Pediatric Bipolar Disorder
Abstract
In the past decade, interest in and research on pediatric bipolar disorder (BD) has increased substantially. Prevalence rates of the disorder have doubled in outpatient settings, while twice as many research articles on pediatric BD were published in the past five years as in the prior decade. This review focuses on recent developments in the study of pediatric BD. We examine current research on the diagnostic boundaries of BD in youths, in particular the issues of episodicity and irritability, and provide assessment guidelines. We review data elucidating the pathophysiology of pediatric BD, with a focus on how these results may inform diagnosis. Finally, we discuss treatment approaches for pediatric BD, particularly psychotherapeutic interventions. Throughout the review, we pay particular attention to youths with severe chronic irritability, hyperarousal, and hyperreactivity, who reflect the population in whom the diagnosis of BD is most debated.
(Reprinted with permission from the Annual Review of Clinical Psychology 2008; 4:163–87)
INTRODUCTION AND PREVALENCE
The past decade has witnessed a dramatic increase of interest, by both researchers and clinicians, in pediatric bipolar disorder (BD). Twice as many research articles investigating pediatric BD were published in the past five years as in the entire prior decade. Clinically, the rate of pediatric BD diagnosis has doubled in outpatient clinical settings (up to 6%) (Youngstrom et al. 2005), and has quadrupled in U.S. community hospitals (up to 40%) (Blader & Carlson 2006, Case et al. 2007).
This marked upsurge of interest in pediatric BD may reflect a variety of factors. First, the suggestion of developmental variations in the presentation of BD has raised questions regarding the appropriate diagnostic criteria. Second, increased recognition of marked impairment in BD youths, and suboptimal responses to treatment, has fueled research on the disorder (Biederman et al. 2005, Geller et al. 2004, Kowatch et al. 2005b). Finally, the advent of safe and noninvasive techniques for studying brain function in children has resulted in pediatric BD becoming the focus of pathophysiology-based research (Rich et al. 2006).
This review focuses on recent developments in research on pediatric BD. First, we examine different approaches to diagnosis and discuss what we view as the optimal diagnostic strategy. We then review recent advances in pathophysiological research and discuss how these data may inform the diagnosis of BD in youths. Finally, we discuss emerging research regarding treatment. A particular focus of this review is the status of youths with severe, chronic irritability, hyperarousal, and hyperreactivity, in whom the diagnosis of BD is much debated.
DIAGNOSIS
Researchers and clinicians continue to discuss the diagnostic boundaries of pediatric BD. Over the past quarter century, the suggestion that BD may present differently in children versus adults has shaped pediatric BD research, resulting in the suggestion that modified diagnostic criteria and assessment techniques should be used in youths. The two primary developmental differences that have been suggested are (a) a distinction between discrete mood episodes separated by periods of euthymia or subsyndromal symptoms in adults, in contrast to chronic symptoms and/or rapid mood cycles in youths, and (b) the characterization of pediatric mania by severe irritability instead of by euphoria, which is seen as the cardinal symptom of adult mania. Given suggestions of developmental differences in the presentation of mania, in the mid-1990s some investigators adopted diagnostic guidelines for youths that differed from those for adults (Geller et al. 1995, Wozniak et al. 1995).
Since the mid-1990s, three approaches have dominated research on the diagnosis of BD in youths: (a) applying adult criteria to children and adolescents; (b) emphasizing cardinal symptoms (i.e., euphoria and grandiosity) and brief, rapid cycles, and (c) emphasizing severe irritability (see Table 1). We begin by reviewing diagnostic criteria provided in the Diagnostic and Statistical Manual, Fourth Edition, Text Revision (DSM-IV-TR) (Am. Psychiatr. Assoc. 2000), and then discuss each of these three approaches.
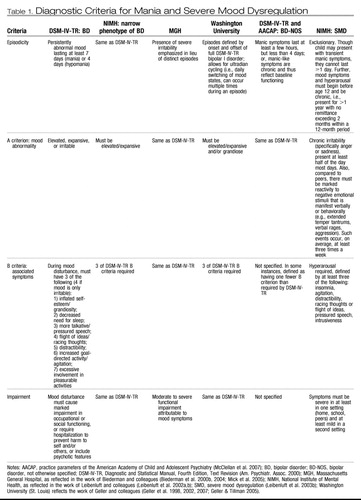 |
Table 1. Diagnostic Criteria for Mania and Severe Mood Dysregulation
DIAGNOSTIC AND STATISTICAL MANUAL DIAGNOSTIC CRITERIA
One goal of psychiatric research is the identification of biological markers that can be used to aid diagnosis. Until that time, diagnosis relies on the report of clinical descriptors and observation of symptoms. The consolidation of these clinical data into a diagnosis relies on guidelines established in the DSM-IV-TR.
Since disagreement regarding the diagnosis of BD in youths centers on the criteria for mania, our discussion focuses on mania rather than depression. The DSM-IV-TR divides mania criteria into primary and secondary symptoms. Primary symptoms, labeled criterion “A,” require “a distinct period of abnormally and persistently elevated, expansive, or irritable mood” lasting at least seven days for mania or four days for hypomania (Am. Psychiatr. Assoc. 2000). BD-I is diagnosed when a full manic episode occurs (i.e., duration ≥7 days, with symptoms resulting in marked impairment), regardless of a history of a depressive episode. BD-II is diagnosed when a hypomanic episode (duration ≥4 days, symptoms noticeable to others but not causing marked impairment) and at least one depressive episode occur.
In requiring a “distinct period,” the DSM indicates that this mood presentation must occur during a discrete episode of time. The primary “A” criterion, which defines the mood itself, is accompanied by the “B” behavioral and cognitive symptoms of mania. The “B” criteria of mania consist of grandiosity, decreased need for sleep, pressured speech, racing thoughts, distractibility, increased goal-directed activity, and excessive pleasure seeking/risky behavior. For a diagnosis of mania, a minimum of three “B” symptoms must be present if the “A” mood symptom is euphoria, whereas four “B” symptoms are required if the mood is irritability. Whereas mania requires that these “B” symptoms be “severely impairing,” hypomania requires that they be “noticeable to others.” When diagnosing mania in adults using semistructured interviews, the interviewer ascertains a distinct time frame during which the patient had a change in mood to determine if “A” criteria are met, and then determines whether a sufficient number of criterion “B” symptoms occurred concurrently.
To what extent do these guidelines accurately identify children and adolescents with BD and thus, to what extent should they be used when diagnosing a pediatric patient? The following describes the three primary approaches to this question.
APPLYING DSM-IV “ADULT” CRITERIA
One approach to diagnosing BD in youths assumes that the DSM-IV criteria identify mania in children as well as adults. Therefore, this approach follows techniques typically used in adults, with the proviso that the thresholds for viewing symptoms as “abnormal” must be developmentally appropriate. Thus, episodes during which a distinct mood change occurred are ascertained, and the interviewer determines whether the requisite number of “B” criteria occurred concurrently. This strategy is recommended by the American Academy of Child and Adolescent Psychiatry (AACAP) in its recently published practice parameter guidelines for the assessment of BD in youths (McClellan et al. 2007). It is also the approach employed by our research group at the National Institute of Mental Health (NIMH) (Dickstein et al. 2007, Leibenluft et al. 2003b, Rich et al. 2007b) (see Table 1) in the large, multisite Course and Outcome of Bipolar Youth (COBY) study (Axelson et al. 2006, Birmaher et al. 2006) as well as by other researchers (Findling et al. 2001, Kafantaris et al. 2003).
One simple question is: do children meeting strict DSM criteria for mania exist? The answer is unequivocally yes. The NIMH research group now follows nearly 100 BD youths, while the COBY study has recruited nearly 300 children meeting DSM-IV-TR criteria. Importantly, data from multiple studies indicate that both elevated mood and irritability are common in youths with mania, as in adults. An NIMH study of strictly defined BD youths (i.e., episodic euphoric mood was required for inclusion, so that the criteria were narrower than DSM-IV) found that irritability was prevalent (77%) (Bhangoo et al. 2003). Similarly, although the COBY study allowed for either euphoria or irritability to meet the “A” criterion of mania, 92% of subjects had elated mood and 84% had irritability (Axelson et al. 2006, Birmaher et al. 2006).
It is important to consider developmentally appropriate thresholds for symptoms when determining the presence of mania. As noted in the AACAP guidelines, it may be particularly challenging to differentiate symptoms including euphoria, grandiosity, psychomotor agitation, and risky behavior from normal fluctuations of extreme excitement, fantasy play and ideation, overactivity, and “youthful indiscretions” (McClellan et al. 2007). Using children of similar age and/or developmental level as a reference group may help to determine if symptoms deviate from that which would be considered age appropriate. Research on the normal developmental progression of symptoms is needed to inform diagnostic decisions.
EMPHASIZING “CARDINAL SYMPTOMS” AND BRIEF, FREQUENT CYCLES
Geller and colleagues (Geller et al. 1998) proposed a conceptualization of pediatric BD that suggested that rapid mood changes and complex cycling patterns characterize the disorder in youths (see Table 1). This definition of pediatric BD is based on their clinical experience, which found that most bipolar youths presented with very rapid mood cycles, as well as their contention that DSM-IV criteria for BD fail to adequately differentiate the disorder from attention deficit-hyperactivity disorder (ADHD) in youths. This conceptualization of pediatric BD emphasizes cycles, initially defined as mood changes lasting a minimum of four hours (Geller et al. 2003), but more recently defined as the daily switching of mood states (Geller et al. 2007). Episodes refer to the interval between the onset and offset of symptoms meeting DSM criteria, where offset is defined by the presence of eight weeks of symptoms that fail to meet full DSM criteria (Geller et al. 2007). Furthermore, to differentiate mania from ADHD, the diagnosis of mania requires either elevated mood or grandiosity (Geller et al. 1998).
