Posttraumatic Stress Disorder: From Phenomenology to Clinical Practice
Abstract
The psychological and behavioral consequences of exposure to traumatic events—particularly combat—have been recognized throughout the ages. Since its 1982 introduction in the Diagnostic and Statistical Manual of Mental Disorders, 3rd edition much has been learned about the pathophysiology of PTSD. Current theories focus on brain regions and neurobiological systems regulating stress and fear response, fear memory formation and retrieval. Effective pharmacologic and psychotherapeutic interventions target these systems. New treatments must prove more effective in specific subpopulations of patients with PTSD. These will include combinations of pharmacologic agents and psychotherapy, and treatments targeting different regions, receptors, or mechanisms involved in the traumatic stress response.
Despite increasing public and professional attention since its 1982 introduction in the Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, posttraumatic stress disorder (PTSD) is often seen as a controversial diagnosis. Research over the last quarter century has contributed to a growing awareness of the nature and extent of human exposure to traumatic events and to our understanding of the neurobiology of threat perception, fear response, and altered brain functional regions associated with PTSD. Clinical research has demonstrated effective treatments across the three symptom clusters of intrusion, hyperarousal, and emotional numbing that characterize PTSD. The concept of the “traumatic stressor” necessary for the diagnosis of PTSD has also evolved. Subsequent editions of the DSM have altered the definition of traumatic stressor and the inclusion criteria for this disorder. APA published the Practice Guideline for the Treatment of Adults with Acute Stress Disorder (ASD) and PTSD in October 2004. By then, a number of well-designed randomized controlled trials of interventions for PTSD had been conducted in various populations. These studies and other meta-analyses support the efficacy of both psychopharmacological and psychotherapeutic approaches to PTSD. Still, expert consensus and practice guidelines have articulated some differing positions on effective treatments. The classification of PTSD as an anxiety disorder (rather than an “event-related disorder” or a mood disorder), the presently required A2 criterion of a specific immediate response, and the duration of symptoms necessary to constitute a diagnosis are areas of ongoing study and consideration in the DSM-V planning committee.
In this overview, we describe the 30-year evolution of the diagnosis of PTSD, highlighting the current conceptualization of core clinical features and epidemiologic findings based on this definition. The molecular neurobiology and genetics of PTSD are then summarized. In light of the current theories of pathology, evidence-based treatments of PTSD are summarized with particular attention to evidence emerging since publication of APA's initial practice guideline. Finally, areas of future research that may clarify existing controversies and identify novel treatment strategies are identified.
PHENOMENOLOGY
The diagnostic criteria for PTSD are shown in Table 1. PTSD is distinguished from most other axis I mental disorders by the requirement for a traumatic environmental exposure as part of the diagnostic criteria. This stressor must involve either witnessing or experiencing threatened or actual death, serious injury, or threat to physical integrity of self or others (A1 criterion). Moreover, the diagnostic criteria require that an individual's initial response must involve intense fear, helplessness, or horror (A2 criterion). Descriptions of the psychopathological consequences of traumatic events—particularly combat—date back thousands of years as noted, for example, in Homer's Iliad. Terms such as “nostalgia” and “soldier's heart” described these phenomena in the Civil War, whereas “shell shock” was used in World War I and “battle fatigue” or “combat neurosis” in World War II. However, the formal diagnosis of PTSD was introduced to the DSM-III in 1980, largely in recognition of the psychiatric symptoms observed in Vietnam veterans. Earlier conceptualizations of PTSD required that the traumatic event be “outside the range of normal human experience,” but this description of the traumatic event was eliminated in DSM-IV. Broadening the concept of the traumatic exposure has resulted in increased prevalence of PTSD in epidemiologic surveys, even as others questioned the diagnostic significance of requiring fear, hopelessness, or horror in the initial response.
 |
Table 1. Diagnostic Criteria for PTSD (DSM-IV-TR)
The diagnosis of PTSD requires the presence of at least one specified reexperiencing symptom from a list including recurrent and distressing recollections or dreams of the event, at least three avoidance symptoms (e.g., efforts to avoid thoughts, feelings, or conversations associated with the trauma or efforts to avoid activities, places, or people that evoke recollections of the trauma) and at least two symptoms of increased arousal (e.g., difficulty falling or staying asleep or exaggerated startle). Symptoms must result in clinically significant distress or impairment in occupational, psychosocial, or other important areas of function. Finally, the diagnosis of PTSD is made only when symptoms and resultant distress and/or impairment persist for at least 1 month. The 1-month duration has been challenged based on the lack of empirical evidence demonstrating this duration of symptoms to be more valid than other constructs and at present there is little epidemiologic evidence to support this cutoff (1)
EPIDEMIOLOGY
In the United States, rates of exposure to one or more A1 criterion traumas range from 39% (2) to 90% (3), with women generally reporting less exposure than men. Ten percent of men and 6% of women report being exposed to at least four events (3). Subpopulations such as combat troops appear to be at high risk. Hoge et al. (4) reported that 95% of U.S. army and marine service members who served in the Iraq war and 84% of army service members who served in Afghanistan were exposed to life-threatening combat situations. Despite high rates of traumatic exposure, relatively few people develop PTSD. Using DSM-III-R criteria for PTSD, Breslau et al. (2) reported a lifetime prevalence of 11% (31% of respondents exposed to trauma) in women and 6% (14% of trauma exposed) in men. Kessler et al. (3) reported a lifetime prevalence of 7.8%, with women being more than twice as likely to meet the criteria (10.4%) than men (5%) despite men being more likely to be exposed to traumatic events. The 12-month prevalence of Kessler et al. was 3.9% (5, 6) and more recently was 3.5% with approximately one third of cases being serious, one third of cases being moderate, and one third of cases being mild (7). Hoge et al. (4) published rates of PTSD (determined by a self-report) in combat-exposed troops ranging from 4% to 20%, with those experiencing the greatest intensity of combat reporting the highest prevalence of PTSD. In an Australian sample, the 12-month prevalence was 1.2% (8).
Different types, severities, and frequencies of trauma in different populations contribute to variable prevalence in population studies. Kessler et al. (3) reported the conditional risk of PTSD by trauma type in a U.S. sample. In men, rape and combat exposure in a war were associated with the highest risk of developing PTSD, 65% and 39%, respectively. Being threatened with a weapon or being physically attacked was associated with the lowest risk (1% and 2%, respectively). In women, rape (46%) and being threatened with a weapon (33%; significantly higher than men) were associated with the highest risk, whereas exposure to a natural disaster at 5% was the lowest and not significantly higher than for men. In general, interpersonal violence, more than other trauma, is associated with the highest risk for PTSD.
NEUROBIOLOGY
PTSD symptoms are presently hypothesized to reflect either pathological changes in neurobiological stress-response systems or failure of neurobiological systems to recover from or adapt to extreme stressors (9). Neurobiological research in PTSD has focused on stress-regulating neuroendocrine systems, such as the hypothalamic-pituitary-adrenal (HPA) axis, and on the neurotransmitters and neuropeptides that connect and regulate brain regions involved in fear and stress response (Figure 1). These fear and memory circuits include the prefrontal cortex, hippocampus, amygdala, and brainstem nuclei.
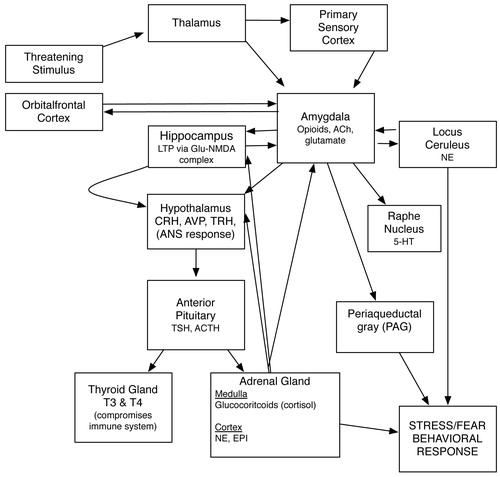
Figure 1. Neurotransmitters and HPA Axis in Stress/Fear Response.
