A Brief Overview of the Genetics of Bipolar Disorder
Abstract
The contribution of genetic factors to the development of bipolar disorder is now well established. Efforts to identify specific genes that are important in the etiology of bipolar disorder are currently underway and will be aided by the convergence of advances in our knowledge of the human genome and improvements in genotyping technology. Although the applications of genetic information to clinical practice are limited at present, they are expected to grow rapidly.
Psychiatric disorders, including bipolar disorder, are thought to be genetically complex, meaning that effects of multiple genes, combined with environmental risk factors, lead to the development of illness. It is thought that each gene probably contributes only a small amount to the overall risk for a disorder, making finding the individual genes that underlie major psychiatric disorders difficult. Coupled to the complexity of the underlying genetics, additional methodological issues that are of special importance to the study of psychiatric genetics also pose further challenges. For example, early studies of affective disorders did not adopt the modern distinction between unipolar and bipolar illness, and questions still remain regarding the validity of our current diagnostic system (1, 2). In addition, we lack identifiable biological markers for psychiatric disorders, and it is likely that our syndromic definitions of disease result in the inclusion of a heterogeneous group of disorders under various diagnostic headings, which would be expected to decrease our ability to find associated genes. Furthermore, even recent studies will sometimes adopt “broad” and “narrow” definitions of their phenotype (i.e., observable characteristics of an individual) of interest, which may change which individuals are counted as being affected with a disorder. Also, because psychiatric disorders may develop across an individual’s lifetime, it is sometimes difficult to conclusively establish “affected” from “unaffected” populations at any one point in time. Dividing patients into subcategories based on clinical factors (e.g., age of onset, treatment response, or comorbid symptoms) may aid in genetic studies of psychiatric disorders by selecting more homogeneous groups. Despite these challenges, the study of psychiatric genetics is an active area of investigation, and there is a wealth of evidence that supports the role of genetic factors in the development of bipolar disorder, which is briefly summarized here.
Establishing the role of genetic factors in bipolar illness
Family, twin, and adoption studies all help in the investigation of the relative contributions of genetic and environmental factors to a disorder. A first step in establishing the heritable nature of bipolar disorder comes from family studies, which look at rates of diagnoses in relatives of those affected with bipolar disorder, compared with rates in relatives of unaffected individuals. Family studies of bipolar disorder have consistently shown increased risks for the development of bipolar disorder among family members of affected patients in comparison with control groups. Estimations of the risk of bipolar disorder among first-degree family members of patients with bipolar disorder have ranged from 5 to 10 times greater than the general population baseline risk of approximately 1% (1, 3, 4). Of note, family studies of bipolar disorder also demonstrate an increased risk of unipolar depression (approximate twofold increased risk above that for the general population) and schizoaffective disorder (5, 6). In addition, further increased risks for bipolar disorder have been seen in family members of individuals with early-onset bipolar disorder compared with those with later-onset symptoms, perhaps indicating a stronger genetic form of the disorder in select families (7–10).
However, establishment of a disorder as familial does not guarantee that genes are involved, as disorders may aggregate in families for nongenetic reasons as well (i.e., shared environment). For this reason, twin studies may help to determine the relative contributions of genes and the environment to the development of a disease. Comparisons of the rates of bipolar disorder among twins who are assumed to share similar environmental influences but differ by the amount of shared genetic factors (100% shared genes in monozygotic/identical twins versus 50% shared genes in dizygotic/fraternal twins) have consistently shown higher concordance among monozygotic twins, attributed to the influence of genetic factors. It should be noted, however, that the concordance rate among monozygotic twins for bipolar disorder is not 100%, thus indicating that genetic factors are not solely responsible for the development of the disorder. In fact, heritability, defined as the proportion of phenotypic variance that can be attributed to genetic influences in a given population (not an individual) is estimated to be 60%–90% for bipolar disorder (1, 4, 6, 11, 12). Further teasing apart of genetic and environmental influences may be accomplished through adoption studies; results of the few adoption studies of bipolar disorder also support the importance of genetic factors in bipolar disorder, with increased rates of bipolar disorder observed among biological, compared with adoptive, family members of individuals with bipolar disorder (6).
