Interactions Between Physicians and Industry: A Guide for Clinicians
Abstract
Physicians and the pharmaceutical industry share a convergence of interest in providing safe and effective medications to patients, but differ in their practices and ethical standards. Interactions between them are inevitable and desirable, but may create conflicts of interest for physicians. Marketing and medical education are fundamentally different and must not be confused with one another. Studies show that marketing affects physician practice, that information from industry is biased in favor of the manufacturer, and that physicians are not skilled in identifying the impact of marketing on their clinical decisions. Self-serving bias is pervasive in these interactions and is particularly difficult to detect in oneself. Guidelines regarding gifts are based on studies demonstrating their impact and generally recommend that they be limited or avoided altogether. Free samples may be of benefit to patients, but must be used with caution to avoid inappropriate drug choice. Contract services, such as research or speakers bureaus, require strict conformity to ethical and regulatory standards. Disclosure, peer review, adherence to policies and guidelines, and frank self-examination are essential to ensure the objectivity of physicians engaged in clinical care, research, and teaching.
The field of medicine exists for the sole purpose of providing care to patients who might benefit from its ministrations. Research, teaching, clinical care, and all related activities ultimately serve this single purpose and must be judged on the basis of how much and how well they contribute to the process.
Discovery and innovation, the acquisition of new knowledge, and translation of that knowledge into practical applications, have been the great strengths of American medicine. This enterprise is built on a combination of public and private investment in research and development, made possible by the commitment of tax dollars from the public sector and the promise of return on investment in the private arena.
Most basic science is the responsibility of academic and research centers, such as universities and the National Institutes of Health (NIH), the mission of which is the discovery and dissemination of knowledge. Although academic research may be sponsored by public or private monies, the largest source of public funding for medical research is the NIH, which provided $28.6 billion for intramural and grant-funded research in 2006 (1). The majority of this research remains in the public domain, mainly in the form of peer-reviewed publications. Little of this work, however, leads directly to the development of medications to benefit patients.
Industrial research, in contrast, is most often internally funded with the aim of developing a commercially viable product. The Pharmaceutical Research and Manufacturers Association (PhRMA) estimates that its member corporations invested $43 billion in research and development of new treatments in 2006 (2). Some of this research remains proprietary, but much of it enters the public domain through the U.S. Food and Drug Administration (FDA) review process, marketing, and peer-reviewed publications. The large majority of newly introduced medications are developed at least in part through this industrial enterprise.
This alliance of public and private funding, of academic and industrial research efforts, has been remarkably effective in driving the development of new drugs and other treatment innovations. In 2006 there were 22 new drugs approved in the United States, about a quarter of which were significant advances over previously available treatments (3), representing more than half of all drugs developed worldwide (2). However, this system has significant problems that must be addressed to ensure the integrity of research and the clinical care it is designed to serve.
Foremost among these issues is the quality of information available to clinicians in the treatment of their patients. Compromise in this area is especially likely in a highly competitive marketing environment in which financial stakes are large. Ironically, or perhaps inevitably, the very market forces that drive innovation also create pressures to ensure high levels of sales and return on investment.
Prescription drug costs totaled $233 billion in 2003, representing 12.4% of health care expenditures. Physician fees constituted 25.7% of the health care budget during this same period, and hospital costs, the largest single item in the health care budget, reached 35.8% (4). The single largest payor for these services is the federal government, which covers 46% of health care expenditures through Medicare, Medicaid, the Department of Veterans Affairs, and other public programs. Private insurance, including both for-profit and nonprofit companies, covered 42% of health care expenses (4).
Each of these groups approaches health care from a unique perspective and with somewhat different priorities. All have an interest in promoting patients' welfare, but each group also has financial interests that complicate health care decisions and conflict with one another. Each group seeks input into the practice of medicine, which may take the form of direct regulation, financial incentives, or control of information to physicians. The motives and limitations of each group must be assessed to make reasonable judgments regarding its contribution to medical education and health care. Perhaps most important, under the current system these groups are largely interdependent, and none can survive in the absence of the others.
MEDICAL EDUCATION AND MARKETING
The goal of medical education at all levels is the provision of accurate, objective, and useful information to clinicians who will use it to provide the highest possible quality of care to their patients. Objectivity and accuracy are essential in both research and teaching to maintain the integrity of this information.