HPA AXIS
Acute stressors activate the HPA. Neurons in the hypothalamic paraventricular nucleus (PVN) secrete corticotrophin-releasing factor (CRF), which stimulates the production of adrenocorticotropin in the pituitary gland, and the release of glucocorticoids (e.g., cortisol) from the adrenal cortex. The hippocampus and prefrontal cortex inhibit the HPA axis, whereas the amygdala and brainstem monoamines stimulate the PVN to begin this cascade. Although acute stressors activate the HPA, the majority of studies in combat veterans, refugees, and holocaust survivors with PTSD have found reduced blood concentrations of cortisol (10). Studies show that hypocortisolism in PTSD occurs in the context of sustained increases in CRF levels, suggesting increased sensitivity of the HPA axis to negative feedback from cortisol (11, 12). The majority of PTSD studies have also demonstrated reduced volume of the hippocampus, the brain region responsible for inhibition of the HPA (13). Together, these findings suggest that PTSD may reflect chronic sensitization of the HPA axis to the effects of stressors (10). Recent studies have demonstrated that low cortisol levels during exposure to traumatic stressors predict development of PTSD. Because glucocorticoids also interfere with the retrieval of traumatic memories, they may eventually prove to be effective treatment for PTSD. CRF may also play a role in conditioned fear response, increased startle, and hyperarousal; thus, antagonism of CRF receptors may prove to be an effective approach to PTSD treatment.
NEUROCIRCUITS AND FEAR CONDITIONING
Norepinephrine is one of the principle mediators of the stress response in the CNS. Noradrenergic cell bodies of the locus ceruleus are responsible for production of the majority of CNS norepinephrine and through extensive branching project to the major brain regions involved in the stress response including the prefrontal cortex, amygdala, hypothalamus, periaqueductal gray, and thalamus. Converging evidence from animal models of fear conditioning and neuromolecular and neuroimaging techniques suggest that norepinephrine interacts with CRF to initiate the fight or flight response including increased heart rate, blood pressure, and skin conductance in the peripheral nervous system and also enhanced arousal and vigilance and facilitated encoding of fear-related memories in the CNS. Glucocorticoids appear to inhibit this response (14). In the peripheral nervous system, adrenal activation during exposure to stressors results in release of norepinephrine and epinephrine from the adrenal medulla and from sympathetic nerve endings. Hyperactivity of the sympathetic branch of the autonomic nervous system has been demonstrated in various PTSD samples and is also exhibited by patients with PTSD in response to reminders of traumatic experiences (15, 16).
Serotonergic (5-HT) neurons originating in the dorsal and median raphe nuclei also project to the amygdala, the hippocampus, and the prefrontal cortex. Current theories suggest that 5-HT neurons projecting from the dorsal raphe to the amygdala and hippocampus mediate anxiogenic responses via 5-HT2 receptors, whereas neurons from the median raphe facilitate extinction learning (i.e., the suppression of responses associated with fear memories) via 5-HT1A receptors (17). This mechanism may contribute to the demonstrated efficacy of selective serotonin reuptake inhibitor (SSRI) medications in PTSD.
OTHER NEUROTRANSMITTERS
γ-Aminobutyric acid also inhibits the CRF/norepinephrine circuits, mediating fear and stress responses through the inhibitory actions of the GABAA/benzodiazepine (BZ) receptor complex. Decreased BZ receptor density or affinity may contribute to the pathophysiology of PTSD in a nonspecific manner (18–20). However, treatment with BZs immediately after trauma does not prevent PTSD, and the few studies of PTSD involving BZs do not suggest efficacy as monotherapy (21). Glutamate, the primary excitatory neurotransmitter in the brain, binds to a number of excitatory amino acid receptors including the N-methyl-d-aspartate (NMDA) receptor. The glutamate/NMDA receptor system is believed to be critical to the process of long-term potentiation and memory consolidation (including traumatic memories). The partial NMDA receptor antagonist d-cycloserine has been shown to facilitate extinction of fear in animal models and in phobic human subjects receiving exposure therapy (22). Its role in facilitating exposure-based therapies for PTSD is an area of active investigation.
Other neuropeptides implicated in the pathophysiology of PTSD include neuropeptide Y (NPY) and the endogenous opioids. Elevated levels of NPY have been found in combat veterans without PTSD compared with veterans with PTSD (23). Because NPY reduces the release of norepinephrine from sympathetic nerve cells and inhibits CRF/norepinephrine circuits involved in the fear response, it has been suggested that NPY may serve a protective role or contribute to recovery from PTSD (21). Endogenous opioids (e.g., endorphins and enkephalins), such as synthetic and naturally occurring opiates, bind to opiate receptors that may contribute to symptoms such as emotional numbing and dissociative phenomena (24). An increase in B-endorphin levels in patients with PTSD has been demonstrated, suggesting increased activation of the endogenous opioid system (25), and the opioid receptor antagonist naltrexone has shown efficacy in treating the dissociative symptoms of PTSD in small series (26). Randomized controlled trials of opioid receptor antagonists in the treatment of PTSD have not yet been conducted.
GENETICS
The findings regarding alterations in hormone and neurotransmitter or neuropeptide levels outlined above raise the question of whether the identified neurobiological changes represent predispositions (risk factors) for developing PTSD or biological markers of the disease process itself. Evidence from family, twin, and molecular genetic studies suggests a role for genetic influences in the etiology of PTSD and may help answer questions regarding why certain individuals exposed to specific stressors develop PTSD, whereas others exposed to the same stressors do not.
Genetic studies in PTSD conducted to date have generally been small samples and require replication. Family studies demonstrate an increased risk for PTSD in first-degree relatives of patients with PTSD (27). Twin studies have identified the risk of trauma exposure, the development of PTSD after exposure, and the contribution of comorbidity before and after traumatic exposure as distinct areas of genetic influence (28). Zhang et al. (29) recently demonstrated differential expression of p11, a member of the S-100 protein family, in the prefrontal cortex of postmortem PDTD subjects and the peripheral blood mononuclear cells of patients with PTSD, major depression, bipolar disorder, and schizophrenia, and control subjects.
In a small clinical sample, Comings et al. (30) demonstrated that a dopamine D2 receptor variant in linkage disequilibrium with the D2A1 allele confers an increased risk for PTSD, and the absence of the variant confers a relative resistance to PTSD. Recently, Kilpatrick et al. (31) found that the low expression variant of the serotonin transporter gene 5-HTTLPR increased the risk of posthurricane PTSD but only under the conditions of high hurricane exposure and low social support. Binder et al. (32) found that four single nucleotide polymorphisms (SNPs) in the FKBP5 locus significantly interacted with the severity of child abuse to predict level of adult PTSD symptoms after correcting for multiple testing. There were no main effects of the SNPs on PTSD symptoms and no significant genetic interactions with level of non-child abuse trauma as predictors of adult PTSD symptoms. These results suggest a potential FKBP5 gene-childhood environment interaction important for the development of adult PTSD in that individuals with these SNPs have a greater risk to develop adult PTSD with specific childhood environmental stressors.
TREATMENT APPROACHES
The recommendations of various PTSD treatment practice guidelines and consensus statements are summarized in Table 2 (33–40). Generally, these analyses support the use of some forms of cognitive behavior therapy (CBT) and also of pharmacological treatment (usually with SSRIs). Recent studies are highlighted below.
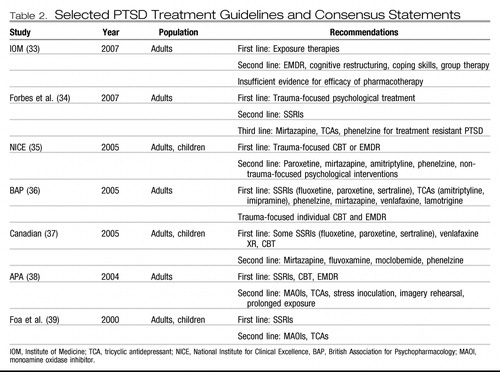 |
Table 2. Selected PTSD Treatment Guidelines and Consensus Statements
PSYCHOTHERAPY
Most of the well-designed randomized controlled trials of psychotherapy published have examined variations of CBT. As described in the 2007 report of the Institute of Medicine (33), therapeutic approaches and techniques overlap across psychotherapies, and there is no consensus on how these psychotherapies should be categorized. Cognitive processing therapy is considered by some experts (including the Institute of Medicine) to be one form of effective exposure-based CBT, whereas prolonged exposure is another.