Using these techniques, Geller and colleagues (Geller et al. 2004) studied BD cases presenting to multiple psychiatric and pediatric sites. The BD sample (N = 86) was quite homogenous: elated mood (90%) and grandiosity (86%), at least one of which was required for inclusion, were prominent, as were irritability (98%) and mixed mania (88%). Also consistent with Geller's conceptualization of pediatric BD, most BD patients (78%) had daily cycling, with a mean of 3.5 (±2.0) cycles per day. Subjects averaged 1.2 (±0.4) lifetime episodes, and the average length of the current episode at intake was 79.2 (±66.7) weeks. Thus, a sizeable number of subjects had experienced episodes lasting approximately three years.
EMPHASIZING IRRITABILITY
Another group of researchers argue that the irritability of pediatric mania is qualitatively and quantitatively distinct from other forms of irritability and thus can be used to identify BD (Biederman et al. 2005, Mick et al. 2005, Wozniak et al. 2005) (see Table 1). These researchers state that irritability is the most debilitating symptom in pediatric BD and that accompanying aggression is the most common reason for psychiatric hospitalization of manic children (Biederman et al. 2000a, Wozniak et al. 1995). Thus, this diagnostic approach deemphasizes distinct episodes while stressing persistent and severe irritability (Biederman et al. 1998, Wozniak et al. 1995). When assessing irritability, these investigators ask specifically about severe, extremely impairing irritability (“super angry, grouchy, or cranky”). If such irritability is endorsed, then the “A” criterion of mania is considered to be met, even if the irritability does not represent a distinct change from the patient's usual level of function (Mick et al. 2005).
Using these techniques, Biederman and colleagues describe several large samples of youths with BD recruited to a large psychiatry clinic (Biederman et al. 2005, Wozniak et al. 1995). For example, consistent with their diagnostic conceptualizations, of 129 children with BD, 92% had irritability when manic, whereas 33% had euphoria, and 61% were reported to have mixed episodes (Biederman et al. 2005). In another, similar sample, these investigators reported that none of their patients had daily cycling, but more than 75% had a chronic course characterized by rapid cycling (≥4 episodes/year) or episodes longer than a year (Biederman et al. 2004).
THE RECOMMENDED APPROACH
The dilemma facing clinicians and researchers is to determine which of the approaches described above is optimal for diagnosing BD in youths. The recent practice parameter guidelines established by AACAP for assessing, diagnosing, and treating pediatric BD state that when diagnosing hypo/mania in youths, clinicians should adhere to the DSM-IV-TR, including duration criteria, i.e., requiring an episodic change in mood lasting at least four days for hypomania or seven days for mania (McClellan et al. 2007). In commenting on particular affective symptoms, the guidelines support the use of both elevated mood and/or irritability in meeting “A” criteria, but note that irritability and emotional reactivity are present in a variety of conditions and therefore “lack specificity.” For this reason, mood disturbance must present as a “marked change” from baseline. Further, the mood disturbance should be accompanied by associated psychomotor, sleep, and cognitive features (i.e., the requisite number of DSM “B” criteria). Finally, although this is not specified in DSM-IV-TR, the AACAP guidelines note the importance of impairment occurring in at least two settings.
Thus, consistent with AACAP recommendations, assessment should focus first on determining the presence of mood episodes. Though the reporter may not be capable of identifying the precise onset or offset of symptoms, it is important to ascertain the extent to which symptoms presented during an identifiable time frame, as compared to a persistent and unremitting presentation. If the family cannot identify distinct episodes, then a BD diagnosis is likely to be inappropriate. In addition, it is critical that the clinician determine that “B” symptoms occurred simultaneous with the “A” mood changes. Furthermore, “B” symptoms should represent a distinct change from the child's usual level of function. For example, if distractibility is characteristic of a child, this presentation would be consistent with ADHD. In contrast, distractibility that occurs only in association with the onset of other mood, cognitive, and behavioral changes, or becomes markedly worse at the same time that uncharacteristic affective and behavioral symptoms are present, would be consistent with mania or hypomania. Focusing on episodicity and the presence of symptoms concurrent with the primary mood change will best allow the clinician to establish a BD diagnosis consistent with DSM criteria.
Adopting DSM adult diagnostic criteria is also the preferred approach because it best promotes research consistency. That is, employing standard adult DSM criteria allows for direct comparisons among different pediatric BD research groups, as well as between adults and children with BD. In addition, it is clear that youths can be recruited who meet DSM-IV (i.e., adult) criteria for BD. It is logical to use these youths as a gold standard against which other definitions can be tested. If, for example, youths are identified who do not meet DSM-IV criteria for BD, but resemble those who do in clinical course, family history, treatment response, and pathophysiology, it would be logical to expand the definition of pediatric BD to include that new group.
BIPOLAR DISORDER-NOT OTHERWISE SPECIFIED
The above discussion has focused on bipolar I and II. However, the populations in whom the diagnosis of BD is most controversial are those who present with unremitting, nonepisodic dysregulation in affect and behavior or whose episodes are too short to meet DSM criteria. With regard to the first group, these youths present with neither discrete mood episodes nor sustained euphoria, and thus they fail to meet DSM-IV-TR criteria for mania or BD. Should either or both of these groups be diagnosed with BD-Not Otherwise Specified (BD-NOS) or other DSM disorders, or should new categories be defined to capture them?
The diagnosis of BD-NOS was addressed in the recent AACAP practice parameter guidelines (McClellan et al. 2007) (see Table 1). These guidelines note that irritability and emotional reactivity are nonspecific symptoms found in multiple behavioral, affective, and developmental disorders and are therefore not diagnostic of mania. The AA-CAP guidelines suggest that the BD-NOS diagnosis be given to youths with either (a) manic symptoms of insufficient duration (i.e., lasting less than four days) or (b) youths with “chronic manic-like symptoms which constitute baseline functioning” (McClellan et al. 2007). However, prominent differences between these two classifications may indicate that BD-NOS, as defined by the AACAP guidelines, is clinically heterogeneous.
In the COBY study, the designation BD-NOS was used to refer to youths whose manic episodes were too short to meet DSM criteria (Birmaher et al. 2006). Specifically, BD-NOS patients had (a) elated mood plus two “B” mania symptoms, or irritable mood plus three “B” symptoms; (b) change in level of function associated with mood symptoms; (c) at least four hours of symptoms within 24 hours; and (d) at least four cumulative lifetime days meeting criteria.
The second description in the AACAP guidelines, of youths with chronic symptoms that reflect baseline functioning, is comparable to the NIMH sample defined as severely mood dysregulated (SMD) (see Table 1). Leibenluft et al. (2003b) suggested a classification system that differentiates youths with strictly defined BD (i.e., narrow phenotype) from SMD youths (i.e., the “broad phenotype” of pediatric BD). Specifically, SMD youths have chronic, severe, impairing irritability and anger. This irritability is unremitting and therefore reflects the child's baseline mood. SMD youths also present with ADHD-like hyperarousal symptoms (including at least three of the following: insomnia, intrusiveness, pressured speech, flight of ideas/racing thoughts, distractibility, and psychomotor agitation). Finally, these SMD youths have developmentally inappropriate reactivity to negative emotional stimuli: outbursts characterized by yelling and/or aggression, occurring at least three times a week. Symptoms have to begin prior to age 12 and must be chronic (i.e., present for at least one year without remission of two months or longer). Symptoms have to cause severe impairment (i.e., hospitalization, repeated grade, marked family discord) in at least one setting (home, school, peers) and mild impairment (i.e., poor academic performance, school disciplinary problems, disrupted family activities) in another. Euphoric mood or distinct episodes lasting ≥1 day are exclusionary (Leibenluft et al. 2003b). In sum, the SMD category operationalizes criteria for the chronically, severely irritable children whose diagnosis is unclear. These children often receive a BD diagnosis despite the fact that they fail to meet DSM criteria for BD (Pogge et al. 2001).
Should the diagnosis of BD-NOS be applied to both youths with episodes too short to meet DSM-IV-TR criteria and those with nonepisodic severe irritability? Research indicates that these two presentations may be distinct. In the COBY study, 25% of BD-NOS subjects converted to BD-I or BD-II after just two years of follow-up (Birmaher et al. 2006). In contrast, a posthoc analysis of the Great Smoky Mountain Study indicates that over an eight-year follow-up, only 1% of youths meeting SMD criteria converted to BD-I or BD-II (Brotman et al. 2006), a conversion rate comparable to that seen in the general population. Furthermore, BD-NOS youths from the COBY study did not differ from those with BD-I on a variety of clinical variables, including family BD history (Axelson et al. 2006). In contrast, SMD youths from the NIMH study differed from youths with strictly defined BD in the likelihood that they had a parent with BD (BD: 33%; SMD: 3%) (Brotman et al. 2007). Taken together, these data indicate that children with episodes shorter than four days may differ in clinical course and family history from those with nonepisodic irritability and hyperarousal, and that the former may resemble BD-I and BD-II patients more than do the latter. Thus, it may be inaccurate to classify both groups as having BD-NOS. Diagnosing SMD youths with BD may result in the inappropriate use of medications indicated for treating BD, or conversely, the withholding of medications indicated for ADHD and/or depression/anxiety.
Unfortunately, no single DSM diagnosis adequately captures the symptoms of severely irritable youths with symptoms of ADHD, thus rendering them “nosological orphans” (Carlson et al. 2004). Although not ideal, diagnosing SMD youths with the varied DSM-IV behavioral [e.g., ADHD or oppositional defiant disorder (ODD)] and/or mood disorders [major depressive disorder (MDD), an anxiety disorder, or mood disorder-NOS] that capture their symptomatology is recommended. Although these may not sufficiently indicate the level of impairment, they most accurately describe the constellation of symptoms afflicting SMD youths.
DIAGNOSING BIPOLAR DISORDER IN PRESCHOOLERS
As the data reviewed above indicate, it appears that the DSM diagnostic criteria for mania can be applied reliably and validly to children. The question is how young is too young for diagnosing mania and, hence, BD?