Molecular studies in bipolar disorder
The establishment of genetic factors as important in the etiology of bipolar disorder has now led to the search for specific genes that may be involved. Overall, two main avenues of clinical investigation are commonly used: positional and candidate gene approaches. Most commonly, genetic markers known as single nucleotide polymorphisms (SNPs) are used to identify areas of interest or specific genetic changes that may have functional significance. Positional approaches allow one to search for chromosomal areas important in the development of a disorder without a priori knowledge of etiology. As such, these methods are often an important tool in genetic investigations of psychiatric disorders for which relatively little is known about the pathophysiological basis of symptoms. With linkage studies, the combination of molecular genetics techniques and statistical linkage analysis allows the identification of regions of chromosomes that appear to be coinherited with a disease within families. The strength of a given linkage is measured by a LOD score (the log10 of the odds for linkage), with a LOD of 3 indicating significant linkage, corresponding to 1,000:1 odds in favor of linkage and a p value of 0.05. Although not necessarily consistent across studies, linkage results in bipolar disorder and meta-analyses of whole genome scans have identified a number of chromosomal regions as promising areas of further exploration (reviewed in 13–16). Encouragingly, a recent meta-analysis combining pooled original genotype data from more than 5,000 individuals in 11 studies showed genome-wide significance for loci on chromosomes 6q (bipolar I diagnosis; LOD 4.19) and 8q (bipolar I and II diagnoses; LOD 3.40) and suggestive linkage on chromosomes 9 and 20 (17). In addition, incorporation of new genotyping technologies is anticipated to add power to our ability to detect significant linkage results, illustrated in a linkage study by Middleton et al. (18), who performed an analysis of 25 Portuguese bipolar pedigrees using a 10,000 SNP gene chip (simultaneous genotyping of 11,560 SNPs spaced across the human genome). By using this high-density genotyping, two chromosomal regions (6q22 and 11p11) were found to have significant linkage that had not been detected by a previous, less extensive, investigation of the same sample.
In contrast to positional studies, candidate gene approaches rely on the determination of genes of interest based on a variety of sources (e.g., biological or pharmacological plausibility, regions identified by linkage analysis, or animal models) before investigation. The method of association studies allows the examination of the coinheritance of alleles and disease across case patients and control subjects and is more powerful than linkage in the detection of smaller genetic effects. Despite this increased power, association studies in bipolar disorder have yet to identify a gene as being conclusively involved in the etiology of the disorder. Recent studies of possible candidate genes in bipolar disorder were summarized by Hayden and Nurnberger in 2006 (15), and the genes are listed in Table 1. Advances in genotyping throughput and increasing knowledge of the haplotype structure of the genome are making the examination of more and more genes possible. An exciting new method of investigation is whole genome association studies, in which genetic markers are spread across the entire genome and compared in case patients and control subjects. These are currently underway for bipolar disorder, and it is hoped that they will help to clarify the genes involved.
Clinical utility of genetic information for bipolar disorder
At present, the applications of genetics to the clinical care of psychiatric patients are limited. Early-onset Alzheimer’s disease remains the only disorder for which genes have been conclusively linked to etiology and for which testing is available. Testing is also available for a number of Mendelian disorders with prominent psychiatric symptoms (e.g., patients with velocardiofacial syndrome who exhibit psychosis and mood disorders, fragile X syndrome, and symptoms of autistic spectrum disorders), but these conditions are quite rare, even in the aggregate, and would be expected to be relevant in only a small number of individuals or families.
In the absence of specific genes known to be related to psychiatric disorders, family history currently acts as a proxy for genetic markers and may also incorporate as yet unknown factors related to the environmental risk for illness. Currently, having a family history of a disorder is the best predictor of risk for development of a psychiatric disorder among family members (2). In addition to basic uses of family history information in practice (e.g., basing medication choices for a patient on a treatment response in a relative), APA treatment guidelines for the management of bipolar disorder state that genetic counseling may be of use to individuals with bipolar disorder (19). In addition to risk estimations, genetic counseling may include discussion of topics such as the etiology of psychiatric disorders, course of illness, available treatments, and issues of guilt or stigma, among others (20). Several researchers have documented interest on the part of patients with bipolar disorder and their family members in genetic counseling and genetic testing (when or if it becomes available), although factors such as the probabilistic nature of test results, severity of illness, and the availability of treatment were seen to influence this interest (21–24). Currently, risk estimations used in genetic counseling are based on empiric recurrence risks derived from family studies of bipolar disorder and are most useful for providing information on the relative magnitude of risk. In bipolar disorder, some basic information regarding recurrent risks is available for use in discussions with patients (Table 2). At present, individualization of risk is not possible, although adjustments of risk based on various factors (e.g., sex, age of onset, and number of affected relatives) may be discussed. For families with early onset bipolar disorder or families that are densely affected, risks may be even greater than those generally reported for first-degree relatives (5, 8–10, 25).