In the best of all worlds, medical information would come from completely objective sources whose only motivation is the benefit of patients. In the real world, a variety of complicating factors and competing objectives not only pose risks to the quality of information, but even complicate the process by which that quality can be assessed.
Under the current system, the pharmaceutical industry markets its products to physicians, patients, health care systems, and government agencies to receive a return on its research investment. The appropriate outcome of this process is the provision of safe and effective drugs to patients who would benefit from them. This goal is best achieved through carefully conducted clinical studies, critical review of those data by independent experts and regulatory bodies, education of clinicians on the results of those studies, and sometimes dissemination of information directly to the public. Industry has a large and legitimate interest in providing this information to those in a position to act on it. Physicians have an interest in receiving this information to stay current on pharmaceutical development and regulatory actions. Thus, interactions between physicians and industry are both inevitable and desirable. Marketing, however, is not synonymous with education and should not be confused with it.
Medical education is about the establishment, maintenance, and certification of competence among clinical practitioners. Industry is among the largest sponsors of formal medical education. The Accreditation Council for Continuing Medical Education (ACCME) reported that $1.35 billion was spent by industry in direct payment or advertising fees for continuing medical education (CME) programs in 2005, representing 60% of all CME funding (5).
In addition, manufacturers spent $7 billion promoting their products through direct marketing contacts with physicians and more than $20 billion marketing them through advertisements in professional journals and meetings, direct-to-consumer advertising, and product samples (6). It is not surprising in the current system that the boundaries between medical education and marketing are frequently blurred.
Physicians face the difficult challenge of sorting through this information to determine what is of value for their patients. Although physicians collectively wield greater financial power than the pharmaceutical industry, the individual clinician may not sense such empowerment. Professional medical organizations and other coalitions may be a better approach (7) but have not consistently been active or effective in this area. Medical school and residency do little to train physicians in the difference between marketing and medical education or in how to ethically and responsibly interact with industry representatives as individuals or in professional groups (8).
Much of the discussion of this issue has gravitated toward extreme positions that do not fairly represent the nuances of marketing or lead to optimal decisions by physicians. The first of these positions asserts that physicians are immune to marketing and are able to maintain objectivity despite gifts, financial incentives, and other marketing factors. The opposite position, equally untenable, is that industry is by nature corrupt and physicians cannot interact with it without being corrupted by it. Neither of these perspectives is realistic or leads to behavior conducive to optimal patient care.
MARKETING GOALS AND PRACTICES
Marketing clearly affects physicians' practices (9–11). More difficult to ascertain is the degree to which that influence is in the best interest of patients. Legitimate marketing factors include the notification of physicians that pharmaceutical products are available, clinical efficacy data, safety data, regulatory information (e.g., recent FDA actions regarding the drug), cost structures, and cost effectiveness data. It is appropriate for physicians to receive and review these data and to allow them to influence clinical decision-making. Indeed, it would be irresponsible of physicians not to do so.
Marketing may also include a number of illegitimate factors in reference to patient care. These include personal relationships with marketers, gifts and other incentives, biased information, and motivational activities. Within the field of marketing, these are standard and acceptable practices. Medical ethics, in contrast, clearly demand the exclusion of these factors from clinical decision making, as they may lead to prescription of unnecessarily expensive drugs, less rational medication choices, or inappropriate recommendations for hospital formulary additions.
The primary use of marketing visits for the individual physician is to receive the latest information on recommendations regarding the manufacturer's drugs. These interactions are governed by the FDA, which regulates drug marketing, and by policies of companies that seek to keep their employees within regulatory laws. Physicians are not bound by FDA regulations in these visits or in their use of medications. It is illegal for companies to market their drugs for off-label uses, but it is neither illegal nor unethical for physicians to use them that way. Physicians should be aware of FDA rules, however, and not place pharmaceutical representatives in compromising positions by asking for unapproved information, inappropriate gifts, or other favors. Ideal topics for discussion are introductory materials to new medications or recent FDA actions regarding existing drugs, including new indications, new warnings, or revised package inserts. The physician may choose to hear the sales representative's marketing pitch, designed to highlight the positive factors regarding the drug but should also take time to review accompanying safety data and should not accept biased marketing claims at face value (12, 13). Questions regarding other information that the company has on the drug may generate a formal request for contact with a company medical liaison and may yield informative additional data. In an appropriately regulated setting, samples may be delivered.
