://www.nuffieldbioethics.orgGoogle Scholar
23 Annas GJ, Glantz LH, Roche PA: Drafting the Genetic Privacy Act: science, policy, and practical considerations. J Law Med Ethics 1995; 23: 360– 366Crossref, Google Scholar
24 Brenner S: Genomics. Hunting the metaphor. Science 2001; 291: 1265– 1266Crossref, Google Scholar
25 Meslin EM, Thomson EJ, Boyer JT: The Ethical, Legal, and Social Implications Research Program at the National Human Genome Research Institute. Kennedy Inst Ethics J 1997; 7: 291– 298Crossref, Google Scholar
26 Burke W, Pinsky LE, Press NA: Categorizing genetic tests to identify their ethical, legal, and social implications. Am J Med Genet 2001; 106: 233– 240Crossref, Google Scholar
27 Pääbo S: Genomics and society. The human genome and our view of ourselves. Science 2001; 291: 1219– 1220Crossref, Google Scholar
28 Lerman C, Croyle RT, Tercyak KP, Hamann H: Genetic testing: psychological aspects and implications. J Consult Clin Psychol 2002; 70: 784– 797Crossref, Google Scholar
29 McCormick RA, Korf BR, Wexler NS: Case vignette: genetic secrets. Ethics Behav 1992; 2: 129– 139Crossref, Google Scholar
30 Hakimian R: Disclosure of Huntington's disease to family members: the dilemma of known but unknowing parties. Genet Test 2000; 4: 359– 364Crossref, Google Scholar
31 Kopelman LM: Using the Best Interests Standard to decide whether to test children for untreatable, late-onset genetic diseases. J Med Philos 2007; 32: 375– 394Crossref, Google Scholar
32 Malpas PJ: Why tell asymptomatic children of the risk of an adult-onset disease in the family but not test them for it? J Med Ethics 2006; 32: 639– 642Crossref, Google Scholar
33 Borry P, Stultiens L, Nys H, Cassiman JJ, Dierickx K: Presymptomatic and predictive genetic testing in minors: a systematic review of guidelines and position papers. Clin Genet 2006; 70: 374– 381Crossref, Google Scholar
34 Hoop JG, Cook J, Edwin H, Dinwiddie SH, Gershon ES: Neurogenetics, behavior, and neurodegenerative disorders, in Genetic Testing: Care, Consent, and Liability. Edited by Sharpe NF, Carter RF. Hoboken, NJ, John Wiley & Sons-Liss, 2006, pp 223– 238Google Scholar
35 Yang Q, Khoury MJ, Botto L, Friedman JM, Flanders WD: Improving the prediction of complex diseases by testing for multiple disease-susceptibility genes. Am J Hum Genet 2003; 72: 636– 649Crossref, Google Scholar
36 McGorry PD: Early intervention in psychotic disorders: beyond debate to solving problems. Br J Psychiatry 2005; 48( Suppl): S108– S110Google Scholar
37 Yung AR, Killackey E, Hetrick SE, Parker AG, Schultze-Lutter F, Klosterkoetter J, Purcell R, Mcgorry PD: The prevention of schizophrenia. Int Rev Psychiatry 2007; 19: 633– 646Crossref, Google Scholar
38 Corcoran C, Malaspina D, Hercher L: Prodromal interventions for schizophrenia vulnerability: the risks of being “at risk.” Schizophr Res 2005; 73: 173– 184Crossref, Google Scholar
39 Holtzman NA, Marteau TM: Will genetics revolutionize medicine? N Engl J Med 2000; 343: 141– 144Crossref, Google Scholar
40 Psynomics. https://www.psynomics.comGoogle Scholar
41 SureGene LLC. http://www.suregene.netGoogle Scholar
42 Fagerlin A, Ubel PA, Smith DM, Zikmund-Fisher BJ: Making numbers matter: present and future research in risk communication. Am J Health Behav 2007; 31( Suppl 1): S47– S56Crossref, Google Scholar
43 Fagerlin A, Zikmund-Fisher BJ, Ubel PA: “If I'm better than average, then I'm ok?”: comparative information influences beliefs about risk and benefits. Patient Educ Couns 2007; 69: 140– 144Crossref, Google Scholar
