End-of-Life Care: Issues Relevant to the Geriatric Psychiatrist
Abstract
Most deaths in the United States occur in the context of chronic diseases in later life and are too often accompanied by potentially remediable emotional or physical suffering. Geriatric psychiatrists and other mental health professionals can contribute meaningfully to the provision of optimal care during the final phases of life. This review provides an overview of end-of-life care, focusing on issues most relevant to the geriatric psychiatrist. The author examined palliative care textbooks and review papers to determine the topics to be included in this article, and searched computerized literature databases on these topics. Many of the recommendations provided herein stem from experts’ clinical experience; however empirical evidence is also incorporated and critiqued. Topics covered include conversations with patients and families about end-of-life care; the evaluation and treatment of suffering, including pain, depression, suicidality, anxiety, and delirium; the role of individual and family therapies in caring for dying patients; capacity determination; advance care planning; withholding life-sustaining treatments; and “last resort” (and, in some cases, quite controversial) options, such as terminal sedation, assisted suicide, and euthanasia. The author also notes the relevance of such end-of-life-care considerations to patients with dementia. Geriatric psychiatrists’ skills across these multiple domains are of particular usefulness. Through such clinical skills and the application of empirical research tools to the many unanswered questions in the care of dying patients, geriatric psychiatry can make increasingly valuable and visible contributions to improving quality of life for people suffering from life-threatening illnesses.
“I am about to, or I am going to, die. Either expression is used.”—reported last words of 17th-century grammarian Dominique Bonhours.
As wonderfully exemplified by the last words of Bonhours, how we die is very much emblematic of how we live. This is so for individuals and for society as a whole. It is thus not surprising that recent years have seen members of the baby-boom generation, approaching the latter decades of life, stimulate an increasingly public examination of the ways in which we die, insisting that society and the medical profession try to ensure a “good death” for as many of us as possible (1–4).
Such examination and demands occur in the context of crucial demographic and clinical shifts in the context of dying. With the remarkable success of 20th-century medicine and public health efforts, most life-threatening acute diseases in younger persons have been prevented or controlled, such that 80% of deaths in the United States now occur among persons age 65 years and older (5). Concomitant with this, the majority of deaths also occur in the setting of chronic illnesses associated with functional decline. Partly related to these shifts, we have seen the so-called “medicalization” of death in our society (6). Concerns raised about such medicalization include fears that suffering will be worsened or prolonged by medical procedures or treatments, as well as recognition that death is often removed from the contexts (i.e., social, cultural, religious) that in former generations helped give the dying process meaning for the terminally ill person and her or his family. Indeed, despite the fact that 90% of Americans would prefer to die at home (7), 60%–80% actually die in institutional settings (5, 8, 9), often in the context of invasive procedures, artificial support of basic human functions (e.g., nutrition, ventilation), or resuscitation efforts.
Yet the medicalization of death offers unique opportunities to improve the dying process. Physicians and other healthcare providers have the opportunity to work with patients and families in the last months, weeks, and days of their lives. What should comprise such work? Several authors and groups have addressed the measurement and implementation of quality outcomes in end-of-life care (10–12). One such approach is summarized in Table 1. It is telling that this list was published in the Journal of the American Geriatrics Society. In recent years, thanks in no small part to the success of earlier efforts in countering ageism, it has become possible for geriatrics to openly examine the dying process and the care of dying persons. Geriatric psychiatrists are uniquely poised to contribute to this work as direct providers of care, as consultants, and as clinical researchers. Clinicians from many specialties and disciplines possess knowledge and skills in some of these areas, but as will be discussed throughout this article, I believe that geriatric psychiatrists’ training and expertise across the multiple domains shown in Table 1 should allow them to be particularly prominent and useful contributors.
Accordingly, in this article, I will review several important areas of palliative care medicine, as particularly applicable to geriatric psychiatrists and, in many aspects, to other mental health professionals. Limitations in empirical research necessitate that much of the content of this review stems from experts’ recommendations and clinical experience, although available empirical support will be incorporated and critiqued when possible. The invaluable work of previous summaries or statements of purpose relevant to psychiatry also must be acknowledged (13–15). Of course, an overview paper like this cannot do justice to the larger field of palliative medicine. The interested reader is referred to the many excellent available reference sources (16–18).
Palliative “versus” curative or disease-focused care
Much of modern medicine targets treatments at disease cure, or at least at limiting or slowing disease progression. In so doing, improvement in the patient’s symptoms or functional status are welcome and expected byproducts of the disease-focused treatment. By contrast, the paramount goal in palliative care is alleviation of symptoms or functional morbidity, that is, suffering. Improvement in the underlying disease process is not expected, or is attempted only in the interest of reducing suffering, without expectations of prolonging survival. Therapies that produce discomfort are not attempted, or are abandoned when the suffering produced exceeds the relief provided.