An increasing number of studies suggest that BD may occur in preschool children (Dilsaver & Akiskal 2004, Luby & Belden 2006, Scheffer & Niskala Apps 2004). In general, studies documenting BD in preschoolers report very high rates of ADHD (80% to 95%) along with mixed mood states in approximately 80% of youths (Scheffer & Niskala Apps 2004, Wilens et al. 2003). Impairing irritability is highly prevalent, and grandiosity and elated mood are also frequently reported. Importantly, these studies do not require mood episodicity when making the BD diagnosis. In fact, most studies describe a chronic, unremitting symptom presentation comparable to that seen in SMD youths, which, as previously discussed, is distinct from BD I, II, or even BD-NOS.
Most importantly, there is considerable debate regarding differentiating developmentally appropriate affect and behavior from manic symptoms or mood cycling in children younger than 6 years. For example, how can a clinician distinguish normative extreme joyfulness or giddiness in a preschooler from manic elation? What distinguishes a preschooler's developmentally appropriate talk of being a superhero from grandiosity? The question of whether it is possible to identify mania in preschoolers remains an important, but still unanswered, one. At present, the AACAP guidelines state that, given that the validity of a BD diagnosis in preschoolers has not been established, caution should be taken before diagnosing a child under age 6 with BD (McClellan et al. 2007).
COMORBIDITY
As with most childhood psychological disorders, comorbid psychopathology is common in pediatric BD (Biederman et al. 1999). For example, ADHD is thought to co-occur in approximately 70% of BD youths (Axelson et al. 2006, Dickstein et al. 2005b, Geller et al. 2004), rates of ODD range from 46% to over 80% (Axelson et al. 2006, Biederman et al. 2005), CD ranges from 12% to 41% (Axelson et al. 2006, Biederman et al. 1999), and high rates of comorbid anxiety, ranging from 45% to 78%, are also reported (Dickstein et al. 2005b, Harpold et al. 2005).
In attempting to differentiate the symptoms of BD from those of comorbid illnesses, the clinician must determine if a symptom is present solely during a mood episode or worsens significantly during a mood episode, as opposed to being present only when the child is otherwise euthymic or subsyndromally ill. A symptom isolated to a particular mood episode, or one that becomes markedly more impairing during an episode, could be “counted” toward the diagnosis of mania. Conversely, a symptom present between episodes would be considered to be a symptom of another psychological disorder.
For example, to a certain degree, diagnostic confusion between ADHD and pediatric BD reflects the overlap between the symptoms of ADHD and the “B” criteria of mania (e.g., distractibility, decreased need for sleep, talkativeness, increased goal-directed activity, excessive involvement in pleasurable activities). The critical distinction is that ADHD symptoms are constant (i.e., nonepisodic) and reflect the youth's typical behavior. In contrast, these symptoms reflect mania only if they are inconsistent with the child's baseline behavior. Thus, for certain symptoms, the DSM uses qualifying words such as “decreased need for sleep,” “more talkative than usual,” and “increase in goal-directed activity” (underline added). With regard to ODD, although negativistic and defiant behavior may occur in youths with BD, it is critical to distinguish episodic, mood-related behavioral changes consistent with mania from the unremitting behavior problems of ODD.
In sum, a child or adolescent with BD is more likely than not to have an additional disorder. If symptoms are evident solely during a particular mood episode, or if they worsen during the mood episode, they may indicate mania. Alternatively, if the symptoms are chronic and characteristic of the youth's baseline level of function, the presentation is more consistent with a nonepisodic disorder such as ODD, ADHD, or anxiety disorder.
PATHOPHYSIOLOGY
Our goal in this section is to summarize neuroscientific research that is beginning to discriminate youths with strictly defined BD from those in whom the BD diagnosis is most controversial: youths with chronic irritability and ADHD/ODD symptoms (i.e., SMD). Neuroimaging research has begun to implicate multiple regions in the pathophysiology of pediatric BD, as detailed in several recent reviews (Kowatch et al. 2005b, Pavuluri et al. 2005, Strakowski et al. 2005). Of the varied neural structures, fronto-limbic regions (e.g., the amygdala, prefrontal cortex, and striatum), which are thought to regulate cognitive and behavioral symptoms, may best differentiate the pathophysiology of BD and SMD youths.
BEHAVIORAL DATA
Standardized behavioral paradigms allow cognitive and affective function to be assessed reliably in a laboratory setting. By coupling these data with neuroimaging techniques, such as structural magnetic resonance imaging (sMRI), functional MRI (fMRI), or psychophysiological measurement, the neural circuitry mediating between-group differences in behavior can be identified.
Behavioral paradigms may be used to elucidate the neural circuitry mediating pediatric BD. These paradigms typically assess emotional function, often by measuring responses to reward, punishment, or emotional stimuli such as faces. One line of studies of emotional processing in BD youths finds that, compared to typically developing youths, children with BD are deficient in adjusting their behavior to changing contingencies (Dickstein et al. 2004, Gorrindo et al. 2005, McChure et al. 2005). Thus, consistent with clinical descriptions, BD youths struggle when required to stop one activity and switch to another with different rules. Such behavioral adaptation is mediated by the ventral prefrontal cortex-striatal-amygdala circuit, which has been implicated in neuroimaging studies of pediatric BD (see below). This inflexible response style may provide clues as to the mood changes that define the disorder. Specifically, the fluctuating mood states that characterize BD may reflect aberrant reward processing during mania (i.e., excessive goal-directed activity and pleasure seeking) and during depression (i.e., anhedonia) (Ernst et al. 2004).
Another line of behavioral studies finds that youths with BD have deficits processing emotional faces. For example, compared to healthy controls, youths with BD make significantly more errors when asked to identify the emotion displayed on both child and adult faces (McClure et al. 2005). Interestingly, a follow-up study using this same task found that misinterpretation of emotional face expressions was significantly greater in BD youths than in those with ADHD or combined anxiety and depressive disorders; the latter two groups performed comparably to controls (Figure 1) (Guyer et al. 2007). A third study found that BD youths negatively misinterpret neutral faces, in that they rate them as more hostile and fear-producing than do controls (Rich et al. 2006). Finally, a fourth study that used face stimuli in which the expression gradually morphed from completely neutral to 100% emotion found that youths with BD required significantly more intense displays of facial emotion than did controls in order to identify the expression (Rich et al. 2007a). Thus, a variety of paradigms finds that BD youths are deficient when processing emotional and nonemotional facial expressions. Given the association between social deficits and impaired face labeling in healthy individuals (De Sonneville et al. 2002), the misinterpretation of face emotions seen in these studies in BD youths may contribute to their social dysfunction.
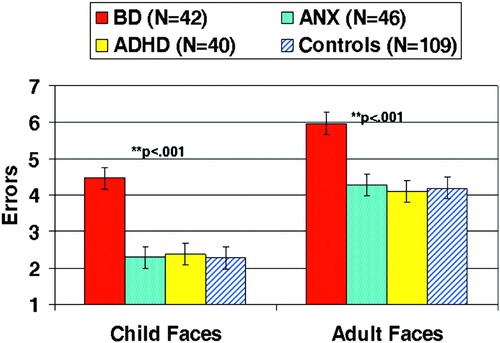
Figure 1. Significantly More Misidentifications of Emotional Facial Expressions Occur in Youths with Pediatric Bipolar Disorder than in Healthy Controls and Youths with Attention Deficit-Hyperactivity Disorder and Anxiety/Depression.
These tasks provide important data on the nature of the affective, behavioral, and cognitive deficits in pediatric BD. These results also indicate domains worthy of more detailed study via the use of neuroimaging methodology.
STRUCTURAL MAGNETIC RESONANCE IMAGING
Most published pediatric BD neuroimaging studies report sMRI data. sMRI allows for the identification of volumetric differences in brain structures between BD youths and controls. Although size does not correlate directly with function, sMRI research may identify regions of interest (ROIs) mediating the symptoms of pediatric BD.
The amygdala is of particular interest in research on developmental psychopathology. Located in the temporal lobe and part of the limbic system, the amygdala plays a central role in processing emotional stimuli and regulating subsequent emotional and behavioral responses (LeDoux 2000). The most replicated finding in sMRI research on pediatric BD is significantly decreased amygdala volume in BD youths compared with controls (Blumberg et al. 2003a, DelBello et al. 2004b, Chang et al. 2005, Dickstein et al. 2005a). However, select other studies fail to identify amygdala volume impairments (Frazier et al. 2005, Kaur et al. 2005), including the study with the largest sample, though this study's young BD population may explain the discrepancy in results (Frazier et al. 2005). In contrast to most child data, sMRI studies of BD adults have reported both increased and unchanged amygdala volume (Nugent et al. 2006).
Results of sMRI studies in other frontal and limbic regions of interest, e.g., the prefrontal cortex (PFC) and striatum, are less consistent. Regions of the PFC, including the ACC, dorsolateral PFC (DLPFC), and orbitofrontal cortex, are thought to shape behavior by regulating attention toward emotionally evocative stimuli and mediating stimulus-reward relationships (Botvinick et al. 2004, Clark et al. 2004, Fuster 2000). Volumetric deficits have been identified in the ACC (Kaur et al. 2005, Wilke et al. 2004), DLPFC (Dickstein et al. 2005a), and orbitofrontal cortex (Wilke et al. 2004), but other studies fail to identify reduced volumes in these regions in BD youths (Dickstein et al. 2005a, Kaur et al. 2005, Sanches et al. 2005).