44 Ubel PA: Emotions, decisions, and the limits of rationality: symposium introduction. Med Decis Making 2005; 25: 95– 96Crossref, Google Scholar
45 Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A: Validation of the Subjective Numeracy Scale: effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making 2007; 27: 663– 671Crossref, Google Scholar
46 Wertz DC: Ethical issues in the application of knowledge from molecular genetics to mental disorders, in
47 Targum SD, Dibble ED, Davenport YB, Gershon ES: The Family Attitudes Questionnaire. patients' and spouses' views of bipolar illness. Arch Gen Psychiatry 1981; 38: 562– 568Crossref, Google Scholar
48 Schulz PM, Schulz SC, Dibble E, Targum SD, van Kammen DP, Gershon ES: Patient and family attitudes about schizophrenia: implications for genetic counseling. Schizophr Bull 1982; 8: 504– 513Crossref, Google Scholar
49 Smith LB, Sapers B, Reus VI, Freimer NB: Attitudes towards bipolar disorder and predictive genetic testing among patients and providers. J Med Genet 1996; 33: 544– 549Crossref, Google Scholar
50 Trippitelli CL, Jamison KR, Folstein MF, Bartko JJ, DePaulo JR: Pilot study on patients' and spouses' attitudes toward potential genetic testing for bipolar disorder. Am J Psychiatry 1998; 155: 899– 904Crossref, Google Scholar
51 Milner KK, Han T, Petty EM: Support for the availability of prenatal testing for neurological and psychiatric conditions in the psychiatric community. Genet Test 1999; 3: 279– 286Crossref, Google Scholar
52 Quaid KA, Aschen SR, Smiley CL, Nurnberger JIJ: Perceived genetic risks for bipolar disorder in a patient population: an exploratory study. J Genet Couns 2001; 10: 41– 51Crossref, Google Scholar
53 Jones I, Scourfield J, McCandless F, Craddock N: Attitudes towards future testing for bipolar disorder susceptibility genes: a preliminary investigation. J Affect Disord 2002; 71: 189– 193Crossref, Google Scholar
54 Finn CT, Wilcox MA, Korf BR, Blacker D, Racette SR, Sklar P, Smoller JW: Psychiatric genetics: a survey of psychiatrists' knowledge, opinions, and practice patterns. J Clin Psychiatry 2005; 66: 821– 830Crossref, Google Scholar
55 Meiser B, Mitchell PB, McGirr H, Van Herten M, Schofield PR: Implications of genetic risk information in families with a high density of bipolar disorder: an exploratory study. Soc Sci Med 2005; 60: 109– 118Crossref, Google Scholar
56 DeLisi LE, Bertisch H: A preliminary comparison of the hopes of researchers, clinicians, and families for the future ethical use of genetic findings on schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 110– 115Crossref, Google Scholar
57 Austin JC, Smith GN, Honer WG: The genomic era and perceptions of psychotic disorders: genetic risk estimation, associations with reproductive decisions and views about predictive testing. Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 926– 928Crossref, Google Scholar
58 Laegsgaard MM, Mors O: Psychiatric genetic testing: attitudes and intentions among future users and providers. Am J Med Genet B Neuropsychiatr Genet 2008; 147B: 375– 384Crossref, Google Scholar
59 Hoop JG, Roberts LW, Green Hammond KA, Cox NJ: Psychiatrists' attitudes regarding genetic testing and patient safeguards: a preliminary study. Genet Test 2008; 12: 245– 252Crossref, Google Scholar
60 Marteau TM, Kidd J, Cook R, Michie S, Johnston M, Slack J, Shaw RW: Perceived risk not actual risk predicts uptake of amniocentesis. Br J Obstet Gynaecol 1991; 98: 282– 286Crossref, Google Scholar