EXPOSURE-BASED CBTS
Trials of exposure-based CBTs have generally included components of psychoeducation, breathing, and relaxation training. By definition, these exposure therapies also incorporate some form of reexposure to past traumatic experience (e.g., imaginal, in vivo, written, verbal, or taped narrative recounting) into sessions. In addition, homework is often included.
In 2006, Monson et al. (41) reported the results of a wait-list-controlled study of cognitive processing therapy in 60 combat veterans. The overall dropout rate was 16.6% (20% from cognitive processing therapy and 13% from the wait-list), but analyses of the intention-to-treat sample revealed significant improvements in both PTSD and co-occurring depressive symptoms in the treatment group compared with the wait-list group.
The effectiveness of cognitive processing therapy was also demonstrated in a study reported in 2005 by Chard (42) of 71 adult sexual abuse survivors with PTSD. The control was a minimal-attention wait-list group. Participants were assessed before treatment, immediately after treatment, 3 months after treatment, and 1 year after treatment using the Clinician Administered PTSD Scale (CAPS), considered a “standard” for assessing PTSD symptoms and severity in research settings, and several other rating scales. Analysis demonstrated that cognitive processing therapy was superior to wait-list in reducing PTSD symptoms and that reductions were maintained for at least 1 year.
Resick et al. (43) dismantled the components of cognitive processing therapy recently. They randomly assigned 150 adult women with PTSD to three conditions: 1) full cognitive processing therapy, which included both exposure (i.e., writing and reading a detailed account of the trauma) and cognitive therapy (i.e., challenging patient assertions about the meaning of the trauma and the implications for the patient's life); 2) cognitive therapy without the writing and reading component; and 3) the writing and reading component without cognitive therapy. All conditions included 2 hours of therapy per week for 6 weeks. Blinded raters assessed all participants for PTSD (using CAPS) and depression weekly during the treatment, at 2 weeks after the last session of therapy, and at 6 months. Although all treatment completers still met the criteria for PTSD at the conclusion of the study, substantial improvement was observed in all three treatment groups on primary PTSD and depression outcomes and on secondary measures of anxiety, guilt, and shame. Cognitive therapy without exposure was associated with the greatest improvement. This result suggested that the cognitive component of this therapy (i.e., altering the meaning of the traumatic event) may occur without repeated and explicitly evoked fear memories. It also suggested that cognitive processing therapy might be characterized as more cognitive than exposure-based therapy.
In 2007, Schnurr et al. (44) reported the results of prolonged exposure for female veterans (N=277) and active duty personnel (N=7) across 12 military and veterans affairs centers specializing in medical treatment for military veterans. Patients were randomly assigned to receive prolonged exposure therapy (N=141) or present-centered therapy (N=143) delivered in 10 weekly 90-minute sessions, and outcomes were assessed before and immediately after treatment and again at 3 and 6 months after treatment. Immediately after treatment, the prolonged exposure group was more likely than the present-centered therapy group to no longer meet PTSD criteria (41% compared with 27.8%, odds ratio [OR]=1.80, confidence interval [CI]=95%) and more likely to achieve full remission (15.2% compared with 6.9%, OR=2.43, CI=95%). These results were maintained at the 3- and 6-month follow-up. Although this was a study of military personnel and veterans, 70% of participants indicated sexual trauma as their index (worst) traumatic experience, Seventeen percent more participants dropped out of the prolonged exposure arm than out of the present-centered arm.
A controlled study reported in 2005 by Rothbaum et al. (45) evaluated the relative efficacy of prolonged exposure therapy and eye movement desensitization and reprocessing (EMDR) (see below). In this study, 74 adult female rape victims (index rape occurring either in adulthood or childhood) were randomly assigned to nine-session prolonged exposure, EMDR, and wait-list control groups. Dropout rates across the groups were not significantly different. Immediately after treatment, the groups receiving prolonged exposure and EMDR both demonstrated statistically significant improvement across three outcome measures, including a 50% or more decrease from baseline in CAPS score (p=0.001). After treatment, 95% of participants who received prolonged exposure therapy and 75% of participants who received EMDR no longer met the criteria for PTSD. Individuals who received both treatments showed significantly reduced depressive symptoms and dissociative symptoms immediately and at 6 months. Results were maintained at the 6-month follow-up for the prolonged exposure group across PTSD, depressive, and dissociative symptoms but were maintained to a significantly lesser extent for the EMDR group with regard to PTSD.
The effectiveness of brief exposure therapy has been demonstrated in two recent studies reported in 2005 and 2007 by Basoglu et al. (46, 47). In the first study, 59 earthquake survivors with PTSD assessed by CAPS were randomly assigned to a single-session exposure-based behavioral therapy intervention (in which the intensity of simulated trauma was adjusted in accordance with the patient's personal feelings of comfort) or to a wait-list (46). At 6, 12, and 24 weeks after treatment, as well as at 1–2 years after treatment, the treatment group was observed to have significant decreases in CAPS score, Beck Depression Inventory score, and other patient self-measures of fear and anxiety, and of overall impression. With regard to CAPS, effect sizes were considerable (Cohen's d=0.7–1.4), and the improvement rate rose from 49% at week 6 to more than 80% at other assessment points. In the second study (47), 31 earthquake survivors with PTSD were randomly assigned to a single-session exposure-based behavioral therapy (N=16) or to repeated assessments (N=15). Participants were assessed at 4, 8, 12, and 24 weeks after treatment and after 1–2 years. Again, significant between-group treatment effects were observed in PTSD (assessed by CAPS) and assessor-rated global improvement (Global Improvement Scale–Assessor), with significant between-group treatment effects observed in both outcome measures at week 8. Improvement rates of 40% at week 4 rose to 80% by week 24 and at the 1–2 year follow-up, with large effect sizes (Cohen's d=0.9–1.7) noted across primary measures at week 8.
EMDR
EMDR continues to be examined as a treatment for victims of trauma; however, many of the studies published since 2004 include participants without a formal diagnosis of PTSD. An exception is a study reported in 2007 by van der Kolk et al. (48), in which 88 patients with PTSD were randomly assigned to 8 weeks of EMDR, fluoxetine, or placebo. Symptoms were assessed using the CAPS and Beck Depression Inventory II immediately after treatment and at 6 months. At the 6-month follow-up, 75% of the patients with adult-onset PTSD (compared with 33% of those with childhood-onset PTSD) receiving EMDR achieved remission compared with none of the patients receiving fluoxetine. Neither treatment produced complete symptom remission in the majority of the patients with childhood-onset PTSD. It should be noted that fluoxetine was discontinued at termination of the 8-week treatment phase, so the poor SSRI outcomes at 6 months should not be surprising.
In a study reported in 2007, Högberg et al. (49) examined 24 transportation workers who had either been assaulted or who had witnessed a person-under-train accident and who met DSM-IV-TR criteria for PTSD. Participants were randomly assigned to either five sessions of EMDR or to a wait-list. After treatment, 8 of 13 patients receiving EMDR (67%) no longer met the criteria for PTSD compared with 1 of 11 (11%) patients on the wait-list (p=0.02). Significant differences were also observed in Global Assessment of Functioning and Hamilton Depression Rating Scores (HAM-D) scores.
Neither of these studies dismantled the effects of exposure compared with eye-movement components of the treatment. Previous studies have shown that the eye movements are not critical to the treatment effect. These small studies suggest efficacy of brief EMDR in sexual assault victims and witnesses to vehicular accidents but cannot be generalized to combat veterans.