Similarly inconsistent sMRI results are seen in studies of the striatum. The striatum mediates several functions relevant to BD, including regulation of attention and motor responses as well as adjustment of behavior in response to rewarding stimuli (O'Doherty et al. 2004). The striatum can be divided into three regions: the caudate, putamen, and accumbens. Although one study found increased caudate volume in BD youths compared with controls (Wilke et al. 2004), others found no differences between these samples (Chang et al. 2005, DelBello et al. 2004b, Sanches et al. 2005). Studies of the putamen find increased volume in BD youths (DelBello et al. 2004b, Wilke et al. 2004), whereas the accumbens may be volumetrically deficient in BD youths (Dickstein et al. 2005a).
In sum, the most consistent sMRI alteration found in BD youths is decreased amygdala volume. Although some data indicate volumetric aberrations in the PFC and striatum in BD youths, there is variability in the nature of these perturbations.
FUNCTIONAL MAGNETIC RESONANCE IMAGING
Whereas sMRI provides measurement of brain structure and volume, fMRI enables the study of brain activity associated with specific psychological constructs, such as attention or the processing of emotional stimuli. An fMRI experiment typically consists of scanning while a subject performs a behavioral/cognitive task assessing a single skill (e.g., processing face stimuli).
Although structural amygdalar deficits are consistently reported in BD youths, reports of functional amygdalar aberrations are few. Our own work with strictly defined BD youths found that when BD youths negatively misinterpreted neutral faces as threatening, they simultaneously displayed amygdala hyperactivation, in comparison with controls (see Figure 2). Further, in pediatric BD subjects, but not in controls, greater amygdala activity was associated with more hostile ratings of neutral faces (Rich et al. 2006). Thus, there was a direct relationship between amygdala hyperactivity and behavioral face processing deficits in BD youths. Pavuluri et al. (2006) recently reported amygdala hyperactivation in BD youths when processing angry and happy faces. These data contrast with those from our study, in which there were no perturbations in amygdala activity in BD subjects when processing happy or fearful faces (Rich et al. 2006). Differences in methodology (e.g., event-related versus block design, subjects rating the faces versus responding when stimuli appeared) and clinical features of the patients (e.g., mood state, medication status) may explain the inconsistency in results. Clearly, further research is required to determine if amygdala hyperactivation in BD youths is associated with the processing of specific facial expressions.
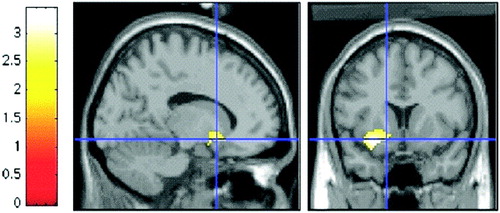
Figure 2. Significantly Greater Left Amygdala Activation is Observed in BD Youths than in Healthy Controls When Rating Their Fear of Neutral Faces.
Differences in methodology and task demands may explain the discrepant nature of fMRI results in the PFC. For example, in our study of face processing described above, we found increased activation of the ventrolateral PFC (VPFC) when BD youths rated the hostility of neutral faces (Rich et al. 2006). In contrast, decreased activation of the VPFC in BD youths was seen in response to angry and happy faces (Pavuluri et al. 2006). This same study found ACC hyperactivation in pediatric BD subjects, which was also documented in a study using tasks involving visuospatial working memory and viewing positively valenced pictures (Chang et al. 2004). Thus, comparable to sMRI results, fMRI results examining the PFC in BD subjects are inconsistent, possibly due to differences in the paradigms employed and the manner in which samples are defined as having BD.
As with the amygdala and PFC, there is variability in fMRI data examining striatal activation in BD youths. Greater activation in BD youths than controls in the accumbens and putamen regions of the striatum has been documented in studies of cognitive interference (Blumberg et al. 2003b), working memory (Chang et al. 2004), and when negatively perceiving neutral faces (Rich et al. 2006). In contrast, our group recently found that compared with controls, BD youths had reduced striatal activation during failed motor inhibition (Leibenluft et al. 2007). Thus, greater striatal activation is seen in BD youths during cognitive tasks and processing of emotional stimuli, but reduced activation is seen during a behavioral task, perhaps reflecting a failed “error signal” associated with BD patients' impulsivity.
In sum, current fMRI studies in pediatric BD subjects implicate aberrant patterns of activation in the amygdala, PFC, and striatum in the pathophysiology of the disorder. However, given that both hypo- and hyperactivation of these regions have been identified, it is not possible at this time to draw conclusions regarding the precise role of these regions in the deficits that characterize pediatric BD. Variability of the results may reflect variability in the neuroimaging methodology employed, the demands of the tasks, and the extent to which they involve emotional or nonemotional stimuli. Furthermore, discrepant findings may reflect differences in the clinical composition of the BD samples, which itself may result from varied applications of diagnostic criteria. Thus, one goal of future neuroimaging research is to attempt to improve cross-study consistency in these research variables.
DIFFERENTIATING BIPOLAR DISORDER FROM SEVERE MOOD DYSREGULATION
Research may eventually identify biological markers that can complement clinical data in the formulation of diagnoses. Indeed, recent neurocognitive research is beginning to elucidate the pathophysiological relationship between youths with clearly defined BD and SMD youths; the criteria for the latter are described above.
Behavioral data indicate that SMD and BD youths have shared and divergent pathophysiology. For example, neurocognitive similarities are seen using face emotion processing tasks. Both BD and SMD samples misidentify face emotions significantly more than do controls and youths with ADHD or anxiety/depression, and both BD and SMD subjects require more intense displays of face emotion than do controls in order to identify face emotions (Guyer et al. 2007, Rich et al. 2007a). Thus, some pathophysiological mechanisms may be common to both SMD and BD.
At the same time, other behavioral tasks suggest that the pathophysiology of SMD and BD may differ. For example, both BD and SMD subjects display deficits in altering their motor responses in response to changing task demands. However, the deficits are more consistent in subjects with BD than in those with SMD, perhaps suggesting more dysfunction in the fronto-limbic neural network, in particular the PFC (Dickstein et al. 2007). More specifically, given the role of the ACC in monitoring conflict between competing responses and shaping resulting behavior (Botvinick et al. 2004), these results suggest that the ACC may be differentially involved in the pathophysiology of BD and SMD. Thus, reward-related processes may affect decision-making more in BD than in SMD youths, perhaps consistent with the increased goal-directed activity and pleasure-seeking behaviors evident in BD mania.
The ACC is also implicated in a recent magnetic resonance spectroscopy study (Davanzo et al. 2003), which compared neurochemistry in youths with BD and those with intermittent explosive disorder, which has a clinical presentation similar to that of SMD. The pediatric BD sample had decreased ACC myo-inositol in comparison to those with intermittent explosive disorder and healthy controls. ACC myo-inositol is of interest because it is thought to be relevant to lithium's antimanic mechanism of action (Moore et al. 1999).
Paradigms eliciting irritable affect are particularly germane to the pathophysiology of both BD and SMD, since irritability is prominent in both disorders. For a child to meet criteria for SMD, chronic irritability is required. In contrast, for irritability to be counted toward a BD diagnosis, it must be present during discrete mood episodes, and at a level that is significantly above the child's baseline state; although irritability is often present between episodes in BD youths, this baseline irritability would not count toward the diagnosis of mania or depression. These distinct presentations of irritability in SMD and BD youths make it a symptom worthy of study. Therefore, we conducted a study in which we manipulated the feedback associated with a basic attention task in an effort to frustrate subjects and elicit irritability in BD (N = 35) and SMD (N = 21) youths (Rich et al. 2007b). We measured affect based on self-report, behavioral performance based on reaction time and accuracy, and brain function using psychophysiology [event-related potentials (ERPs)]. The task began with a nonemotional baseline condition, and a subsequent task used rigged feedback to frustrate subjects.
As predicted, both patient groups reported significantly greater arousal than did controls following punishment on the frustration task. However, despite the similar affective response, SMD and BD subjects differed in their behavioral and psychophysiological performance. Specifically, the deficits seen in the SMD subjects were consistent with those seen in children with ADHD: poorer accuracy and lower N1/P1 ERP amplitude (a measure of visual information processing at the initial stages of attention), regardless of emotional context. In contrast, BD subjects displayed slower reaction time and lower P3 ERP amplitude (a measure of allocation of attention), but only during the frustration task (see Figure 3). These results indicate that the affective response to frustration, similar in the BD and SMD youths, was mediated by different brain mechanisms and resulted in different patterns of behavioral deficits (Rich et al. 2007b). Overall, whereas BD youths failed to properly deploy their attention when frustrated, similar to deficits seen in youths with mood disorders (Pause et al. 2003), SMD youths showed initial attention deficits similar to those seen in ADHD youths (Jonkman et al. 2000); furthermore, these impairments were mediated by ODD in the SMD sample.
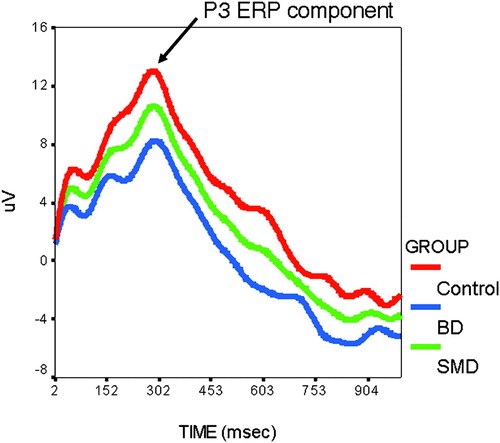
Figure 3. Youths with Pediatric Bipolar Disorder (BD), as Compared with Youths with Severe Mood Dysregulation (SMD) and Healthy Controls, Displayed Significantly Lower P3 Parietal Event-Related Potential (ERP) Amplitude When Completing an Attentional Task in a Frustrating Context.
Future neuroimaging research will continue to elucidate the neural circuitry of these disorders. At present, emerging data suggest some clinical and pathophysiological distinctions between SMD and BD youths. At the same time, the two populations share deficits in face emotion processing. Pathophysiological similarities and differences suggest that the ultimate answer to the question of whether a patient is bipolar may be dimensional, rather than categorical, reflecting the multigenic nature of these disorders' symptoms.