61 Bluman LG, Rimer BK, Berry DA, Borstelmann N, Iglehart JD, Regan K, Schildkraut J, Winer EP: Attitudes, knowledge, and risk perceptions of women with breast and/or ovarian cancer considering testing for BRCA1 and BRCA2. J Clin Oncol 1999; 17: 1040– 1046Crossref, Google Scholar
62 Wiggins S, Whyte P, Huggins M, Adam S, Theilmann J, Bloch M, Sheps SB, Schechter MT, Hayden MR: The psychological consequences of predictive testing for Huntington's disease. Canadian Collaborative Study of Predictive Testing. N Engl J Med 1992; 327: 1401– 1405Crossref, Google Scholar
63 Codori AM, Slavney PR, Young C, Miglioretti DL, Brandt J: Predictors of psychological adjustment to genetic testing for Huntington's disease. Health Psychol 1997; 16: 36– 50Crossref, Google Scholar
64 Dorval M, Patenaude AF, Schneider KA, Kieffer SA, DiGianni L, Kalkbrenner KJ, Bromberg JI, Basili LA, Calzone K, Stopfer J, Weber BL, Garber JE: Anticipated versus actual emotional reactions to disclosure of results of genetic tests for cancer susceptibility: findings from p53 and BRCA1 testing programs. J Clin Oncol 2000; 18: 2135– 2142Crossref, Google Scholar
65 Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Green RC, REVEAL (Risk Evaluation and Education for Alzheimer's Disease) Study Group: Genetic risk assessment for adult children of people with Alzheimer's disease: the Risk Evaluation and Education for Alzheimer's Disease (REVEAL) Study. J Geriatr Psychiatry Neurol 2005; 18: 250– 255Crossref, Google Scholar
66 Chao S, Roberts JS, Marteau TM, Silliman R, Cupples LA, Green RC: Health behavior changes after genetic risk assessment for Alzheimer disease: the REVEAL Study. Alzheimer Dis Assoc Disord 2008; 22: 94– 97Crossref, Google Scholar
67 LaRusse S, Roberts JS, Marteau TM, Katzen H, Linnenbringer EL, Barber M, Whitehouse P, Quaid K, Brown T, Green RC, Relkin NR: Genetic susceptibility testing versus family history-based risk assessment: impact on perceived risk of Alzheimer disease. Genet Med 2005; 7: 48– 53Crossref, Google Scholar
68 Marteau TM, Roberts S, LaRusse S, Green RC: Predictive genetic testing for Alzheimer's disease: impact upon risk perception. Risk Anal 2005; 25: 397– 404Crossref, Google Scholar
69 Lapham EV, Kozma C, Weiss JO: Genetic discrimination: perspectives of consumers. Science 1996; 274: 621– 624Crossref, Google Scholar
70 Jeffords JM, Daschle T: Policy issues. Political issues in the genome era. Science 2001; 291: 1249– 1251Crossref, Google Scholar
71 Roberts LW, Geppert CM, Warner TD, Green Hammond KA, Rogers M, Smrcka J, Roberts BB: Perspectives on use and protection of genetic information in work settings: results of a preliminary study. Soc Sci Med 2005; 60: 1855– 1858Crossref, Google Scholar
72 Wong JG, Lieh-Mak F: Genetic discrimination and mental illness: a case report. J Med Ethics 2001; 27: 393– 397Crossref, Google Scholar
73 Hudson KL, Holohan MK, Collins FS: Keeping pace with the times—the Genetic Information Nondiscrimination Act of 2008. N Engl J Med 2008; 358: 2661– 2663Crossref, Google Scholar
74 Harper PS: Genetic testing, life insurance, and adverse selection. Philos Trans R Soc Lond B Biol Sci 1997; 352: 1063– 1066Crossref, Google Scholar
75 Raithatha N, Smith RD: Disclosure of genetic tests for health insurance: is it ethical not to? Lancet 2004; 363: 395– 396Crossref, Google Scholar