OTHER PSYCHOTHERAPIES
Studies of other types of psychotherapy, including coping skills therapy, eclectic psychotherapy, psychodynamic psychotherapy, cognitive restructuring, and brainwave neurofeedback, have also been published in recent years, but the utility and generalizability of conclusions from these studies are limited by methodological issues such as lack of formalized diagnostic procedures, inclusion of patients who did not have PTSD, very high dropout rates, unspecified handling of dropouts or missing data, and use of nonblinded assessors. A study reported in 2004 by Neuner et al. (50) of coping skills therapy in 43 war refugees, although methodologically sound, failed to demonstrate a differential effect of treatment.
Case reports (51, 52) have recently suggested that exposure-based therapy may be facilitated through the use of computerized audiovisual simulations of a traumatic combat environment. The effectiveness of this facilitated CBT—termed “virtual reality therapy”—in disaster workers with PTSD has also been demonstrated in a small controlled trial. In 2007, Difede et al. (53) assigned 21 September 11 terrorist attack workers to either virtual reality treatment (N=13) or wait-list control (N=8). The treatment group showed a significant decline in CAPS scores compared with the wait-list group. Although these reports are encouraging, larger randomized controlled trails must replicate such findings before virtual reality therapy can be recommended with high confidence.
GROUP PSYCHOTHERAPY
The majority of psychotherapies may be delivered in either individual or group formats. Of the studies reviewed above, the 2005 study by Chard (42) comparing cognitive processing therapy with minimal attention wait-list used both individual and group therapy formats (participants in the treatment group received both individual and group therapy in the first 9 weeks, followed by 7 weeks of group therapy and then one session of individual therapy). Effects of group therapy compared with individual therapy were not clearly demonstrated in this study. Although there is a substantial descriptive literature for group therapy for PTSD, well-designed studies of cognitive processing therapy and other psychotherapies delivered in group formats are needed in the future to validate the efficacy of this method of delivery.
PHARMACOLOGICAL TREATMENT
ANTIDEPRESSANTS
SSRIs for non-combat-related PTSD.
Meta-analyses and several randomized controlled trials published since 2004 generally support the superiority of SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) over placebo for non-combat-related PTSD as well as exposure-based CBTs. However, recent reviews have questioned the extent to which these medications are effective in combat-related PTSD (54).
In 2006, Stein et al. (55) reviewed 35 short-term randomized controlled trials (of 14 or fewer weeks in duration) involving a total of 4,597 participants. In 17 of the trials, symptom severity was significantly reduced in the medication groups relative to the placebo group. Evidence of efficacy was most convincing for the SSRIs, across all symptom clusters and for co-occurring depression and disability.
In a study reported in 2007, Marshall et al. (56) evaluated the efficacy of paroxetine for treating symptoms and associated features of chronic PTSD. Of 70 patients initially enrolled, 52 (mostly minority) adult patients rated as not significantly improved after 1 week of placebo were randomly assigned to receive flexibly dosed paroxetine (maximum 60 mg/day by week 7) or continued placebo. After 10 weeks, significantly more patients treated with paroxetine responded to treatment, as rated by the Clinical Global Impression–Improvement scale. Patients treated with paroxetine were also observed to have a significantly greater reduction in their total score on the CAPS and the Dissociative Experience Scale; self-reported interpersonal problems were also noted to be significantly decreased. During a 10-week maintenance phase, the paroxetine response but not the placebo response continued to improve.
In a 2006 reanalysis of two previously published trials, Stein et al. (57) examined 395 adult patients with PTSD who were randomly assigned to double-blind treatment with flexibly dosed sertraline (50–200 mg/day) or placebo. After 12 weeks, sertraline was significantly more effective than placebo on most primary efficacy variables including Part 2 of the CAPS, irrespective of whether the patients had experienced childhood abuse or interpersonal trauma, suggesting the efficacy of medication treatment in individuals whose precipitating trauma is either childhood abuse in particular or interpersonal trauma in general.
In a 2005 study, Davidson et al. (58) compared the relapse rates of 57 patients who responded to 6 months of open-label fluoxetine and who were subsequently blindly randomly assigned to continue receiving fluoxetine (mean dosage=42.1 mg/day) or placebo. Relapse rates were 22% for fluoxetine compared with 50% for placebo (p=0.02); the odds ratio for relapse with placebo relative to fluoxetine was 3.50, and time to relapse with fluoxetine was longer than with placebo (p=0.02, log-rank statistic).
These newer studies augment the evidence base for SSRI efficacy previously established in samples of patients with PTSD (predominantly women) resulting from civilian trauma, including childhood and adult sexual assault, other interpersonal traumas, and motor vehicle accidents.
SSRIs for combat-related PTSD.
Randomized controlled trials have called into question the efficacy of SSRIs for the treatment of PTSD in combat veterans. Some of this evidence was described in the 2004 guideline, including the 1994 study of van der Kolk et al. (59) of 31 veterans with chronic PTSD randomly assigned to fluoxetine or placebo. In this study, fluoxetine was significantly superior to placebo for symptoms of co-occurring depression as measured by the HAM-D, but the change in the total PTSD score did not differ between placebo and fluoxetine. In a similar randomization of 88 veterans with PTSD, none of those receiving 8 weeks of fluoxetine treatment achieved an asymptomatic state as measured by the CAPS at the 6-month follow-up (45). Negative results were reported in a placebo-controlled, randomized controlled trial by Hertzberg et al. (60) of fluoxetine in 12 Vietnam War veterans.
Friedman et al. (61) completed a multicenter trial of sertraline in 169 combat veterans with PTSD recruited from 10 Veterans Affairs medical centers. After 1 week of placebo, the patients were randomly assigned to receive 12 weeks of flexibly dosed sertraline (mean dosage=156 mg/day among completers) or continued placebo. Total PTSD symptom reduction as measured by the CAPS did not significantly differ between the sertraline (−13.1, ±3) and placebo (−15.4, ±3.1) groups, and in both groups combat-related PTSD was associated with poorer outcome compared with non-combat-related PTSD. In a 2002 study, Zohar et al. (62) randomly assigned 42 Israeli combat veterans to sertraline (mean dosage=120 mg/day, ±60 mg) or placebo. At 10 weeks, no significant differences were noted in the total score on the CAPS-2 or on any of the three CAPS symptom cluster scores.
These findings contrast with those from a 2006 randomized controlled trial by Martenyi and Soldatenkova (63) of 144 combat veterans of the Balkan Wars recruited at eight sites in Bosnia-Herzegovina and Croatia and randomly assigned to fluoxetine (20–80 mg/day) or placebo for both a 12-week acute phase and 24-week relapse prevention phase. In the acute phase, fluoxetine was superior to placebo as measured by total score on the Treatment Outcome PTSD (TOP-8) scale (−9.05 compared with −5.20, p=0.001), total score on the CAPS (−31.12 compared with −16.07, p<0.001), all CAPS subscores, and total score on the Davidson Trauma Scale (DTS). Fluoxetine was also more effective for depression as measured by the Montgomery-Åsberg Depression Rating Scale and for anxiety symptoms as measured by the Hamilton Anxiety Scale. In the relapse prevention phase of the trial, fluoxetine was superior to placebo in sustaining improvement in TOP-8 and CAPS scores, and the risk of relapse was significantly greater in the placebo arm than in the fluoxetine arm (log-rank test χ2 = 4.9, df=1, p=0.048). The veterans of the Balkan Wars were younger than the Israeli and American combat veterans, were somewhat more recently traumatized, and had probably received less treatment for their symptoms before study entry. It is possible that negative results with older combat veterans may be due to the chronic nature of their PTSD (and co-occurring disorders) rather than a unique resistance to SSRI treatment among individuals with combat-related PTSD. Taken as a whole, these studies suggest that further research is needed to understand why these populations may have differential responses to SSRI treatment.
Other antidepressants.