TREATMENT
Clinicians wishing to implement evidence-based pharmacological or psychotherapeutic treatment for their pediatric BD patients face a scarcity of data. The lack of replicated findings from randomized placebo-controlled trials, or trials comparing different psychotherapeutic approaches, precludes designating any treatment as having “strong” evidentiary support for treating pediatric BD. In the discussion below, we review separately psychopharmacological and psychotherapeutic interventions that show promise for treating pediatric BD.
PSYCHOPHARMACOLOGICAL TREATMENT
Medication is often the first intervention with a BD child, with the goal to provide an immediate reduction in symptom severity. Stabilization via medication may also better prepare the child to benefit from psychotherapeutic interventions (Fristad et al. 2003, Miklowitz et al. 2003a, Pavuluri et al. 2004). Despite the widespread use of medication in youths with BD, there are few double-blind, placebo-controlled studies.
The AACAP practice parameters (McClellan et al. 2007) recommend that treatment begin with either lithium or a medication approved by the FDA for BD in adults, such as anticonvulsants/mood stabilizers (e.g., valproate, divalproex, carbamazepine, topiramate, and lamotrigine), or atypical antipsychotics (e.g., olanzapine, quetiapine, risperidone, and aripiprazole) (McClellan et al. 2007). A separate review of psychopharmacological treatment of BD youths recommends two algorithms, based on the presence or absence of psychosis (Kowatch et al. 2005a). For the child with BD-I, manic or mixed, without psychosis, monotherapy with traditional mood stabilizers and atypical antipsychotics is advised, followed by combination therapy if there is a partial, or no response. When psychosis is present, the guidelines advise beginning with a combination of a mood stabilizer and atypical antipsychotic. With both algorithms, clozapine and/or electroconvulsive therapy are recommended as final treatment options if other medication trials fail.
In addition to the presence of psychosis, other considerations when selecting a medication include the youth's mood state, clinical variables (e.g., rapid cycling, comorbidities), risk of side effects, prior response to medication, and preferences of the patient and his/her family (McClellan et al. 2007). Patients and their families must be educated about the risks associated with medication and the manner in which adverse effects may present themselves. In general, a six- to eight-week trial of a particular medication is required before an adequately informed decision can be made regarding changing or adding medications (Kowatch et al. 2005b, McClellan et al. 2007).
Chronic medication treatment may be required to prevent relapse in youths with BD. Noncompliance with lithium treatment was associated with a 90% relapse rate in adolescents; even those who were treatment adherent relapsed 38% of the time (Strober et al. 1990). Thus, symptoms often fail to resolve on medication alone; rather, comprehensive treatment requires both psychopharmacology and psychotherapy.
PSYCHOTHERAPEUTIC TREATMENT
As with pharmacotherapy, there are few controlled studies of psychotherapy in BD youths. The AACAP practice parameters (McClellan et al. 2007) and a prior report by Goldberg-Arnold & Fristad (2002) suggest that psychotherapeutic interventions with BD youths should aim to improve (a) the child and parent's understanding of the causes, symptoms, and treatment options of BD (psychoeducation); (b) symptom management; (c) coping skills; (d) social and family relationships; (e) academic and occupational functioning; and (f) the prevention of relapse.
To these aims, a select number of interventions are beginning to demonstrate efficacy in treating young patients with BD. Treatment programs include child- and family-focused cognitive-behavioral therapy (CFF-CBT) (Pavuluri et al. 2004), multifamily psychoeducation groups (MFPG) (Fristad et al. 2002), and family-focused psychoeducational therapy (FFT) (Miklowitz et al. 2000). Whereas CFF emphasizes individual psychotherapy with children and parents, parent training and support, and family therapy, MFPG uses child and parent group therapy, and FFT adopts a family-therapy format. Though these approaches differ somewhat in the extent to which they focus on individual treatment or incorporate group and/or family approaches, many of the therapeutic strategies and aims are shared across treatments.
At their core, these treatments incorporate basic tenants of psychoeducation, interpersonal therapy, and CBT to address the affective instability and psychosocial deficits in children with BD. Sessions initially focus on educating participants about BD symptoms, suspected neurobiological causes, medications, and systems of care. Psychoeducation must be developmentally appropriate to insure that children with BD fully comprehend the educational material. These treatments also take a cognitive-behavioral approach to helping BD youths better understand their emotions, thoughts, and actions, and learn the skills to regulate their affect and behavior, cope with distorted cognitions, and improve self-efficacy. Social dysfunction is targeted via social skill building, interpersonal problem solving, and perhaps most importantly, role-playing to learn to enact these skills in social situations.
Finally, improving family functioning is a primary goal of these interventions. Using behavioral rehearsal and role-playing, family members learn to reduce conflict and augment the positive nature of family interactions. Family interventions often focus on communication enhancement, including improving active listening skills, effective delivery of positive and negative feedback, and learning ways to seek changes in others' behaviors. An additional focus is improving problem-solving skills by learning how to appropriately define and label problems, brainstorm solutions, evaluate pros and cons of alternative solutions, identify the option most likely to be successful, and implement the identified solution (Miklowitz et al. 2003b).
Initial studies support the efficacy of these psychotherapies in BD youths and their families. A 12-week course of CFF-CBT is associated with significant symptom reduction and improved overall functioning (Pavuluri et al. 2004). A recent follow-up study of CFF-CBT using psychosocial booster sessions and optimized pharmacotherapy found that, over three years, intervention resulted in the maintenance of clinically significant improvements in affect and functioning; 83% of participants responded to treatment and were experiencing no or minimal symptoms (West et al. 2007). The eight-session MFPG is associated with improvement in multiple domains, including increased knowledge about BD and ability to obtain appropriate services, overall improved family environment, more positive attitudes in both children and parents, and increased social support for BD children (Fristad 2006; Fristad et al. 2002, 2003). Finally, most studies with FFT have involved adult BD patients; these find improved compliance with medication treatment (Miklowitz 1996) and one-year maintenance of significantly reduced depression and mania symptomatology (Miklowitz et al. 2004). Recently, the completion of a two-year open trial of FFT and pharmacotherapy in adolescents resulted in significant reductions in mania and depression symptoms in youths and reduced negative parental behavior (Miklowitz et al. 2006).
In addition to these treatments, interpersonal social rhythm therapy (IPSRT) (Frank et al. 2000) may be applicable to pediatric BD patients. Based on data showing that changes in daily routine are associated with increased risk for mania and mood lability in BD adults (Leibenluft et al. 1996), IPSRT seeks to minimize the effects of life stressors on individuals' schedules by helping the patient establish regular patterns of sleep, exercise, and social interactions. IPSRT in adults has been shown to be superior to intensive clinical management (e.g., supportive psychotherapy plus psychoeducation) (Frank et al. 2005). There are no studies of IPSRT in youths with BD, but current research is studying a developmentally appropriate version of IPSRT for BD youths ages 12 to 18. This intervention targets interpersonal functioning, role transitions, regularity in sleep and social interactions, and family involvement (Hlastala & Frank 2006).
Although these therapies show promise in treating pediatric BD, it is important to emphasize that these studies are uncontrolled, meaning these have yet to display efficacy in comparison with a randomly assigned alternate treatment. Research is currently underway to determine the extent to which these interventions differ from treatment as usual, wait-list controls, or other therapeutic approaches.
Which of these psychosocial interventions is ideal? A recent study of depressed BD adults suggests they may be equally successful. A randomized controlled study examined the benefits of four disorder-specific psychotherapies in conjunction with pharmacotherapy in depressed adult patients with BD I or II (Miklowitz et al. 2007). Patients were randomly assigned either to collaborative care (N = 130), which consisted of a brief psychoeducational intervention, or intensive psychotherapy (N = 163), which consisted of either FFT, IPSRT, or CBT given weekly and biweekly for up to 30 sessions in nine months. Results found that the intensive psychotherapy was superior to collaborative care in terms of significantly higher year-end recovery rates and shorter times to recovery. Importantly, the three forms of intensive psychotherapies were comparable in their treatment efficacy. Given the extensive overlap in treatment goals, these results suggest that the active treatment component may be more important than the specific format used. Specifically, psychotherapeutic success with a BD patient is best achieved by improving the patient's knowledge of his disorder and providing him with skills to regulate his mood and behavior, better interact with peers, and better communicate and problem solve with family members. Given that this study was conducted with depressed BD adults, it is unclear if similar results would be seen in BD youths, in whom depressive states may be less common. An extension of this type of research to child and adolescent samples may delineate optimal components and potential developmental modifications of therapy for pediatric BD and determine if one form of psychotherapy is superior to another in youths with BD.
TREATING SEVERE MOOD DYSREGULATION
The above review informs treatment of youths with BD, but it is unclear the extent to which such strategies would achieve comparable efficacy in youths with SMD. Given that the above psychotherapies are nonspecific in the symptoms they target, it is possible that SMD youths would benefit from treatments that aim to improve family functioning and regulation of affect and behavior. At the same time, it is advisable that clinicians use psychotherapeutic treatments that are implicated with those DSM-IV disorders that are common in youths with SMD, namely ADHD and ODD. This is likely to include behaviorally oriented approaches, such as contingency management, clinical behavior therapy, and sociotherapy, which involve parental education and training (Jensen 2000, Steiner & Remsing 2007, Wells et al. 2000). The extent of individual therapy will depend upon the developmental level of the child or adolescent. The typical principles involved in parent management training, the approach with strongest empirical support for disruptive behavior disorders in children, include reducing positive reinforcement of disruptive behaviors while increasing reinforcement of prosocial and compliant behavior, and the consistent and immediate application of consequences and/or punishment for disruptive behaviors (Steiner & Remsing 2007). In addition, given the prominent mood disorders often seen in youths with SMD, incorporating cognitive-behavioral strategies proven effective in youths with depression and/or anxiety may be indicated (Kendall et al. 2004, Ryan 2005). As the study of SMD youths increases, incorporating investigation of research strategies will be of great benefit.