76 Zick CD, Mathews CJ, Roberts JS, Cook-Deegan R, Pokorski RJ, Green RC: Genetic testing for Alzheimer's disease and its impact on insurance purchasing behavior. Health Aff (Millwood) 2005; 24: 483– 490Crossref, Google Scholar
77 Corrigan PW, Watson AC: At issue: Stop the stigma: call mental illness a brain disease. Schizophr Bull 2004; 30: 477– 479Crossref, Google Scholar
78 Mehta S, Farina A: Is being ‘sick’ really better? Effect of the disease view of mental disorder on stigma. J Soc Clin Psychol 1997; 16: 405– 419Crossref, Google Scholar
79 Walker I, Read J: The differential effectiveness of psychosocial and biogenetic causal explanations in reducing negative attitudes toward “mental illness.” Psychiatry 2002; 65: 313– 325Crossref, Google Scholar
80 Phelan JC: Geneticization of deviant behavior and consequences for stigma: the case of mental illness. J Health Soc Behav 2005; 46: 307– 322Crossref, Google Scholar
81 Safer v. Pack, 677 A 2d 1188 ( NJ Super Ct App Div 1996)Google Scholar
82 Mathews CA, Reus VI, Bejarano J, Escamilla MA, Fournier E, Herrera LD, Lowe TL, McInnes LA, Molina J, Ophoff RA, Raventos H, Sandkuijl LA, Service SK, Spesny M, León PE, Freimer NB: Genetic studies of neuropsychiatric disorders in Costa Rica: a model for the use of isolated populations. Psychiatr Genet 2004; 14: 13– 23Crossref, Google Scholar
83 Harmon A: In DNA era, new worries about prejudice. New York Times, November 11, 2007, p 1Google Scholar
84 Harmon A: Indian tribe wins fight to limit research of its DNA. New York Times, April 21, 2010, p 1Google Scholar
85 International HapMap Consortium: A haplotype map of the human genome. Nature 2005; 437: 1299– 1320Crossref, Google Scholar
86 Knoppers BM: Biobanking: international norms. J Law Med Ethics 2005; 33: 7– 14Crossref, Google Scholar
87 Sheremeta L: Population genetic studies: is there an emerging legal obligation to share benefits? Health Law Rev 2003; 12: 36– 38Google Scholar
88 Biesecker BB, Peay HL: Ethical issues in psychiatric genetics research: points to consider. Psychopharmacology (Berl) 2003; 171: 27– 35Crossref, Google Scholar
89 Kapp MB: Ethical and legal issues in research involving human subjects: do you want a piece of me? J Clin Pathol 2006; 59: 335– 339Crossref, Google Scholar
90 Furness PN, Nicholson ML: Obtaining explicit consent for the use of archival tissue samples: practical issues. J Med Ethics 2004; 30: 561– 564Crossref, Google Scholar
91 Lipworth W, Ankeny R, Kerridge I: Consent in crisis: the need to reconceptualize consent to tissue banking research. Intern Med J 2006; 36: 124– 128Crossref, Google Scholar
92 Wendler D: One-time general consent for research on biological samples: is it compatible with the health insurance portability and accountability act? Arch Intern Med 2006; 166: 1449– 1452Crossref, Google Scholar
93 Chen DT, Rosenstein DL, Muthappan P, Hilsenbeck SG, Miller FG, Emanuel EJ, Wendler D: Research with stored biological samples: what do research participants want? Arch Intern Med 2005; 165: 652– 655Crossref, Google Scholar
94 Van Diest PJ: No consent should be needed for using leftover body material for scientific purposes. BMJ 2002; 325: 648– 651Crossref, Google Scholar
95 Ashcroft R: The ethics of reusing archived tissue for research. Neuropathol Appl Neurobiol 2000; 26: 408– 411Crossref, Google Scholar
96 White MT, Gamm J: Informed consent for research on stored blood and tissue samples: a survey of institutional review board practices. Account Res 2002; 9: 1– 16Crossref, Google Scholar