In a 2006 study, Davidson et al. (64) randomly assigned 329 adult outpatients from 56 sites with a primary diagnosis of PTSD for 6 months or longer and CAPS scores of 60 or greater to receive venlafaxine, extended release (37.5–300 mg/day), or placebo. At 24 weeks, mean changes in total CAPS score from baseline were −51.7 for the venlafaxine group compared with −43.9 for the placebo group (p=0.006); improvement was significantly greater for the venlafaxine group in symptom cluster scores for reexperiencing (p=0.008) and avoidance/numbing (p=0.006) but not for hyperarousal. Remission rates (defined as a CAPS score of 20 or lower) were 50.9% for venlafaxine and 37.5% for placebo (p=0.01). A 12-week, multicenter double-blind trial (14) compared venlafaxine, extended release (37.5–300 mg/day) with sertraline (25–200 mg/day) or placebo in adult outpatients with PTSD. Mean changes from baseline CAPS-SX17 scores were −41.8, −39.4, and −33.9 for venlafaxine, sertraline, and placebo, respectively, with only venlafaxine separating from placebo in a statistically significant manner (p<0.05).
In a 2007 study, Becker et al. (65) found no between-group differences in 30 patients with civilian- or military-related PTSD who were randomly assigned to placebo or bupropion, sustained release in addition to usual pharmacological care. About half of these patients were already receiving an SSRI at the time of randomization.
In a 2004 study, Davis et al. (66) randomly assigned 41 predominantly male combat veterans with PTSD to nefazodone or placebo. After 12 weeks, they found significant improvement in the percent change in the total CAPS score from baseline in those receiving nefazodone compared with those receiving placebo using a repeated analysis of variance with last observation carried forward (p=0.04, effect size=0.6).
Finally, in a double-blind, randomized, placebo-controlled trial of 29 patients with PTSD reported in 2003 by Davidson et al. (67), mirtazapine (up to 45 mg/day) was found to be more effective than placebo on the Global Improvement item of the Short PTSD Rating Interview (but not on total Short PTSD Rating Interview score nor on the DTS total score), as well as on the Structured Interview for PTSD and anxiety subscale of the Hospital Anxiety and Depression Scale.
Head-to-head comparisons.
Studies have been published comparing nefazodone and sertraline (68), venlafaxine and sertraline (69), the SNRI reboxetine and fluvoxamine (70), and fluoxetine, moclobemide, and tianeptine (71). These studies have generally demonstrated the superiority of antidepressants to placebo but have done little to clarify the relative utility of these different antidepressants.
These data build on the relatively robust evidence basis for pharmacological treatment with antidepressant medications (particularly SSRIs and SNRIs for noncombat PTSD) compared with other classes of medications. However, the data also suggest that more effective pharmacological treatments must be identified, particularly for veterans with combat-related PTSD. It is also important to note that comparison of other pharmacotherapies with the SSRIs and SNRIs is complicated by methodological differences in the available studies.
ADRENERGIC AGENTS
β-Blockers.
A potential role for propranolol in preventing PTSD was suggested by a pilot study reported in 2002 by Pitman et al. (72), in which 32 emergency department patients received a 10-day course of propranolol or placebo, beginning within 6 hours of a trauma. Propranolol treatment did not change CAPS scores at 1 month but did decrease physiological response to script-driven imagery 3 months after the trauma. However, a 14-day randomized controlled trial reported in 2007 by Stein et al. (73) of propranolol compared with gabapentin and placebo failed to demonstrate the superiority of either medication over placebo.
Prazosin.
Among the most promising advances in the pharmacological treatment of PTSD have been a series of placebo-controlled augmentation trials demonstrating the efficacy of the α-adrenergic antagonist prazosin for the treatment of trauma-related nightmares and sleep disruption (74–76). Here, patients were allowed to continue maintenance medications, including SSRIs, as the primary outcome variables were related to sleep disturbance. However, the studies also assessed total PTSD symptoms using either the CAPS or the PTSD Checklist–Civilian Version.
The first study, reported in 2003 by Raskind et al. (75), was a double-blind, crossover trial in which 10 Vietnam combat veterans with PTSD received placebo or prazosin (mean dosage=9.6 mg/night) over a 3-week dose-titration phase and a 6-week maintenance phase. Prazosin was significantly superior to placebo in reducing nightmares (CAPS “recurrent distressing dreams” item) and sleep disturbance (CAPS “difficulty sleeping” item) and in improving global clinical status (Clinical Global Impression of Change [CGIC]), with effect size z>1.0 on all measures. The change in the total CAPS score and scores on all three CAPS cluster items was also significantly greater with prazosin than with placebo.
The second study, reported in 2007 by Raskind et al. (75), was a parallel-group trial in 40 veterans with chronic PTSD, most of whom experienced combat-related trauma in Vietnam. Patients received placebo or prazosin (mean dosage=13.3 mg/night) during a 4-week dose-titration phase and an 8-week maintenance phase. Similar improvements in nightmares, sleep disturbance, and CGIC scores (effect size=0.9) were observed. A numerically greater reduction in total CAPS score was observed with prazosin, but this did not reach statistical significance.
Finally, in a double-blind, placebo-controlled crossover study of 13 civilians with trauma-related PTSD, reported in 2008 by Taylor et al. (76), prazosin was rapidly titrated to 3 mg/night during each 3-week treatment phase. Along with clinical outcomes, sleep time and sleep latency were recorded in the final 3 nights of the treatment phase. Total sleep time was 94 minutes longer with prazosin than with placebo (374±86 minutes compared with 280±105 minutes, p<0.01, effect size=0.98), and total REM sleep and mean REM duration were also longer with prazosin. Once again, reductions in trauma nightmares, total PTSD symptoms (using the PTSD Checklist–Civilian Version), and CGIC scores were significantly changed compared with placebo.
Further investigation may clarify an optimal dosage and titration for prazosin, which, based on the above studies, appears to be effective in a range of 3 to 15 mg/night.
SECOND-GENERATION (ATYPICAL) ANTIPSYCHOTIC MEDICATIONS
In 2006, Padala et al. (77) reported the results of a small pilot study in which 20 women aged 19–94 years with PTSD from sexual and domestic abuse were randomly assigned during the acute phase to receive risperidone or placebo. A significant difference was observed between baseline and subsequent visit TOP-8 total scores beginning in week 6 and persisting through the 12th week of the study. This response pattern was also observed in the secondary outcome measures of CAPS, the HAM-D, and the Hamilton Anxiety Scale. Risperidone was also studied in an 8-week randomized controlled trial reported in 2004 by Reich et al. (78) of 19 women who met the DSM-III-R criteria related to childhood abuse. Significant differences in reduction from baseline total CAPS-2 score (z=−2.44, p=0.015) and significant reductions in CAPS-2 intrusive (z=−5.71, p<0.001) and hyperarousal (z=−2.74, p=0.006) subscores were associated with flexible dosing (0.5–8 mg/day) of risperidone. In 2008, Rothbaum et al. (79) randomly assigned 25 adult PTSD patients whose symptoms did not remit (<70% decrease in symptoms, as measured by the CAPS) with 8 weeks of open-label sertraline to augmentation with risperidone compared with placebo for an additional 8 weeks. Patients receiving placebo and risperidone did not differ in their continued improvement in symptoms of depression or PTSD over the 8 weeks of augmentation (both groups improved), although those who received risperidone showed more improvement on the DTS sleep item on post hoc analysis. A randomized, placebo-controlled augmentation study of 73 combat veterans reported in 2005 by Bartzokis et al. (80) demonstrated the superiority of risperidone to placebo in increasing response to SSRIs. These findings are consistent with the limited evidence from previous small randomized controlled trials of risperidone (81) and olanzapine (82). These data are encouraging for adjunctive treatment with a second-generation antipsychotic drug in patients who have partially responded to an SSRI or an SNRI. Patients receiving an antipsychotic medication should be monitored for side effects including weight gain and metabolic changes (83).