As with psychotherapy, the pharmacological treatment of SMD youths has not been studied systematically. Given the prevalence of ADHD in SMD, stimulant medications (Jensen 2000) may be indicated for treating the hyperarousal symptoms seen in SMD. Although there is concern that stimulants may induce mania in BD youths or those at risk of developing BD (DelBello et al. 2001, Carlson & Kelly 2003), it is unknown if this potential risk extends to SMD youths. Data from the large Multi-Modal Treatment of ADHD study found that ADHD youths with severe irritability (e.g., comparable to SMD youths) responded positively to stimulants without increased risk for adverse responses, relative to youths with ADHD but without irritability (Galanter et al. 2003).
The high prevalence of DSM-IV ODD in youths with SMD suggests that medications effective in reducing aggression and irritability in youths with disruptive behavior disorders may be indicated for treating these symptoms in SMD youths. These medications may include atypical antipsychotics such as risperidone (Findling et al. 2000, Schreier 1998), mood stabilizers such as divalproex (Barzman et al. 2005, DelBello et al. 2004a, Donovan et al. 2000, Steiner et al. 2003), or lithium (Campbell et al. 1995, Malone et al. 1994, 2000). Even stimulants have been shown to reduce physical and verbal aggression in youths with conduct disorder and ADHD (Connor et al. 2002, Klein et al. 1997).
Finally, given the prominent mood and anxiety symptoms seen in SMD patients, selective serotonin reuptake inhibitor (SSRI) antidepressants may be indicated, given their efficacy in treating depression and anxiety in youths (Emslie et al. 2002). SSRI use is complicated by recent “black box” warnings and concerns of a potential risk for inducing mania in youths with BD (and perhaps SMD as well). However, the careful use of SSRIs in conjunction with mood stabilizers, especially when mood stabilizers are used first, may improve functioning in BD youths without an increased risk of mania (Wagner 2004). Clearly, it is unknown the extent to which medications implicated for treating BD may be effective or harmful to SMD youths. Randomized controlled trials with SMD samples are required to understand what medications are indicated.
FUTURE DIRECTIONS
Future research on pediatric BD is likely to focus on four primary domains: (a) continuing refinement of the diagnosis of BD in children and adolescents by determining which symptoms characterize the disorder in youths and differentiate it from other psychopathologies; (b) elucidating the neurobiological mechanisms and genetic correlates of pediatric BD; (c) establishing optimal pediatric BD treatments via double-blind, placebo-controlled medication trials and empirically supported therapeutic interventions; and (d) studying youths at risk for BD, given family history of the disorder. These domains have the potential to identify neurobiological markers of susceptibility and behavioral and cognitive precursors to BD, and thus to suggest possible early intervention and/or prevention strategies.
The greatest advances may occur in research combining several of these goals. For example, future work may compare neuropsychological and imaging findings in strictly defined BD youths to SMD youths. This would provide neurobiological data to complement clinical data and thus begin to clarify the diagnostic boundaries of these syndromes. Furthermore, studies determining pathophysiological similarities and differences between youths with BD and those with ADHD, ODD, anxiety, or MDD will enable researchers to ascertain neural and cognitive correlates specific to pediatric BD.
Finally, research designed to identify the genetic correlates of BD and other psychopathologies may be facilitated by the identification of endophenotypes, i.e., behavioral deficits (e.g., face emotion misidentification) or biological findings (e.g., abnormalities in the fronto-limbic circuit) that are familial and associated with risk for a specific disorder (Glahn et al. 2004, Gottesman & Gould 2003). Studies are beginning to indicate cognitive, neuroanatomical, and neurofunctional impairments in youths at risk for BD (Chang et al. 2004, 2005; Clark et al. 2005; Ferrier et al. 2004) and thus are beginning to identify endophenotypic markers for pediatric BD.
CONCLUSION
Research on pediatric BD has increased dramatically in the past decade. Our knowledge of the clinical presentation and pathophysiology of pediatric BD is far greater than even five years ago, but critical issues remain. Perhaps greatest among them, as is seen with many childhood psychopathologies, the diagnostic boundaries of pediatric BD continue to be discussed and studied. The disparate use of assessment and diagnostic techniques is likely a primary contributing factor for the continued heterogeneity in phenomenological studies of pediatric BD (Kowatch et al. 2005b). Consistency in the nosology used to define pediatric BD, and the procedures used to determine the presence of these symptoms, is a critical need. Furthermore, the adoption of standard diagnostic procedures would foster comparative research among pediatric studies and, if adult procedures are used, comparative research with the clinical and pathophysiological literature on adult BD.
| 1. | The diagnosis of BD in youths has increased dramatically over the past decade in community clinics and hospitals. | ||||
| 2. | Although some have advocated diagnosing BD in youths by adjusting criteria to emphasize rapid cycling or severe irritability, we advise adhering to DSM criteria, in particular as they relate to requiring the presence of episodic symptoms. | ||||
| 3. | SMD youths reflect the sample in whom the diagnosis of BD is most controversial, given their chronic severe irritability and ADHD and ODD symptomatology, yet these youths appear to differ from those with BD and BD-NOS. | ||||
| 4. | Neurocognitive studies of cognitive flexibility and response to frustration are beginning to identify divergent pathophysiologies between BD and SMD youths, indicating they may be different disorders. | ||||
| 5. | Comparable deficits in face emotion identification highlight neurocognitive overlap between BD and SMD. | ||||
| 6. | More randomized control treatment studies are needed in BD youths, but initial evidence indicates that group, family, and individually based cognitive-behavioral and psychoeducational approaches may be efficacious for treating pediatric BD. | ||||
Am. Psychiatric Assoc. 2000. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: Am. Psychiatric Press. 4th ed., text rev.Google Scholar
Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, et al. 2006. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch. Gen. Psychiatry 63 ( 10): 1139– 48Crossref, Google Scholar
Barzman DH, McConville BJ, Masterson B, McElroy S, Sethuraman G, et al. 2005. Impulsive aggression with irritability and responsive to divalproex: a pediatric bipolar spectrum disorder phenotype? J. Affect. Disord. 88 ( 3): 279– 85Crossref, Google Scholar
Bhangoo RK, Dell ML, Towbin K, Myers FS, Lowe CI I, et al. 2003. Clinical correlates of episodicity in juvenile mania. J. Child Adolesc. Psychopharmacol. 13 ( 4): 507– 14Crossref, Google Scholar
Biederman J, Faraone SV, Chu MP, Wozniak J. 1999. Further evidence of a bidirectional overlap between juvenile mania and conduct disorder in children. J. Am. Acad. Child Adolesc. Psychiatry 38 ( 4): 468– 76Crossref, Google Scholar
Biederman J, Faraone SV, Wozniak J, Mick E, Kwon A, Aleardi M. 2004. Further evidence of unique developmental phenotypic correlates of pediatric bipolar disorder: findings from a large sample of clinically referred preadolescent children assessed over the last 7 years. J. Affect. Disord. 82 ( Suppl. 1): S45– 58Crossref, Google Scholar
Biederman J, Faraone SV, Wozniak J, Mick E, Kwon A, et al. 2005. Clinical correlates of bipolar disorder in a large, referred sample of children and adolescents. J. Psychiatr. Res. 39 ( 6): 611– 22Crossref, Google Scholar
Biederman J, Faraone SV, Wozniak J, Monuteaux MC. 2000a. Parsing the association between bipolar, conduct, and substance use disorders: a familial risk analysis. Biol. Psychiatry 48 ( 11): 1037– 44Crossref, Google Scholar
Biederman J, Klein RG, Pine DS, Klein DF. 1998. Resolved: mania is mistaken for ADHD in prepubertal children. J. Am. Acad. Child Adolesc. Psychiatry 37 ( 10): 1091– 96Crossref, Google Scholar
Biederman J, Mick E, Faraone SV, Spencer T, Wilens TE, Wozniak J. 2000b. Pediatric mania: a developmental subtype of bipolar disorder? Biol. Psychiatry 48 ( 6): 458– 66Crossref, Google Scholar
Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, et al. 2006. Clinical course of children and adolescents with bipolar spectrum disorders. Arch. Gen. Psychiatry 63 ( 2): 175– 83Crossref, Google Scholar
Blader JC, Carlson GA. 2006. BPD diagnosis among child and adolescent U.S. psychiatric inpatients, 1996–2003. Presented at NIMH Pediatr. Bipolar Disord. Conf., ChicagoGoogle Scholar
Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, et al. 2003a. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch. Gen. Psychiatry 60 ( 12): 1201– 8Crossref, Google Scholar
Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, et al. 2003b. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am. J. Psychiatry 160 ( 7): 1345– 47Crossref, Google Scholar
Botvinick MM, Cohen JD, Carter CS. 2004. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 8 ( 12): 539– 46Crossref, Google Scholar
Brotman MA, Kassem L, Reising M, Guyer AE, Dickstein DP, et al. 2007. Parental diagnoses in youth with narrow phenotype bipolar disorder or severe mood dysregulation. Am. J. Psychiatry 164 ( 8): 1238– 41Crossref, Google Scholar
Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, et al. 2006. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol. Psychiatry 60: 991– 97Crossref, Google Scholar
Campbell M, Adams PB, Small AM, Kafantaris V, Silva RR, et al. 1995. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J. Am. Acad. Child Adolesc. Psychiatry 34 ( 4): 445– 53Crossref, Google Scholar
Carlson GA, Kelly KL. 2003. Stimulant rebound: How common is it and what does it mean? J Child Adolesc. Psychopharmacol. 13 ( 2): 137– 42Crossref, Google Scholar
Carlson GA, Pine DS, Nottelmann E, Leibenluft E. 2004. Defining subtypes of childhood bipolar disorder: response and commentary. J. Am. Acad. Child Adolesc. Psychiatry 43: 3– 4Crossref, Google Scholar
Case BG, Olfson M, Marcus SC, Siegel C. 2007. Trends in the inpatient mental health treatment of children and adolescents in US community hospitals between 1990 and 2000. Arch. Gen. Psychiatry 64 ( 1): 89– 96Crossref, Google Scholar
Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. 2004. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch. Gen. Psychiatry 61 ( 8): 781– 92Crossref, Google Scholar
Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. 2005. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 44 ( 6): 565– 73Crossref, Google Scholar
Clark L, Cools R, Robbins TW. 2004. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 55 ( 1): 41– 53Crossref, Google Scholar
Clark L, Sarna A, Goodwin GM. 2005. Impairment of executive function but not memory in first-degree relatives of patients with bipolar I disorder and in euthymic patients with unipolar depression. Am. J. Psychiatry 162 ( 10): 1980– 82Crossref, Google Scholar
Connor DF, Glatt SJ, Lopez ID, Jackson D, Melloni RII Jr. 2002. Psychopharmacology and aggression. I: A meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. J. Am. Acad. Child Adolesc. Psychiatry 41 ( 3): 253– 61Crossref, Google Scholar
Davanzo P, Yue K, Thomas MA, Belin T, Mintz J, et al. 2003. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am. J. Psychiatry 160 ( 8): 1442– 52Crossref, Google Scholar
DelBello MP, Adler C, Strakowski SM. 2004a. Divalproex for the treatment of aggression associated with adolescent mania. J. Child Adolesc. Psychopharmacol. 14 ( 2): 325– 28Crossref, Google Scholar
DelBello MP, Soutullo CA, Hendricks W, Niemeier RT, McElroy SL, Strakowski SM. 2001. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 3 ( 2): 53– 57Crossref, Google Scholar
DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. 2004b. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 6 ( 1): 43– 52Crossref, Google Scholar
De Sonneville LM, Verschoor CA, Njiokiktjien C, Op het Veld V, Toorenaar N, Vranken M. 2002. Facial identity and facial emotions: speed, accuracy, and processing strategies in children and adults. J. Clin. Exp. Neuropsychol. 24 ( 2): 200– 13Crossref, Google Scholar
Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, et al. 2005a. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch. Gen. Psychiatry 62 ( 7): 734– 41Crossref, Google Scholar
Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, et al. 2007. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 46 ( 3): 341– 55Crossref, Google Scholar
Dickstein DP, Rich BA, Binstock AB, Pradella AG, Towbin KE, et al. 2005b. Comorbid anxiety in phenotypes of pediatric bipolar disorder. J. Child Adolesc. Psychopharmacol. 15 ( 4): 534– 48Crossref, Google Scholar
Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, et al. 2004. Neuropsychological performance in pediatric bipolar disorder. Biol. Psychiatry 55 ( 1): 32– 39Crossref, Google Scholar
Dilsaver SC, Akiskal HS. 2004. Preschool-onset mania: incidence, phenomenology and family history. J. Affect. Disord. 82 ( 1001): S35– 43Crossref, Google Scholar
Donovan SJ, Stewart JW, Nunes EV, Quitkin FM, Parides M, et al. 2000. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am. J. Psychiatry 157 ( 5): 818– 20Crossref, Google Scholar
Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, et al. 2002. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J. Am. Acad. Child Adolesc. Psychiatry 41 ( 10): 1205– 15Crossref, Google Scholar
Ernst M, Dickstein DP, Munson S, Eshel N, Pradella AG, et al. 2004. Reward-related processes in pediatric bipolar disorder: a pilot study. J. Affect. Disord. 82 ( 1001): S89– 101Crossref, Google Scholar
Ferrier IN, Chowdhury R, Thompson JM, Watson S, Young AH. 2004. Neurocognitive function in unaffected first-degree relatives of patients with bipolar disorder: a preliminary report. Bipolar Disord. 6 ( 4): 319– 22Crossref, Google Scholar
Findling RL, Gracious BL, McNamara NK, Youngstrom EA, Demeter CA, et al. 2001. Rapid, continuous cycling and psychiatric comorbidity in pediatric bipolar I disorder. Bipolar Disord. 3 ( 4): 202– 10Crossref, Google Scholar
Findling RL, McNamara NK, Branicky LA, Schluchter MD, Lemon E, Blumer JL. 2000. A double-blind pilot study of risperidone in the treatment of conduct disorder. J. Am. Acad. Child Adolesc. Psychiatry 39 ( 4): 509– 16Crossref, Google Scholar
Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, et al. 2005. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch. Gen. Psychiatry 62 ( 9): 996– 1004Crossref, Google Scholar
Frank E, Swartz HA, Kupfer DJ. 2000. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol. Psychiatry 48 ( 6): 593– 604Crossref, Google Scholar
Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, et al. 2005. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am. J. Psychiatry 162 ( 7): 1256– 65Crossref, Google Scholar
Fristad MA. 2006. Psychoeducational treatment for school-aged children with bipolar disorder. Dev. Psychopathol. 18 ( 4): 1289– 306Crossref, Google Scholar
Fristad MA, Gavazzi SM, Mackinaw-Koons B. 2003. Family psychoeducation: an adjunctive intervention for children with bipolar disorder. Biol. Psychiatry 53 ( 11): 1000– 8Crossref, Google Scholar
Fristad MA, Goldberg-Arnold JS, Gavazzi SM. 2002. Multifamily psychoeducation groups (MFPG) for families of children with bipolar disorder. Bipolar Disord. 4 ( 4): 254– 62Crossref, Google Scholar
Fuster JM. 2000. Prefrontal neurons in networks of executive memory. Brain Res. Bull. 52 ( 5): 331– 36Crossref, Google Scholar
Galanter CA, Carlson GA, Jensen PS, Greenhill LL, Davies M, et al. 2003. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J. Child Adolesc. Psychopharmacol. 13 ( 2): 123– 36Crossref, Google Scholar
Geller B, Craney JL, Bolhofner K, DelBello MP, Axelson D, et al. 2003. Phenomenology and longitudinal course of children with prepubertal and early adolescent bipolar disorder phenotype. See Geller & DelBello 2003, pp. 25– 50Google Scholar
Geller B, DelBello MP, eds. 2003. Bipolar Disorder in Childhood and Early Adolescence. New York: GuilfordGoogle Scholar
Geller B, Sun K, Zimerman B, Luby J, Frazier J, Williams M. 1995. Complex and rapid-cycling in bipolar children and adolescents: a preliminary study. J. Affect. Disord. 34 ( 4): 259– 68Crossref, Google Scholar
Geller B, Tillman R. 2005. Prepubertal and early adolescent bipolar I disorder: review of diagnostic validation by Robins and Guze criteria. J. Clin. Psychiatry 66 ( Suppl. 7): 21– 28Google Scholar
Geller B, Tillman R, Bolhofner K. 2007. Proposed definitions of bipolar I disorder episodes and daily rapid cycling phenomena in preschoolers, school-aged children, adolescents, and adults. J. Child Adolesc. Psychopharmacol. 17 ( 2): 217– 22Crossref, Google Scholar
Geller B, Tillman R, Craney JL, Bolhofner K. 2004. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch. Gen. Psychiatry 61 ( 5): 459– 67Crossref, Google Scholar
Geller B, Williams M, Zimerman B, Frazier J, Beringer L, Warner KL. 1998. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultrarapid or ultradian cycling. J. Affect. Disord. 51 ( 2): 81– 91Crossref, Google Scholar
Geller B, Zimerman B, Williams M, DelBello MP, Bolhofner K, et al. 2002. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J. Child Adolesc. Psychopharmacol. 12 ( 1): 11– 25Crossref, Google Scholar
Glahn DC, Bearden CE, Niendam TA, Escamilla MA. 2004. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 6 ( 3): 171– 82Crossref, Google Scholar
Goldberg-Arnold JS, Fristad M. 2002. Psychotherapy for children with bipolar disorder. See Geller & DelBello 2003, pp. 272– 94Google Scholar
Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. 2005. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am. J. Psychiatry 162 ( 10): 1975– 77Crossref, Google Scholar
Gottesman II, Gould TD. 2003. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160 ( 4): 636– 45Crossref, Google Scholar
Guyer AE, McClure EB, Adler A, Brotman MA, Rich BA, et al. 2007. Specificity of facial expression labeling deficits in childhood psychopathology. J. Child Psychol. Psychiatry 48: 863– 71Crossref, Google Scholar
Harpold TL, Wozniak J, Kwon A, Gilbert J, Wood J, et al. 2005. Examining the association between pediatric bipolar disorder and anxiety disorders in psychiatrically referred children and adolescents. J. Affect. Disord. 88 ( 1): 19– 26Crossref, Google Scholar
Hlastala SA, Frank E. 2006. Adapting interpersonal and social rhythm therapy to the developmental needs of adolescents with bipolar disorder. Dev. Psychopathol. 18 ( 4): 1267– 88Crossref, Google Scholar
Jensen PS. 2000. Current concepts and controversies in the diagnosis and treatment of attention deficit hyperactivity disorder. Curr. Psychiatry Rep. 2 ( 2): 102– 9Crossref, Google Scholar
Jonkman LM, Kemner C, Verbaten MN, Van Engeland II, Camfferman G, et al. 2000. Attentional capacity, a probe ERP study: differences between children with attentiondeficit hyperactivity disorder and normal control children and effects of methylphenidate. Psychophysiology 37 ( 3): 334– 46Crossref, Google Scholar