GIFTS
Gifts to physicians have been the subject of extensive controversy, a small amount of research, and occasional guidelines or policy action. A clear consensus among researchers in this area is that gifts influence physicians, even when they do not believe it to be so and even when the gifts are of negligible value (8, 14). Patients' perceptions of gifts tend to be negative, more so than most physicians recognize (15). Gifts such as pens and note pads labeled with the name of a drug may serve the legitimate goal of reminding physicians of the availability of a specific product, but even gifts of trivial value tend to create positive feelings toward the pharmaceutical representative and the manufacturer or a sense of obligation to them, factors that are not directly related to patient welfare. Furthermore, physicians are not particularly adept at recognizing the factors that influence them (10, 16).
Consequently, both the American Medical Association (AMA) (17, 18) and PhRMA (19) have created guidelines for physicians and pharmaceutical representatives in the presentation and acceptance of gifts. The guidelines, which closely parallel one another, state that gifts should be of minimal value (PhRMA specifies a $100 limit), should be related to the physician's practice, may not include travel to meetings, and should have no strings attached, among other restrictions. Neither set of guidelines is mandatory, although several major pharmaceutical companies have accepted them as policy, creating the ironic situation of pharmaceutical representatives having to place limits on physicians who are either uninformed about or choose to disregard the AMA guidelines.
DRUG SAMPLES
Somewhat more ambiguous are free samples, which do not benefit physicians directly but may be of great value to patients who are unable to afford a potentially helpful medication, a factor cited by physicians as driving their use (20). In addition, samples may indirectly benefit physicians by broadening their base of clinical experience (20), a potentially positive effect. This practice carries the risk, however, of encouraging physicians to prescribe a more expensive drug even after the free samples are no longer available or in cases in which the drug may not be the optimal choice (9, 22–23). It is important to remember, however, that the advantage of most generic drugs is limited to cost, which is the appropriate focus of a discussion of this issue. In that context, it is noteworthy that a cost-effectiveness study of the effects of removal of samples from a health system found no impact on physician prescribing but an overall increase in patients' out-of-pocket drug costs (24). Thus, in situations in which a continued supply of these drugs will be available, they may be justified as beneficial to patients and to physicians.
CONTRACT SERVICES
Contract services provided by physicians to drug companies include management or participation in clinical trials, service on speakers bureaus, and involvement in research and marketing advisory panels. Physicians, especially those in academic settings, have expertise in clinical care, research, and teaching that may appropriately be provided in exchange for reasonable fees. Physicians must exercise care in doing so, however, to ensure that information exchanged is accurate and objective and that it is their expertise that is being provided and not their opinions being sold. Examples of inappropriate services include participation in advisory boards without meaningful input to the company's research or marketing efforts, attachment of one's name to a ghost-written paper, or presentation of a company's marketing materials in a medical education setting. Financial compensation for legitimate services must be appropriate to the time and effort involved lest it cross the sometimes diffuse boundary between contract service and gift, as might be the case when a large honorarium is offered in exchange for a trivial service. Useful tools in achievement of these goals include candid disclosure of financial interests, peer review, scrupulous adherence to regulatory guidelines, and incessant self-examination.
SPEAKERS BUREAUS
Many drug companies maintain lists of physicians and other experts with whom they contract to speak in programs involving their products. These programs are of two fundamentally different types, marketing and CME, which differ substantially in speakers' legal and ethical obligations. Marketing programs are regulated by the FDA, which strictly controls their content, requires review of every slide used, and limits commentary to approved information. Speakers are permitted to answer unsolicited questions from the audience with information from peer-reviewed publications but are not allowed to digress into topics such as off-label medication uses or alternative therapies (especially competing products) or to minimize safety concerns associated with the drugs. Most companies are aggressive about training their speakers in these rules and ensuring that they comply with them, lest they run afoul of FDA regulations. Speakers in these programs should be clear in their presentations that they are engaged in a marketing exercise, should adhere to the FDA rules completely, and should do so only if they are comfortable in that role and with the data they have contracted to present. Speakers with clinical care responsibilities have an additional duty to ensure that their contract work does not bias their clinical decisions, a goal best achieved through peer review and careful self-assessment.