ANTICONVULSANTS
Randomized controlled trials of anticonvulsant medications are limited in number and have shown mixed results. In a study reported in 2007 by Tucker et al. (83), 38 civilian patients with PTSD were randomly assigned to placebo or flexibly dosed topiramate (25–400 mg/day). There were no significant differences in total CAPS scores or total Clinical Global Impression Scale scores, although patients treated with topiramate demonstrated clinically significant decreases in the TOP-8 total score and CAPS reexperiencing symptoms subscale score.
In a continuation study reported in 2006 by Connor et al. (84), 29 patients with PTSD who completed an open-label trial of tiagabine and demonstrated at least minimal improvement were randomly assigned to continued tiagabine or placebo. Benefits of treatment were maintained in the tiagabine group, and tiagabine was associated with a greater trend toward remission, but there was no statistically significant difference in remission rates, nor was there a change in rate of relapse in comparison with the placebo group.
In 2007, Davidson et al. (85) also evaluated the efficacy of tiagabine (2–4 mg/day in divided doses) in a 12-week randomized, placebo-controlled, multisite trial of 232 adult patients with PTSD. They found neither a statistically significant change from baseline CAPS score in either group nor a significant difference in any other outcome measure.
Most recently, Davis et al. (86) randomly assigned 85 older male military veterans with PTSD to an 8-week trial of divalproex compared with placebo. No difference in outcomes was noted for either group, and no improvement was noted.
FUTURE DIRECTIONS
PTSD is currently conceptualized as a response to a traumatic stressor (or stressors) that becomes generalized and maladaptive. It may be viewed as an injury that results in alterations in the neurobiological systems mediating fear response or a failure of a mechanism involved in extinction of a generalized response to a traumatic experience (i.e., a failure of extinction learning). The course and trajectory of illness in patients with PTSD are highly variable. In some cases (with or without treatment) symptoms may permanently resolve, whereas in others full-blown reoccurrence of disabling symptoms may occur over time. Symptom scales used to measure treatment response may not adequately account for the loss of productivity and diminished social function and quality of life experienced by persons with chronic symptoms. Perhaps future researchers will identify specific risk factors or biological markers for disease status and disease severity that may aid in disability assessment. At present, such evaluations must be supported by objective data regarding the effect of illness on social and occupational function, which may be impossible to obtain from the patient alone. A number of organizations provide updated information on PTSD and PTSD treatment on their web sites (Table 3). Recent studies have advanced our understanding of neurobiology of the traumatic stress response and PTSD as these relate to the processes of emotional memory, and impairment of extinction learning (53,87–90). These studies provide a theoretical basis for the mechanism of action of exposure-based CBTs as interventions that promote reprocessing and reconsolidation of emotionally laden memories of traumatic experiences and facilitate the extinction of conditioned responses to reminders of these experiences. Current pharmacological agents for PTSD also target the neurocircuitry involved in fear-related learning and memory formation and extinction. Studies point to the involvement of NMDA receptors in the process of extinction learning, suggesting a potential role for NMDA agonists such as d-cycloserine as enhancers of exposure-based psychotherapies (91,92). Trials under way at this time may augment the emerging data from pilot studies that suggest the possible benefits of NMDA agonist treatment in combination with exposure-based psychotherapies (92). Community level observations of the variability of human responses to stressful exposures have led to exploration of the genetic basis of PTSD and also to a search for the genetic polymorphisms that may explain the range of individual human responses to stress in terms of gene by environment interactions. PTSD is often present in the context of comorbid depression or substance abuse and may follow traumatic events resulting in severe physical injury (including traumatic brain injury) and chronic pain. Although several of the studies noted above assessed treatment response in terms of depressive symptoms, further study of effective treatments in patients with comorbid conditions is clearly warranted. Further study of resilience to traumatic events may also lead to clarification of the genetics governing resilience in the face of traumatic exposure, to the identification of genetic biomarkers for the disease, and to the identification of additional targets for therapeutic intervention. As novel treatments are identified, their translation into clinical trials must rely on increasingly standardized mechanisms for addressing treatment dropouts and missing data, as well as standardized definitions of treatment outcome and remission. To generalize the treatment to usual clinical populations, studies must include samples with co-occurring conditions—particularly other mood and anxiety disorders. Still other studies must address cross-cultural and multiethnic populations. Present recommendations continue to emphasize SSRIs and exposure-based psychotherapies. New understanding offers the hope of both enhancing present treatments and approaching treatment from different mechanisms.
 |
Table 3. PTSD Information and Treatment Resources
1 Rosen GM, Lilienfeld SO: Posttraumatic stress disorder: An empirical evaluation of assumptions. Clin Psychol Rev 2008; 28: 837– 863Crossref, Google Scholar
2 Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P: Trauma and posttraumatic stress disorder in the community: the 1996. Detroit Area Survey of Trauma. Arch Gen Psychiatry 1998; 55: 626– 632Crossref, Google Scholar
3 Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52: 1048– 1060Crossref, Google Scholar
4 Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL: Combat duty in Iraq and Afghanistan, mental health problems and barriers to care. N Engl J Med 2004; 351: 13– 22Crossref, Google Scholar
5 Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, Breslau N: Epidemiological risk factors for trauma and PTSD, in Risk Factors of Posttraumatic Stress Disorder. Edited by Yehuda R. Washington DC, American Psychiatry Press, 1999, pp 23– 60Google Scholar
6 McFarlane A: The contribution of epidemiology to the study of traumatic stress. Soc Psychiatry Psychiatr Epidemiol 2004; 39: 874– 882Crossref, Google Scholar
7 Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE: Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 617– 627Crossref, Google Scholar
8 Creamer M, Burgess P, McFarlane AC: Post-traumatic stress disorder: Findings from the Australian National Survey of Mental Health and Well-being. Psychol Med 2001; 31: 1237– 1247Crossref, Google Scholar
9 Ursano RJ, Li H, Zhang L, Hough C, Fullerton C, Benedek DM, Grieger T, Holloway H: Models of PTSD and traumatic stress: the importance of research “from bedside to bench to beside.” Progr Brain Res 2008; 167: 203– 215Crossref, Google Scholar
10 Yehuda R: Advances in understanding neuroendocrine alternations in PTSD and their therapeutic implications. Ann NY Acad Sci 2006; 1071: 137– 166Crossref, Google Scholar
11 Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS: Elevated CSF corticotropin-releasing factors concentrations in posttraumatic stress disorder. Am J Psychiatry 1997; 154: 624– 629Crossref, Google Scholar
12 Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD Jr: Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999; 156: 585– 588Google Scholar
13 Bremner JD, Elzinga B, Schmahl C, Vermetten E: Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res 2008; 167: 171– 186Crossref, Google Scholar