Future directions
Our understanding of the genetic basis of psychiatric illnesses such as bipolar disorder is growing rapidly. Advances in genetic knowledge are expected to aid in the validation of current diagnostic schema, illuminate the underlying pathophysiological processes related to disease, highlight targets for new treatment options, identify environmental risk factors for illness, and potentially offer opportunities for earlier intervention or prevention of disease onset. At present, we have not yet identified specific genes that are conclusively associated in the etiology of bipolar disorder, and the applications of genetic knowledge in psychiatry remain limited. However, patients and their families are interested in this information, and clinicians should familiarize themselves with basic information on the genetic basis of major psychiatric disorders.
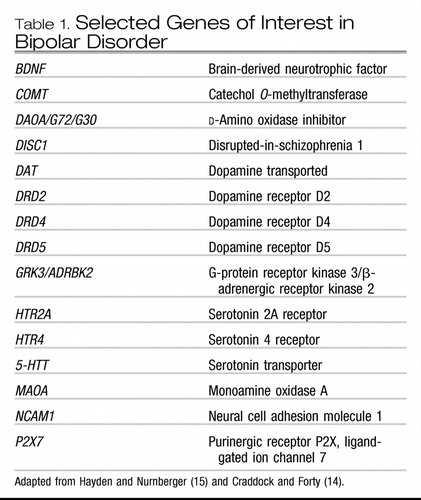 |
Table 1. Selected Genes of Interest in Bipolar Disorder
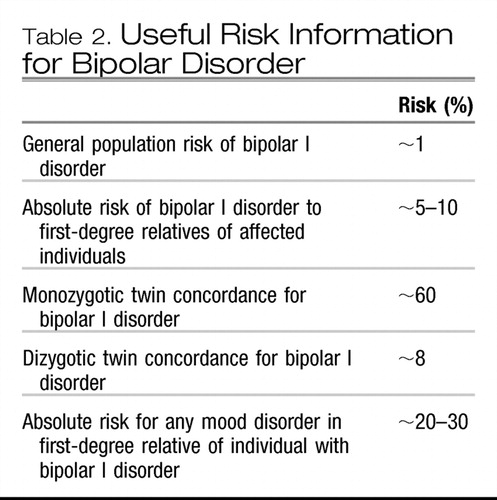 |
Table 2. Useful Risk Information for Bipolar Disorder
1 Tsuang MT, Faraone SV: The Genetics of Mood Disorders. Baltimore, Johns Hopkins University Press, 1990Google Scholar
2 Merikangas KR, Risch N: Will the genomics revolution revolutionize psychiatry? Am J Psychiatry 2003; 160:625– 635Crossref, Google Scholar
3 Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S. Hwu HG, Joyce PR, Karem EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK: Cross-national epidemiology of major depression and bipolar disorder. JAMA 1996; 276:293–299Crossref, Google Scholar
4 Moldin SO: Report of NIMH Genetics Workgroup: summary of research. Biol Psychiatry 1999; 45:573– 602Crossref, Google Scholar
5 Gershon ES, Hamovit J, Guroff JJ, Dibble E, Leckman JF, Sceery W, Tarqum SD, Nurnberger JI Jr, Goldin LR, Bunney WE Jr:: A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry 1982; 39:1157–1167Crossref, Google Scholar
6 Smoller JW, Finn CT: Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet 2003; 123:48–58Crossref, Google Scholar
7 Rice J, Reich T, Andreasen NC, Endicott J., Van Eerdewegh M, Fishman, Hirshfeld RM, Klerman GL: The familial transmission of bipolar illness. Arch Gen Psychiatry 1987; 44:441– 447Crossref, Google Scholar
8 Strober M, Morrell W, Burroughs J, Lampert C, Danforth H, Freeman R: A family study of bipolar I disorder in adolescence: early onset of symptoms linked to increased familial loading and lithium resistance. J Affect Disord 1988; 15:255–268Crossref, Google Scholar
9 Strober M: Relevance of early age-of-onset in genetic studies of bipolar affective disorder. J Am Acad Child Adolesc Psychiatry 1992; 31:606 – 610Crossref, Google Scholar
10 Neuman RJ, Geller B, Rice JP, Todd RD: Increased prevalence and earlier onset of mood disorders among relatives of prepubertal versus adult probands. J Am Acad Child Adolesc Psychiatry 1997; 36:466 – 473Crossref, Google Scholar
11 McGuffin P, Owen MJ, Gottesman II: Psychiatric Genetics and Genomics. New York, Oxford University Press, 2002Google Scholar
12 Althoff RR, Faraone SV, Rettew DC, Morley CP, Hudziak JJ: Family, twin, adoption, and molecular genetic studies of juvenile bipolar disorder. Bipolar Disord 2005; 7:598 – 609Crossref, Google Scholar