Speakers at industry-supported CME programs have a more difficult course to navigate, governed by ACCME regulations but often facing pressures from marketers to highlight or shade data to favor a particular product. The magnitude of this problem has been the subject of a small but consistent body of research showing that bias in favor of the sponsor's product is typical of these presentations (10, 25). The ACCME requires a minimal disclosure of potential conflicts of interest, expertise appropriate to the topic, specific learning objectives for the program, and an effort to provide balanced information (26). Best practices in these programs include full disclosure of financial conflicts of interest, the use of slides made independently of industry, peer review of the material to be presented, and careful self-examination to ensure that opinions expressed are empirically supported and are not interchangeable based on program sponsor (27).
RESEARCH GRANTS
Pharmaceutical companies may contract with clinics and hospitals to enroll patients in clinical trials of their drugs, either for FDA approval or to obtain additional data on the performance of the medications in clinical settings. These studies are regulated by the FDA and by federal research guidelines regarding human subject research (28). Institutional review boards are charged with responsibility to ensure that these rules are followed and that patients' rights and interests, such as privacy, informed consent, and safety, are protected. An additional level of protection is added if these data are to be presented in professional publications, which generally involves peer-review and prior registration of studies to ensure that findings unfavorable to the sponsoring company are not suppressed (29).
BIAS—ORIGINS AND REMEDIES
SELF-SERVING BIAS
A substantial body of research in the social sciences reveals an unconscious tendency to favor one's own position unrealistically when conflicts exist with other possible perspectives. As an elementary school student playing sandlot baseball, I noticed an interesting pattern of runners at first base always appearing “safe” to teammates on the sidelines, but “out” to the opposing team in the field. Little did I know that a similar study involving supporters of college football teams counting penalties by their own and the opposing team found exactly this bias in favor of one's own side (30). Of particular interest in both of these cases was a lack of awareness of any bias on the part of the participants, all of whom believed they were judging fairly and impartially.
This is particularly true when judgments affect one's financial self-interests. In studies of workers comparing the fairness of payment by the hour or by the task completed, workers tended to see the fairer method of payment as the one that paid them more (31). Experimental subjects in a bargaining task that could be resolved by the two parties either agreeing to share the probability of financial gain equally or the expected financial outcome equally, each party saw as fairer the bargain that was more favorable to them (32). Babcock and Loewenstein have applied this principle to bargaining impasses in contract negotiations (33) and legal disputes (34), which may become mired in conflicting views of fairness between parties whose perspectives are skewed by self-interest. As in the previous cases, participants in these studies believed themselves to be objective and fair while consistently making decisions in their own interests.
Although none of these studies was conducted in the context of clinical practice, they create a picture with probable implications for medicine. Dana and Loewenstein (35) have extrapolated these data to the issue of gifts and their impact on medical decision making, concluding that a ban on all gifts is the only remedy. Interestingly, Babcock et al, (36) take a less draconian approach when discussing their own field of legal disputes and have reported effective strategies to reduce bias by individuals simply reviewing the weaknesses of their own perspectives or examining arguments contrary to their current views. It is not clear why a similar approach would not be effective for physicians.
It is hardly a new idea to suggest that physicians may be swayed by self-interest in clinical decisions and recommendations. Within psychiatry, it is appropriate to ask on what basis physicians maintain objectivity when making treatment decisions with major implications for their own time commitment or income, such as psychotherapy versus medication management or time-limited versus open-ended psychotherapy. Studies of self-serving bias should particularly give pause to clinicians who depend on their personal clinical experience and judgment as the primary bases for patient-care recommendations, the most common factor physicians invoke to justify their decisions (20).
These studies have even greater implications for physicians who serve on advisory boards, participate in speakers bureaus, accept contracts for research, or accept payment for other activities. The combination of immersion in a company's perspective on its drugs and receipt of financial compensation are key factors in the creation of self-serving bias. They are compounded by the risk that the profitable relationship will be sacrificed if the wrong opinions are shared.
Traditionally, the profession has depended on training insulated from costs and income, peer review, and controlled research studies to ensure independence of decision making. Within the ethics community, these conflicts are considered “intrinsic” to the practice of medicine and therefore unavoidable, in contrast to “extrinsic” conflicts, which are superimposed on medical practice from outside (37). Little evidence is available, however, to demonstrate that there is any practical difference between intrinsic and extrinsic conflicts of interest or that current training practices are effective for overcoming them. Most concerning is the unconscious nature of this bias, making it largely invisible to the individual involved. At present, the best evidence suggests that role-playing exercises may be the most effective intervention (36), but these have not yet been studied in the medical setting.