14 Pavcovich LA, Valentino RJ: Regulation of a putative neurotransmitter effect of corticotropin-releasing factor: effects of adrenalectomy. J Neurosci 1997; 17: 401– 408Crossref, Google Scholar
15 Strawn JR, Geracioti TD: Norandrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety 2008; 25: 260– 271Crossref, Google Scholar
16 Southwick SM, Bremner JD, Rasmusson A, Morgan CA 3rd, Amsten A, Charney DS: Role of norepinephrine in pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 1999; 46: 1192– 1204Crossref, Google Scholar
17 LeDoux J: The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 2003; 23: 727– 738Crossref, Google Scholar
18 Gavish M, Laor N, Bidder M, Fisher D, Fonia O, Muller U, Reiss A, Wolmer L, Karp L, Weizman R: Altered platelet peripheral-type benzodiazepine receptor in posttraumatic stress disorder. Neuropsychopharmacology 1996; 14: 181– 186Crossref, Google Scholar
19 Bremner JD, Innis RB, Southwick SM, Staib L, Zoghbi S, Charney DS: Decreased benzodiazepine receptor binding in prefrontal cortex in combat related posttraumatic stress disorder. Am J Psychiatry 2000; 157: 1120– 1126Crossref, Google Scholar
20 Geuze E, van Berckel BN, Lammertsma AA, Boellaard R, de Kloet CS, Vermetter E, Westenberg M: Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol Psychiatry 2008; 13: 74– 83Crossref, Google Scholar
21 Practice Guidelines for the Treatment of Patients with Panic Disorders, 2nd ed. Washington, DC, American Psychiatric Association, 2008Google Scholar
22 Davis M, Ressler K, Rothbaum BO, Richardson R: Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry 2006; 60: 369– 375Crossref, Google Scholar
23 Yehuda R, Brand S, Yang RK: Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry 2006; 59: 660– 663Crossref, Google Scholar
24 Glover H: A preliminary trial of nalmefene for the treatment of emotional numbing in combat veterans with posttraumatic stress disorder. Israel J Psychiatry Relat Sci 1993; 30: 255– 263Google Scholar
25 Newport DJ, Nemeroff CB: Neurobiology of posttraumatic stress disorder. Cur Opin Neurobiol 2000; 10: 211– 218Crossref, Google Scholar
26 Pittman RK, Delahanty DL: Conceptually driven pharmacologic approaches to acute trauma, CNS Spectr 2005; 10: 99– 106Crossref, Google Scholar
27 Sack WH, Clarke GN, Seeley J: Posttraumatic stress disorder across two generations of Cambodian refugees. J Am Acad Child Adolesc Psychiatry 34; 1995: 1160– 1166Crossref, Google Scholar
28 Nugent NR, Amstadter AB, Koenen KC: Genetics of posttraumatic stress disorder: Informing clinical conceptualization and promoting future research. Am J Med Genet C Semin Med Genet 2008; 148: 127– 132Crossref, Google Scholar
29 Zhang L, Li H, Su TP, Barker JL, Maric D, Fullerton CS, Webster MJ, Hough CJ, Li XX, Traumatic Stress Brain Study Group, Ursano R: P11 is up regulated in the forebrain of stressed rats by glucocorticoid acting via two specific glucocorticoid response elements in the P11 promoter. Neuroscience 2008; 153: 1126– 1134Crossref, Google Scholar
30 Comings DE, Muhleman D, Glysin R: Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol Psychiatry 1996; 40: 368– 372Crossref, Google Scholar
31 Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J: The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry 2007; 164: 1693– 1699Crossref, Google Scholar
32 Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ: Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 2008; 299: 1291– 1305Crossref, Google Scholar
33 Institute of Medicine: Treatment of PTSD: An Assessment of the Evidence. Washington, DC, National Academies Press, 2007. http://www.iom.edu/?id=47402Google Scholar
34 Forbes D, Creamer M, Phelps A, Bryant R, McFarlane A, Devilly GJ, Matthews L, Raphael B, Doran C, Merlin T, Newton S: Australian guidelines for the treatment of adults with ASD and PTSD. Aust NZ J Psychiatry 2007, 41: 637– 648Crossref, Google Scholar
35 Posttraumatic Stress Disorder (PTSD) the Management of PTSD in Adults and Children in Primary and Secondary Care. London, National Institute for Clinical Excellence, 2005.Google Scholar
36 Baldwin D, Anderson I, Nutt D, Bandelow B, Bond A, Davidson JRT, den Boer, JA, Fineberg NA, Knapp M, Scott J, Wittchen H-U: Evidence based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2005; 19: 567– 576Crossref, Google Scholar
37 Swinson, RP, Antony MM, Bleau PB, Chokka P, Craven M, Fallu A, Kjernisted K, Lanhts R, Manassis K, McIntosh D, Plamondon J, Rabheni K, Van Ameringen M, Walker JR: Clinical practice guidelines: management of anxiety disorders. Can J Psychiatry 2006; 51( Suppl 2): 1– 92Crossref, Google Scholar
38 American Psychiatric Association: Practice guideline for the treatment of patients with schizophrenia, 2nd ed. Am J Psychiatry 2004; 161: 1– 56Crossref, Google Scholar
39 Foa EB, Keane TM, Friedman MJ: Guidelines for treatment of PTSD, J Trauma Stress 2000; 13: 539– 588Crossref, Google Scholar
40 World Federation of the Societies of Biological Psychiatry: Guidelines for the pharmacological treatment of anxiety, obsessive compulsive, and PTSD. World J Biol Psychiatry 2008; 9: 248– 312Crossref, Google Scholar
41 Monson CM, Schnurr PP, Resick PA, Friedman MJ, Young-Xu Y, Stevens SP: Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol 2006; 74: 898– 907Crossref, Google Scholar
42 Chard KM: An evaluation of cognitive processing therapy for the treatment of posttraumatic stress disorder related to childhood sexual abuse. J Consult Clin Psychol 2005; 73: 965– 971Crossref, Google Scholar
43 Resick PA, Galovski TE, O'Brien UM, Scher CD, Clum GA, Young-Xu Y: A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. J Consult Clin Psychol 2008; 76: 243– 258Crossref, Google Scholar
44 Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, Resick PA, Thurston V, Orsillo SM, Haug R, Turner C, Bernardy N: Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA 2007; 297: 820– 830Crossref, Google Scholar
45 Rothbaum BO, Astin MC, Marsteller F: Prolonged exposure versus eye movement desensitization and reprocessing (EMDR) for PTSD rape victims. J Trauma Stress 2005; 18: 607– 616Crossref, Google Scholar
46 Basoglu M, Salcioglu E, Livanou M, Kalender D, Acar G: Single-session behavioral treatment of earthquake-related posttraumatic stress disorder: a randomized waiting list controlled trial. J Trauma Stress 2005; 18: 1– 11Crossref, Google Scholar
47 Basoglu M, Salcioglu E, Livanou M: A randomized controlled study of single-session behavioural treatment of earthquake-related post-traumatic stress disorder using an earthquake simulator. Psychol Med 2007; 37: 203– 213Crossref, Google Scholar
48 van der Kolk BA, Spinazzola J, Blaustein ME, Hopper JW, Hopper EK, Korn DL, Simpson WB: A randomized clinical trial of eye movement desensitization and reprocessing (EMDR), fluoxetine, and pill placebo in the treatment of posttraumatic stress disorder: treatment effects and long-term maintenance. J Clin Psychiatry 2007; 68: 37– 46Crossref, Google Scholar
49 Högberg G, Pagani M, Sundin O, Soares J, Aberg-Wistedt A, Tärnell B, Hällström T: On treatment with eye movement desensitization and reprocessing of chronic post-traumatic stress disorder in public transportation workers—a randomized controlled trial. Nord J Psychiatry 2007; 61: 54– 61Crossref, Google Scholar
50 Neuner F, Schauer M, Klaschik C, Karunakara U, Elbert T: A comparison of narrative exposure therapy, supportive counseling, and psychoeducation for treating posttraumatic stress disorder in an African refugee settlement. J Consult Clin Psychol 2004; 72: 579– 587Crossref, Google Scholar
51 Wood DP, Murphy JA, Center KB, Russ C, McLay RN, Reeves D, Pyne J, Shilling R, Hagan J, Wiederhold BK: Combat related post traumatic stress disorder: a multiple case report using virtual reality graded exposure therapy with physiological monitoring. Stud Health Technol Inform 2008; 132: 556– 561Google Scholar
52 Gerardi M, Rothbaum BO, Ressler K, Heekin M, Rizzo A: Virtual reality exposure therapy using a virtual Iraq: case report. J Trauma Stress 2008; 21: 209– 213Crossref, Google Scholar