97 Haga SB, Burke W: Pharmacogenetic testing: not as simple as it seems. Genet Med 2008; 10: 391– 395Crossref, Google Scholar
98 Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, de Faire U, Danesh J: Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 2007; 298: 1300– 1311Crossref, Google Scholar
99 Alessandro S, Kato M: The serotonin transporter gene and effectiveness of SSRIs. Expert Rev Neurother 2008; 8: 111– 120Crossref, Google Scholar
100 Mayo Clinic offers new genetic test to help manage treatment for patients with depression, July 17, 2006. http://www.mayoclinic.org/news2006-rst/3540.htmlGoogle Scholar
101 Finn CT, Smoller JW: Genetic counseling in psychiatry. Harv Rev Psychiatry 2006; 14: 109– 121 http://www.topix.com/forum/health/depression/TD223E6QT2DH37D7EGoogle Scholar
102 Finn CT: Increasing genetic education for psychiatric residents. Harv Rev Psychiatry 2007; 15: 30– 33Crossref, Google Scholar
103 Austin JC, Honer WG: The genomic era and serious mental illness: a potential application for psychiatric genetic counseling. Psychiatr Serv 2007; 58: 254– 261Crossref, Google Scholar
104 Collins FS: Preparing health professionals for the genetic revolution. JAMA 1997; 278: 1285– 1286Crossref, Google Scholar
105 Peay H, McInerney J: A pilot study on psychiatric genetic counseling: counselor's needs. J Genet Couns 2002; 11: 485Google Scholar
106 Getting personal (editorial). Nature 2008; 455: 1007Google Scholar
107 Braff DL, Freedman R: Clinically responsible genetic testing in neuropsychiatric patients: a bridge too far and too soon. Am J Psychiatry 2008; 165: 952– 955Crossref, Google Scholar
108 Kuehn BM: Risks and benefits of direct-to-consumer genetic testing remain unclear. JAMA 2008; 300: 1503– 1505Crossref, Google Scholar
109 23andMe. https://www.23andme.comGoogle Scholar
110 Hudson K, Javitt G, Burke W, Byers P: ASHG statement* on direct-to-consumer genetic testing in the United States. Obstet Gynecol 2007; 110: 1392– 1395Crossref, Google Scholar
111 Hempelmann LH: Potential dangers in the uncontrolled use of shoe-fitting fluoroscopes. N Engl J Med 1949; 241: 335– 336Crossref, Google Scholar
112 Williams CR: Radiation exposures from the use of shoe-fitting fluoroscopes. N Engl J Med 1949; 241: 333– 335Crossref, Google Scholar
113 Campbell I, Castle C, Curtis H, Lucas S, O'Donahue M, Townsend J: Home testing for HIV. HIV Testing Working Party. UK NGO AIDS Consortium. Lancet 1994; 343: 1293– 1294Crossref, Google Scholar
114 Cubells JF: Clinical genomic psychiatry comes of age in the evaluation and treatment of developmental disabilities: is our nation prepared to make the benefits available to all who need them? Curr Psychiatry Rep 2007; 9: 81– 82Crossref, Google Scholar
115 Emanuel EJ, Wendler D, Grady C: What makes clinical research ethical? JAMA 2000; 283: 2701– 2711Crossref, Google Scholar
116 Roberts LW: Ethical dimensions of psychiatric research: a constructive, criterion-based approach to protocol preparation. The Research Protocol Ethics Assessment Tool (RePEAT). Biol Psychiatry 1999; 46: 1106– 1119Crossref, Google Scholar
117 Hoop JG, Layde J, Roberts LW: Ethical considerations in psychopharmacological treatment and research, in Textbook of Psychopharmacology, 4th ed. Edited by Schatzberg AF, Nemeroff CB. Arlington, VA, American Psychiatric Publishing, 2008Google Scholar
118 Brodwin PE: Introduction, in Biotechnology and Culture: Bodies, Anxieties, Ethics. Edited by Brodwin PE. Bloomington, IN, Indiana University Press, 2000 pp 1– 23Google Scholar