INSTITUTIONAL REGULATIONS
Institutional policies have been recommended to address issues that arise in contacts with marketers, particularly as they relate to trainees (38, 39). In 2003 the University of Michigan became the first of a small but growing number of leading academic medical centers to implement stricter rules regarding gifts and related items, such as meals. The Michigan policy forbids all industry gifts to clinicians (including food), prohibits any unsupervised contact between trainees and pharmaceutical representatives, bars the use of free samples (although vouchers are permitted), and places restrictions on industry sponsorship of educational activities (40). An AMSA survey of medical schools found similar policies more recently adopted at the University of California Davis, University of Pennsylvania, Stanford University, and Yale University (41, 42). The survey also found, however, that the large majority of U.S. medical centers neither recommend nor require such practices. Among psychiatry programs, there is little awareness of institutional policies at most training centers and little sense that they have an impact on physician behavior (8).
Objections have been raised to the absence in these policies of restrictions on faculty members' interactions with industry through contracts and grants for research, teaching, or speakers bureaus (43). These interactions, however, must be considered separately from gifts and marketing through physician visits. Contacts between academia and industry are essential for the development of new medical treatments but are subject to the same types of bias that affect other activities. Additional institutional oversight of these activities is necessary and appropriate.
DISCLOSURE
In all CME programs, most professional journals, many clinical and academic institutions, and some professional organizations (including APA), disclosure of potential conflicts of interest is required for participation in medical education programs, research, and governance. Expected levels of disclosure differ significantly among organizations, the most common being a simple listing of companies with which one has affiliations and the nature of those associations. Higher levels of disclosure may include a list of financial relationships in order of compensation received or a more explicit statement of either the total amount of money received or percentage of income derived from each activity. Examples of each of these, illustrated with the author's financial disclosure information, are shown in Table 1. APA requires the lowest level for CME programs, in accordance with ACCME rules (26) but the highest level of disclosure for participation in committees overseeing this work.
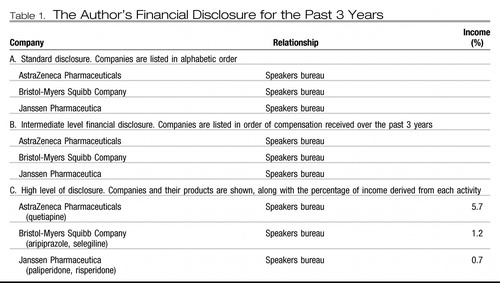 |
Table 1. The Author's Financial Disclosure for the Past 3 Years
Although it is anticipated that such a disclosure would help expose bias in a presentation or official decision, there are no studies in peer-reviewed medical journals examining this topic. One set of studies involving a nonmedical paradigm found that disclosure actually worsened the bias of the presenter, making listeners' judgments more difficult (44, 45). Not taken into account in this research, however, was the long-term impact on those making the disclosure, who, in order to maintain credibility and professional reputation, may take greater care to ensure objectivity if the extent of their involvement with industry were widely known or might be induced to restrict the degree of their involvement if it were subject to public review. Despite the dearth of data, the trend in professional organizations and journals has been toward greater degrees of disclosure. Studies of patients have indicated that they also prefer higher degrees of disclosure in clinical settings (15), although not in research studies (46).
PEER REVIEW
Medical journals have long depended on peer review to maintain the quality and integrity of professional publications. Peer review involves not only the passing of judgment on research but also an opportunity for specific, detailed critique of methodology, perspective, and conclusions. Despite a paucity of evidence that peer review is either valid or reliable, it has become the gold standard of objectivity in the conduct and dissemination of scientific research. Within clinical medicine, peer review is used to ensure the competence of individual clinicians and the integrity of clinical practice.
Peer review has only recently been included, however, in CME programs or other presentations. Some professional organizations now include a formal peer review of slides and other educational materials before their presentation. In 2007, for example, APA piloted a peer-review procedure for selected industry-supported symposia as a step toward ensuring the objectivity of those programs. The effectiveness of this approach is yet to be determined.