53 Difede J, Cukor J, Jayasinghe N, Patt I, Jedel S, Spielman L, Giosan C, Hoffman HG: Virtual reality exposure therapy for the treatment of posttraumatic stress disorder following September 11, 2001. J Clin Psychiatry 2007; 68: 1639– 1647Crossref, Google Scholar
54 Busko M: Early psychotherapy, not SSRI therapy, prevents chronic PTSD in large trial. MedScape. http://www.medscape.com/viewarticle/567859Google Scholar
55 Stein DJ, Ipser JC, Seedat S: Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2006; CD002795Google Scholar
56 Marshall RD, Lewis-Fernandez R, Blanco C, Simpson HB, Lin SH, Vermes D, Garcia W, Schneier F, Neria Y, Sanchez-Lacay A, Liebowitz MR: A controlled trial of paroxetine for chronic PTSD, dissociation, and interpersonal problems in mostly minority adults. Depress Anxiety 2007; 24: 77– 84Crossref, Google Scholar
57 Stein DJ, van der Kolk BA, Austin C, Fayyad R, Clary C: Efficacy of sertraline in posttraumatic stress disorder secondary to interpersonal trauma or childhood abuse. Ann Clin Psychiatry 2006; 18: 243– 249Crossref, Google Scholar
58 Davidson JR, Connor KM, Hertzberg MA, Weisler RH, Wilson WH, Payne VM: Maintenance therapy with fluoxetine in posttraumatic stress disorder: a placebo-controlled discontinuation study. J Clin Psychopharmacol 2005; 25: 166– 169Crossref, Google Scholar
59 van der Kolk BA, Dreyfuss D, Michaels M, Shera D, Berkowitz R, Fisler R, Saxe G: Fluoxetine in posttraumatic stress disorder. J Clin Psychiatry 1994; 55: 517– 522Google Scholar
60 Hertzberg MA, Feldman ME, Beckham JC, Kudler HS, Davidson JR: Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry 2000; 12: 101– 105Crossref, Google Scholar
61 Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM: Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry 2007; 68: 711– 720Crossref, Google Scholar
62 Zohar J, Amital D, Miodownik C, Kotler M, Bleich A, Lane RM, Austin C: Double-blind placebo-controlled pilot study of sertraline in military veterans with posttraumatic stress disorder. J Clin Psychopharmacol 2002; 22: 190– 195Crossref, Google Scholar
63 Martenyi F, Soldatenkova V: Fluoxetine in the acute treatment and relapse prevention of combat-related post-traumatic stress disorder: analysis of the veteran group of a placebo-controlled, randomized clinical trial. Eur Neuropsychopharmacol 2006; 16: 340– 349Crossref, Google Scholar
64 Davidson J, Baldwin D, Stein DJ, Kuper E, Benattia I, Ahmed S, Pedersen R, Musgnung J: Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry 2006; 63: 1158– 1165Crossref, Google Scholar
65 Becker ME, Hertzberg MA, Moore SD, Dennis MF, Bukenya DS, Beckham JC: A placebo-controlled trial of bupropion SR in the treatment of chronic posttraumatic stress disorder. J Clin Psychopharmacol 2007; 27: 193– 197Crossref, Google Scholar
66 Davis LL, Jewell ME, Ambrose S, Farley J, English B, Bartolucci A, Petty F: A placebo-controlled study of nefazodone for the treatment of chronic posttraumatic stress disorder: a preliminary study. J Clin Psychopharmacol 2004; 24: 291– 297Crossref, Google Scholar
67 Davidson JR, Weisler RH, Butterfield MI, Casat CD, Connor KM, Barnett S, van Meter S: Mirtazapine vs. placebo in posttraumatic stress disorder: a pilot trial. Biol Psychiatry 2003; 53: 188– 191Crossref, Google Scholar
68 McRae AL, Brady KT, Mellman TA, Sonne SC, Killeen TK, Timmerman MA, Bayles-Dazet W: Comparison of nefazodone and sertraline for the treatment of posttraumatic stress disorder. Depress Anxiety 2004; 19: 190– 196Crossref, Google Scholar
69 Davidson J, Rothbaum BO, Tucker P, Asnis G, Benattia I, Musgnung JJ: Venlafaxine extended release in posttraumatic stress disorder: a sertraline- and placebo-controlled study. J Clin Psychopharmacol 2006; 26: 259– 267Crossref, Google Scholar
70 Spivak B, Strous RD, Shaked G, Shabash E, Kotler M, Weizman A: Reboxetine versus fluvoxamine in the treatment of motor vehicle accident-related posttraumatic stress disorder: a double-blind, fixed-dosage, controlled trial. J Clin Psychopharmacol 2006; 26: 152– 156Crossref, Google Scholar
71 Onder E, Tural U, Aker T: A comparative study of fluoxetine, moclobemide, and tianeptine in the treatment of posttraumatic stress disorder following an earthquake. Eur Psychiatry 2006; 21: 174– 179Crossref, Google Scholar
72 Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP: Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002; 51: 189– 192Crossref, Google Scholar
73 Stein MB, Kerridge C, Dimsdale JE, Hoyt DB: Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress 2007; 20: 923– 932Crossref, Google Scholar
74 Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM: Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003; 160: 371– 373Crossref, Google Scholar
75 Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O'Connell J, Taylor F, Gross C, Rohde K, McFall ME: A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry 2007; 61: 928– 934Crossref, Google Scholar
76 Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, Peskind ER, Raskind MA: Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry 2008; 63: 629– 632Crossref, Google Scholar
77 Padala PR, Madison J, Monnahan M, Marcil W, Price P, Ramaswamy S, Din AU, Wilson DR, Petty F: Risperidone monotherapy for post-traumatic stress disorder related to sexual assault and domestic abuse in women. Int Clin Psychopharmacol 2006; 21: 275– 280Crossref, Google Scholar
78 Reich DB, Winternitz S, Hennen J, Watts T, Stanculescu C: A preliminary study of risperidone in the treatment of posttraumatic stress disorder related to childhood abuse in women. J Clin Psychiatry 2004; 65: 1601– 1606Crossref, Google Scholar
79 Rothbaum BO, Killeen TK, Davidson JR, Brady KT, Connor KM, Heekin MH: Placebo-controlled trial of risperidone augmentation for selective serotonin reuptake inhibitor-resistant civilian posttraumatic stress disorder. J Clin Psychiatry 2008; 69: 520– 525Crossref, Google Scholar
80 Bartzokis G, Lu PH, Turner J, Mintz J, Saunders CS: Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry 2005; 57: 474– 479Crossref, Google Scholar
81 Hamner MB, Faldowski RA, Ulmer HG, Frueh BC, Huber MG, Arana GW: Adjunctive risperidone treatment in post-traumatic stress disorder: a preliminary controlled trial of effects on comorbid psychotic symptoms. Int Clin Psychopharmacol 2003; 18: 1– 8Crossref, Google Scholar
82 Stein MB, Kline NA, Matloff JL: Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002; 159: 1777– 1779Crossref, Google Scholar
83 Tucker P, Trautman RP, Wyatt DB, Thompson J, Wu SC, Capece JA, Rosenthal NR: Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2007; 68: 201– 206Crossref, Google Scholar
84 Connor KM, Davidson JR, Weisler RH, Zhang W, Abraham K: Tiagabine for posttraumatic stress disorder: effects of open-label and double-blind discontinuation treatment. Psychopharmacology (Berl) 2006; 184: 21– 25Crossref, Google Scholar
85 Davidson JR, Brady K, Mellman TA, Stein MB, Pollack MH: The efficacy and tolerability of tiagabine in adult patients with post-traumatic stress disorder. J Clin Psychopharmacol 2007; 27: 85– 88Crossref, Google Scholar
86 Davis LL, Davidson JR, Ward LC, Bartolucci A, Bowden CL, Petty F: Divalproex in the treatment of posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial in a veteran population. J Clin Psychopharmacol 2008; 28: 84– 88Crossref, Google Scholar
87 Quirk GJ, Garcia R, Gonzalez-Lima F: Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 2006; 60: 337– 343Crossref, Google Scholar
88 Sharot T, Delgado MR, Phelps EA: How emotion enhances the feeling of remembering. Nat Neurosci 2004; 7: 1376– 1380Crossref, Google Scholar
89 Wessa M, Flor H: Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry 2007; 164: 1684– 1692Crossref, Google Scholar
90 Guthrie RM, Bryant RA: Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med 2006; 68: 307– 311Crossref, Google Scholar
91 Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M: Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004; 61: 1136– 1144Crossref, Google Scholar
92 Myers KM, Davis M: Mechanisms of fear extinction. Mol Psychiatry 2007; 12: 120– 150Crossref, Google Scholar