Kafantaris V, Coletti DJ, Dicker R, Padula G, Kane JM. 2003. Lithium treatment of acute mania in adolescents: a large open trial. J. Am. Acad. Child Adolesc. Psychiatry 42 ( 9): 1038– 45Crossref, Google Scholar
Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, et al. 2005. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am. J. Psychiatry 162 ( 9): 1637– 43Crossref, Google Scholar
Kendall PC, Safford S, Flannery-Schroeder E, Webb A. 2004. Child anxiety treatment: outcomes in adolescence and impact on substance use and depression at 7.4-year follow-up. J. Consult Clin. Psychol. 72 ( 2): 276– 87Crossref, Google Scholar
Klein RG, Abikoff H, Klass E, Ganeles D, Seese LM, Pollack S. 1997. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch. Gen. Psychiatry 54 ( 12): 1073– 80Crossref, Google Scholar
Kowatch RA, Fristad M, Birmaher B, Wagner KD, Findling RL, Hellander M. 2005a. Treatment guidelines for children and adolescents with bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 44 ( 3): 213– 35Crossref, Google Scholar
Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. 2005b. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord. 7 ( 6): 483– 96Crossref, Google Scholar
LeDoux JE. 2000. Emotion circuits in the brain. Annu. Rev. Neurosci. 23: 155– 84Crossref, Google Scholar
Leibenluft E, Albert PS, Rosenthal NE, Wehr TA. 1996. Relationship between sleep and mood in patients with rapid-cycling bipolar disorder. Psychiatry Res. 63 ( 2–3): 161– 68Crossref, Google Scholar
Leibenluft E, Charney DS, Pine DS. 2003a. Researching the pathophysiology of pediatric bipolar disorder. Biol. Psychiatry 53 ( 11): 1009– 20Crossref, Google Scholar
Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. 2003b. Defining clinical phenotypes of juvenile mania. Am. J. Psychiatry 160 ( 3): 430– 37Crossref, Google Scholar
Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, et al. 2007. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am. J. Psychiatry 164 ( 1): 52– 60Crossref, Google Scholar
Luby J, Belden A. 2006. Defining and validating bipolar disorder in the preschool period. Dev. Psychopathol. 18 ( 4): 971– 88Crossref, Google Scholar
Malone RP, Delaney MA, Luebbert JF, Cater J, Campbell M. 2000. A double-blind placebocontrolled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch. Gen. Psychiatry 57 ( 7): 649– 54Crossref, Google Scholar
Malone RP, Luebbert J, Pena-Ariet M, Biesecker K, Delaney MA. 1994. The Overt Aggression Scale in a study of lithium in aggressive conduct disorder. Psychopharmacol. Bull. 30 ( 2): 215– 18Google Scholar
McClellan J, Kowatch R, Findling RL. 2007. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 46 ( 1): 107– 25Crossref, Google Scholar
McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, et al. 2005. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am. J. Psychiatry 162 ( 9): 1644– 51Crossref, Google Scholar
Mick E, Spencer T, Wozniak J, Biederman J. 2005. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol. Psychiatry 58 ( 7): 576– 82Crossref, Google Scholar
Miklowitz DJ. 1996. Psychotherapy in combination with drug treatment for bipolar disorder. J. Clin. Psychopharmacol. 16 ( 2 Suppl. 1): S56– 66Google Scholar
Miklowitz DJ, Biuckians A, Richards JA. 2006. Early-onset bipolar disorder: a family treatment perspective. Dev. Psychopathol. 18 ( 4): 1247– 65Google Scholar
Miklowitz DJ, George EL, Axelson DA, Kim EY, Birmaher B, et al. 2004. Family-focused treatment for adolescents with bipolar disorder. J. Affect. Disord. 82 ( Suppl. 1): S113– 28Crossref, Google Scholar
Miklowitz DJ, George EL, Richards JA, Simoneau TL, Suddath RL. 2003a. A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Arch. Gen. Psychiatry 60 ( 9): 904– 12Crossref, Google Scholar
Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Wisniewski SR, et al. 2007. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch. Gen. Psychiatry 64 ( 4): 419– 26Crossref, Google Scholar
Miklowitz DJ, Richards JA, George EL, Frank E, Suddath RL, et al. 2003b. Integrated family and individual therapy for bipolar disorder: results of a treatment development study. J. Clin. Psychiatry 64 ( 2): 182– 91Crossref, Google Scholar
Miklowitz DJ, Simoneau TL, George EL, Richards JA, Kalbag A, et al. 2000. Family-focused treatment of bipolar disorder: 1-year effects of a psychoeducational program in conjunction with pharmacotherapy. Biol. Psychiatry 48 ( 6): 582– 92Crossref, Google Scholar
Moore GJ, Bebchuk JM, Parrish JK, Faulk MW, Arfken CL, et al. 1999. Temporal dissociation between lithium-induced changes in frontal lobe myo-inositol and clinical response in manic-depressive illness. Am. J. Psychiatry 156 ( 12): 1902– 8Google Scholar
Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, et al. 2006. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. NeuroImage 30 ( 2): 485– 97Crossref, Google Scholar
O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. 2004. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304 ( 5669): 452– 54Crossref, Google Scholar
Pause BM, Raack N, Sojka B, Goder R, Aldenhoff JB, Ferstl R. 2003. Convergent and divergent effects of odors and emotions in depression. Psychophysiology 40 ( 2): 209– 25Crossref, Google Scholar
Pavuluri MN, Birmaher B, Naylor MW. 2005. Pediatric bipolar disorder: a review of the past 10 years. J. Am. Acad. Child Adolesc. Psychiatry 44 ( 9): 846– 71Crossref, Google Scholar
Pavuluri MN, Graczyk PA, Henry DB, Carbray JA, Heidenreich J, Miklowitz DJ. 2004. Childand family-focused cognitive-behavioral therapy for pediatric bipolar disorder: development and preliminary results. J. Am. Acad. Child Adolesc. Psychiatry 43 ( 5): 528– 37Crossref, Google Scholar
Pavuluri MN, O'Connor MM, Harral E, Sweeney JA. 2006. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol. Psychiatry 62: 158– 67Crossref, Google Scholar
Pogge DL, Wayland-Smith D, Zaccario M, Borgaro S, Stokes J, Harvey PD. 2001. Diagnosis of manic episodes in adolescent inpatients: structured diagnostic procedures compared to clinical chart diagnoses. Psychiatry Res. 101 ( 1): 47– 54Crossref, Google Scholar
Rich BA, Grimley ME, Schmajuk M, Blair K, Blair RJR, Leibenluft E. 2007a. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev. Psychopathol. In pressGoogle Scholar
Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. 2007b. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am. J. Psychiatry 164 ( 2): 309– 17Crossref, Google Scholar
Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, et al. 2006. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc. Natl. Acad. Sci. USA 103 ( 23): 8900– 5Crossref, Google Scholar
Ryan ND. 2005. Treatment of depression in children and adolescents. Lancet 366 ( 9489): 933– 40Crossref, Google Scholar
Sanches M, Roberts RL, Sassi RB, Axelson D, Nicoletti M, et al. 2005. Developmental abnormalities in striatum in young bipolar patients: a preliminary study. Bipolar Disord. 7 ( 2): 153– 58Crossref, Google Scholar
Scheffer RE, Niskala Apps JA. 2004. The diagnosis of preschool bipolar disorder presenting with mania: open pharmacological treatment. J. Affect. Disord. 82 ( Suppl. 1): S25– 34Crossref, Google Scholar
Schreier HA. 1998. Risperidone for young children with mood disorders and aggressive behavior. J. Child Adolesc. Psychopharmacol. 8 ( 1): 49– 59Crossref, Google Scholar
Steiner H, Petersen ML, Saxena K, Ford S, Matthews Z. 2003. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J. Clin. Psychiatry 64 ( 10): 1183– 91Crossref, Google Scholar
Steiner H, Remsing L. 2007. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J. Am. Acad. Child Adolesc. Psychiatry 46 ( 1): 126– 41Crossref, Google Scholar
Strakowski SM, DelBello MP, Adler CM. 2005. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol. Psychiatry 10 ( 1): 105– 16Crossref, Google Scholar
Strober M, Morrell W, Lampert C, Burroughs J. 1990. Relapse following discontinuation of lithium maintenance therapy in adolescents with bipolar I illness: a naturalistic study. Am. J. Psychiatry 147 ( 4): 457– 61Crossref, Google Scholar
Wagner KD. 2004. Diagnosis and treatment of bipolar disorder in children and adolescents. J. Clin. Psychiatry 65 ( Suppl. 15): 30– 34Google Scholar
Wells KC, Pelham WE, Kotkin RA, Hoza B, Abikoff HB, et al. 2000. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J. Abnorm. Child Psychol. 28 ( 6): 483– 505Crossref, Google Scholar
West AE, Henry DB, Pavuluri MN. 2007. Maintenance model of integrated psychosocial treatment in pediatric bipolar disorder: a pilot feasibility study. J. Am. Acad. Child Adolesc. Psychiatry 46 ( 2): 205– 12Crossref, Google Scholar
Wilens TE, Biederman J, Forkner P, Ditterline J, Morris M, et al. 2003. Patterns of comorbidity and dysfunction in clinically referred preschool and school-age children with bipolar disorder. J. Child Adolesc. Psychopharmacol. 13 ( 4): 495– 505Crossref, Google Scholar
Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. 2004. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 131 ( 1): 57– 69Crossref, Google Scholar
Wozniak J, Biederman J, Kiely K, Ablon JS, Faraone SV, et al. 1995. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J. Am. Acad. Child Adolesc. Psychiatry 34 ( 7): 867– 76Crossref, Google Scholar
Wozniak J, Biederman J, Kwon A, Mick E, Faraone S, et al. 2005. How cardinal are cardinal symptoms in pediatric bipolar disorder? An examination of clinical correlates. Biol. Psychiatry 58 ( 7): 583– 88Crossref, Google Scholar
Youngstrom E, Youngstrom JK, Starr M. 2005. Bipolar diagnoses in community mental health: Achenbach Child Behavior Checklist profiles and patterns of comorbidity. Biol. Psychiatry 58 ( 7): 569– 75Crossref, Google Scholar