RESEARCH LITERACY
The current emphasis on evidence-based medicine in residency training and medical practice is dependent on high-quality research, high standards in professional publications, and the capacity of individual clinicians to review and assess pertinent research findings. Among the factors to be considered in this process is research sponsorship, specifically with regard to industry-supported studies with the potential to enhance the marketing position of a specific drug. Even in journals with the most vigorous standards of review, bias toward the sponsoring companies' products is evident (47). It would be disingenuous, however, of the medical profession to demand ever greater levels of empirical data from pharmaceutical companies concerning their products and then to disregard precisely those data on the basis of their originating in industry. Instead, additional measures of objectivity and scientific rigor are required. The recently established international registry of clinical trials (29) is an excellent step in this direction. In addition, individual physicians should temper their trust in clinical experience and make more extensive use of evidence-based decision-making. To do so, they should be encouraged to increase their familiarity with research design and seek to become more discerning consumers of the evidence-based medical literature.
SUMMARY AND RECOMMENDATIONS
Interactions between clinicians and the pharmaceutical industry are both inevitable and desirable but create potential conflicts of interest for physicians that must be addressed. A significant body of evidence shows that bias is common across a broad range of industry-sponsored activities. Among physicians, self-serving bias is difficult to detect and may be introduced by the acceptance of gifts or contracts, or by alignment with a particular product for other reasons. Physicians often underestimate the degree to which they are influenced by marketing and other nonclinical factors.
Physicians may choose to interact with marketing representatives to stay abreast of new developments with particular drugs, especially as represented by FDA actions, but must take care with other aspects of these contacts. Physicians may not be aware of the amount of control they have over their interactions with industry and would benefit from being more thoughtful and assertive in expressing their expectations and ethical standards. Perhaps the clearest issue involves gifts from marketing representatives, which are difficult to justify in most cases and have been shown to have more impact on clinical practice than physicians realize. The trend among policies in both medicine and industry is toward greater restriction of their use. Other issues also require a thoughtful and careful approach. The following recommendations are consistent with the current research, policies, and guidelines:
| •. | Regarding gifts, it is recommended that physicians familiarize themselves with and scrupulously follow the AMA guidelines, but there is no clear reason why gifts should be accepted under any circumstances. | ||||
| •. | Acceptance of samples must be done with care to ensure that they are provided to appropriate patients and result in enhancement of care. | ||||
| •. | Participation in speakers bureaus requires strict adherence to FDA regulations, frequent self-examination regarding objectivity, and periodic peer review of the consequences for clinical practice. | ||||
| •. | CME programs must be conducting independently of the supporting company, should not incorporate marketing materials, and should be reviewed personally and by peers to ensure objectivity and freedom from bias. Participants should be alert to bias and ask questions to clarify any concerns. | ||||
| •. | Contract relationships for research and consultation require careful attention to FDA and institutional policies, disclosure, peer review, and self-evaluation. | ||||
| •. | Industry-sponsored research has a place in professional journals and medical practice, but individual clinicians must become educated consumers of this literature to detect subtle sources of bias without rejecting legitimate research data. | ||||
| •. | All physicians should be aware of the potential for self-serving bias to affect these and other interactions and should take advantage of peer review, examination of alternative positions, adherence to institutional guidelines, and disclosure requirements to minimize this risk. | ||||