13 Sklar P: Linkage analysis in psychiatric disorders: the emerging picture. Annu Rev Genomics Hum Genet 2002; 3:371– 413Crossref, Google Scholar
14 Craddock N, Forty L: Genetics of affective (mood) disorders. Eur J Hum Genet 2006; 14:660 – 668Crossref, Google Scholar
15 Hayden EP, Nurnberger JI Jr: Molecular genetics of bipolar disorder. Genes Brain Behav 2006; 5:85–95Crossref, Google Scholar
16 Baron M: Manic-depression genes and the new millennium: poised for discovery. Mol Psychiatry 2002; 7:342–358Crossref, Google Scholar
17 McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, Abou Jamra R, Albus M, Bacanu SA, Baron M, Barrett TB, Berrettini W, Blacker D, Byerley W, Cichon S, Coryell W, Craddock N, Daly J, Depaulo JR, Edenberg HJ, Foroud T, Gill M, Gilliam TC, Hamshere M, Jones I, Jones L, Juo SH, Kelsoe JR, Lambert D, Lange C, Lerer B, Liu J, Maier W, Mackinnon JD, McInnis MG, McMahon FJ, Murphy DL, Nothen MM, Nurnberger JI, Pato CN, Pato MT, Potash JB, Propping P, Pulver AE, Rice JP, Rietschel M, Scheftner W, Schumacher J, Segurado R, Van Steen K, Xie W, Zandi PP, Laird NM: Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet 2005; 77:582–595Crossref, Google Scholar
18 Middleton FA, Pato MT, Gentile KL, Morley CP, Zhao X, Eisener F, Brown A, Petryshen TL, Kirby AN, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, Soares MJ, Ferreira CP, Lei M, Azevedo MH, Kennedy JL, Daly MJ, Sklar P, Pato CN: Genomewide linkage analysis of bipolar disorder by use of a high-density single-nucleotide-polymorphism (SNP) genotyping assay: a comparison with microsatellite marker assays and finding of significant linkage to chromosome 6q22. Am J Hum Genet 2004; 74:886 – 897Crossref, Google Scholar
19 American Psychiatric Association: Practice guideline for the treatment of patients with bipolar disorder (revision). Focus 2003; 1:1–50Google Scholar
20 Finn CT, Smoller JW: Genetic counseling in psychiatry. Harv Rev Psychiatry 2006; 14:109–121Crossref, Google Scholar
21 Smith LB, Sapers B, Reus VI, Freimer NB: Attitudes towards bipolar disorder and predictive genetic testing among patients and providers. J Med Genet 1996; 33:544–549Crossref, Google Scholar
22 Trippitelli CL, Jamison KR, Folstein MF, Bartko JJ, DePaulo JR: Pilot study on patients’ and spouses’ attitudes toward potential genetic testing for bipolar disorder. Am J Psychiatry 1998; 155:899–904Crossref, Google Scholar
23 Quaid KA, Aschen SR, Smiley CL, Nurnberger JI: Perceived genetic risks for bipolar disorder in a patient population: an exploratory study. J Genet Counsel 2001; 10:41–51Crossref, Google Scholar
24 Jones I, Scourfield J, McCandless R, Craddock N: Attitudes towards future testing for bipolar disorder susceptibility genes: a preliminary investigation. J Affect Disord 2002; 71:189–193Crossref, Google Scholar
25 Schurhoff F, Bellivier F, Jouvent R, Mouren-Simeoni MC, Bouvard M, Allilaire JF, Leboyer M: Early and late onset bipolar disorders: two different forms of manic depressive illness? J Affect Disord 2000; 58:215–221Crossref, Google Scholar