| •. | Physicians should collectively work to maintain the integrity of clinical practice and academic medicine through professional organizations, institutional regulations, and other activities. | ||||
We would do well to remember the words of J. Reuben Clark, who in the early 20th century left a successful career in government service to become a religious leader. His teenage daughter once objected to his rules regarding her dating by asking, “Daddy … do you not trust me?” He wisely answered, “No, my child … I do not even trust myself.” (48)
1 National Institutes of Health Office of Budget: FY 2007. Final Enacted Appropriation. http://officeofbudget.od.nih.gov/PDF/Final%20Conference%20by%20IC%20for%20Web.pdfGoogle Scholar
2 Pharmaceutical Research and Manufacturers of America: Pharmaceutical Industry Profile 2007. Washington, D.C., PhRMA, 2007Google Scholar
3 Food and Drug Administration: CDER New Molecular Entity (NME) Drug and New Biologic Approvals in Calendar Year 2006. http://www.fda.gov/cder/rdmt/InternetNME06.htmGoogle Scholar
4 Centers for Medicare and Medicaid Services, Office of the Assistant Secretary for Planning and Evaluation: An Overview of the U.S. Health Care System Chart Book; January 31, 2007. http://www.cms.hhs.gov/TheChartSeries/downloads/Chartbook_2007_pdf.pdfGoogle Scholar
5 Accreditation Council for Continuing Medical Education: ACCME Annual Report Data 2005. http://www.accme.org/dir_docs/doc_upload/9c795f02–c470-4ba3–a491-d288be965eff_uploaddocument.pdfGoogle Scholar
6 Pharmaceutical Research and Manufacturers of America: PhRMA 2006–2007. Annual Report, Washington, D.C., PhRMA, 2007. http://www.phrma.org/files/Annual_Report_2006_2007.pdfGoogle Scholar
7 Blumenthal D: Doctors and drug companies. N Engl J Med 2004; 351: 1885– 1890Crossref, Google Scholar
8 Varley CK, Jibson MD, McCarthy M, Benjamin S: A survey of the interactions between psychiatry residency programs and the pharmaceutical industry. Acad Psychiatry 2005; 29: 40– 46Crossref, Google Scholar
9 Wazana A: Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA 2000; 283: 373– 380Crossref, Google Scholar
10 Avorn J, Chen M, Hartley R: Scientific versus commercial sources of influence on the prescribing behavior of physicians. Am J Med 1982; 73: 4– 8Crossref, Google Scholar
11 Lexchin J: Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ 1993; 149: 1401– 1407Google Scholar
12 Lexchin J: What information do physicians receive from pharmaceutical representatives? Can Fam Physician 1997; 43: 941– 945Google Scholar
13 Zeigler MG, Lew P, Singer BC: The accuracy of drug information from pharmaceutical sales representatives. JAMA 1995; 273: 1296– 1298Crossref, Google Scholar
14 Katz D, Caplan AL, Merz JF: All gifts large and small: toward an understanding of the ethics of pharmaceutical industry gift giving. Am J Bioethics 2003; 3: 39– 46Crossref, Google Scholar
15 Gibbons RV, Landry FJ, Blouch DL, Jones DL, Williams FK, Lucey CR, Kroenke K: A comparison of physicians' and patients' attitudes toward pharmaceutical industry gifts. J Gen Intern Med 1998; 13: 151– 154Crossref, Google Scholar
16 Chren M-M, Landefeld CS: Physicians' behavior and their interactions with drug companies: a controlled study of physicians who requested additions to a hospital drug formulary. JAMA 1994; 271: 684– 689Crossref, Google Scholar
17 American Medical Association, Council on Ethical and Judicial Affairs: Gifts to physicians from industry. JAMA 1991; 265: 50 1Google Scholar
18 American Medical Association: Ethical Opinions and Guidelines. http://www.ama-assn.org/ama/pub/category/4001.htmlGoogle Scholar
19 Pharmaceutical Research and Manufacturers of America: PhRMA Code on Interactions with Healthcare Professionals, revised 2004. http://www.phrma.org/files/PhRMA%20Code.pdfGoogle Scholar
20 Schumock GT, Walton SM, Park HY, Nutescu EA, Blackburn JC, Finley JM, Lewis RK: Factors that influence prescribing decisions. Ann Pharmacotherapy 2004; 38: 557– 562Crossref, Google Scholar
21 Adair RF, Holmgren LR: Do drug samples influence resident prescribing behavior? A randomized trial. Am J Med 2005; 118: 881– 884Crossref, Google Scholar
22 Chew LD, O'Young TS, Hazlet TK, Bradley KA, Maynard C, Lessler DS: A physician survey of the effect of drug sample availability on physician's behavior. J Gen Intern Med 2000; 15: 478– 483Crossref, Google Scholar
23 Morelli D, Koenigsberg MR: Sample medication dispensing in a residency practice. J Fam Pract 1992; 34: 42– 48Google Scholar
24 Lurk JT, DeJong DJ, Woods TM, Knell ME, Carroll CA: Effects of changes in patient cost sharing and drug sample policies on prescription drug costs and utilization in a safety-net-provider setting. Am J Health Syst Pharm 2004; 61: 267– 272Crossref, Google Scholar
25 Bowman MA, Pearle DL: Changes in drug prescribing patterns related to commercial funding of continuing medical education. J Cont Educ Health Prof 1982; 8: 13– 20Crossref, Google Scholar
26 Accreditation Council for Continuing Medical Education: ACCME Standards of Commercial Support: Standards to Ensure the Independence of CME Activities. http://www.accme.org/dir_docs/doc_upload/68b2902a-fb73-44d1-8725-80a1504e520c_uploaddocument.pdfGoogle Scholar
27 Rosner F: Pharmaceutical industry support for continuing medical education programs: a review of current ethical guidelines. Mt Sinai J Med 1995; 62: 427– 463Google Scholar
28 The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research: The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. April 18, 1979. http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.htmGoogle Scholar
29 De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJPM, Torben V, Schroeder TV, Hal C, Sox HC, Van Der Weyden MB. Clinical Trial Registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med 2004; 351: 1250– 1251Crossref, Google Scholar
30 Hastorf A, Cantril H: They saw a game: a case study. J Abnorm Psychol 1954; 49: 129– 134Crossref, Google Scholar
31 Messick D, Sentis K: Fairness and preference. J Exp Soc Psychol 1979; 15: 418– 434Crossref, Google Scholar
32 Roth AE, Murnigham KJ: The role of information in bargaining: an experimental study. Econometrica 1982; 50: 1123– 1142Crossref, Google Scholar
33 Babcock L, Wang X, Loewenstein G: Choosing the wrong pond: social comparisons that reflect a self-serving bias. Q J Econ 1996; 111: 1– 19Crossref, Google Scholar
34 Babcock L, Loewenstein G: Explaining bargaining impasse: the role of self-serving bias. J Econ Perspect 1997; 11: 109– 126Crossref, Google Scholar
35 Dana J, Loewenstein G: A social science perspective on gifts to physicians from industry. JAMA 2003; 290: 252– 255Crossref, Google Scholar
36 Babcock L, Loewenstein G, Issacharoff S. Creating convergence: debiasing biased litigants. Law Soc Inquiry 1997; 22: 913– 925Crossref, Google Scholar
37 Sollitto S, Hoffman S, Mehlman M, Lederman RJ, Youngner SJ, Lederman MM: Intrinsic conflicts of interest in clinical research: a need for disclosure. Kennedy Inst Ethics J 2003; 13: 83– 91Crossref, Google Scholar
38 McCormick BB, Tomlinson G, Brill-Edwards P, Detsky AS: Effect of restricting contact between pharmaceutical company representatives and internal medicine residents on posttraining attitudes and behavior. JAMA 2001; 286: 1994– 1999Crossref, Google Scholar
39 Wazana A, Granich A, Primeau F, Bhanji NH, Jalbert M: Using the literature in developing McGill's guidelines for interactions between residents and the pharmaceutical industry. Acad Med 2004; 79: 1033– 1040Crossref, Google Scholar
40 University of Michigan Hospitals and Health Centers Policies and Procedures: Vendor Visitation and Interaction. Policy 01-04-008. http://www.med.umich.edu/i/policies/umh/01-04-008.htmGoogle Scholar
41 American Medical Student Association: AMSA's 2007 PharmFree Scorecard. http://www.amsa.org/prof/scorecard07.pdfGoogle Scholar
42 Brennan TA, Rothman DJ, Blank L, Blumenthal D, Chimonas SC, Cohen JJ, Goldman J, Kassirer JP, Kimball H, Naughton J, Smelser N: Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA 2006; 295: 429– 433Crossref, Google Scholar
43 Cain DM, Loewenstein G, Moore DA: The dirt on coming clean: perverse effects of disclosing conflicts of interest. J Legal Stud 2005; 34: 1– 25Crossref, Google Scholar
44 Coleman DL, Kazdin AE, Miller LA, Morrow JS, Udelsman R: Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school's approach. Acad Med 2006; 81: 154– 160Crossref, Google Scholar
45 Moore DA, Cain DM, Loewenstein G, Bazerman MH (eds): Conflict of Interest: Challenges and Solutions in Business, Law, Medicine, and Public Policy. New York, Cambridge University Press, 2005Google Scholar
46 Hampson LA, Agrawal M, Joffe S, Gross CP, Verter J, Emanuel EJ: Patients' views on financial conflicts of interest in cancer research trials. N Engl J Med 2006; 355: 2330– 2337Crossref, Google Scholar
47 Ridker PM, Torres J: Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000–2005. JAMA 2006; 295: 2270– 2274Crossref, Google Scholar
48 Lee HB: Teachings of Harold B. Lee. Edited by Williams CJ. Salt Lake City, Utah, Deseret Book, 1996, p. 629Google Scholar