It is important to recognize, however, that the distinction between palliative and disease-focused care is often arbitrary. Symptom reduction, of course, may be a direct result of curative therapy, or may be the result of allied therapies administered concurrently. Conversely, disease-focused treatments may be the best approach to palliation in some situations. It may be argued that “traditional” medical management of many chronic diseases is, in reality, palliative in goals and scope. Even in the context of life-threatening diseases, palliative and disease-focused approaches to care often coexist. There may be a gradual transition as the disease progresses, curative interventions fail, and death approaches, as depicted in Figure 1.
Thus, palliative care does not apply solely to those facing imminent death. Indeed, discussions with patients and families about palliative approaches ideally take place relatively early in the course of a life-threatening illness (19), and such discussions should be revisited as often as indicated by changes in the patient’s condition, wishes, or care options. The timing and shape of such discussions also will be affected by the varied “trajectories” toward death (8, 20) produced by different diseases, for example, relatively slow or predictable decline (often seen for example in neurodegenerative dementias, cancers, or amyotrophic lateral sclerosis), or courses marked by more acute exacerbations, any one of which might lead to death or to partial or even full, if temporary, return to baseline, for example, as often seen in pulmonary or cardio- or cerebrovascular diseases.
Figure 1 also indicates that some patients may die after a discrete period of hospice care, that is, a purely palliative approach provided within a clinical, organizational, or funding framework designated as “hospice.” The designation as hospice care depends partly on patient (and provider) decisions to explicitly focus on a purely palliative approach, and partly on disease course; for example, funding for hospice may depend on the physician’s ability to state that the patient is highly likely to die within an arbitrary time-frame. Figure 1 also refers to “bereavement care” after the patient’s death (note the section below on the role of supportive contacts with the family to reduce or prevent subsequent psychiatric morbidity).
All physicians should be versed in the basic tenets of palliative care, along with more specific aspects relevant to their specialty. Training in palliative care during medical school and residency historically has been inadequate, but curricula are increasingly changing to reflect this need (21, 22). Palliative care also has achieved formal specialty status in many nations, recently including the United States.
Talking with patients and families about end-of-life care
Application of our interview skills is particularly crucial, challenging, and rewarding with dying patients and their families. As previously noted, discussions about end-of-life care should begin relatively early in the context of severe debilitating or life-threatening illness. These discussions provide a base upon which to continue discussions as the disease progresses, and often increasingly complex or urgent decisions need to be made about care options. Such “end-of-life conversations” (19) should become routine, structured parts of interventions in health care, as guided both by clinical experience and by growing empirical evidence that these discussions improve outcomes such as elicitation of and adherence to patient wishes (23).
First discussions should begin with the use of open-ended questioning to understand the patient’s lifelong values and views on illness, suffering, medical treatments, and death (24–26). This approach may include queries such as, “Tell me about yourself . . . about your past experiences with illness . . . with doctors or medical treatments . . . .” It is critical to convey empathy and a clear message that the care-provider will 1) respect the patient’s wishes, and 2) not abandon the patient, even (especially) if or when disease-focused treatments no longer prove effective (27, 28). It is also important to help the patient understand the concept of palliative care, especially that a palliative approach may coexist with (i.e., is not necessarily the opposition of) disease-focused care. Elicitation of values and wishes must include an appreciation of the patient’s cultural context (29), including the family system (30), as well as an understanding of the role religion or spirituality may play in the patient’s values and choices (31). All of these discussions should help the patient articulate, and the physician understand, his or her own personal definition of “death with dignity,” so that future interventions may help conserve that dignity (32, 33). Geriatric psychiatrists have particular experience in assisting patients and families conduct life review and reminiscences, which may be a therapeutic part of the interview process as well as a “diagnostic” aid to understanding the patient’s personality, values, and priorities. For example, some persons who have always valued self-reliant independence might choose to assertively “fight” their illness until the end, whereas others might choose to forgo certain treatments (curative or palliative in intent) with an eye toward living their remaining days as autonomously as possible. Also, the geriatric psychiatrist should use her or his skills to understand the ways in which the patient’s religious or spiritual beliefs affect their decisions, serve as a framework for seeking meaning during the final phases of life, or suggest additional sources of support (i.e., clergy or members of the patient’s religious congregation) (31).
Further and ongoing conversations with the patient will include other elements, including
| 1. | Delivering bad news: As the disease progresses, complications develop, or disease-focused treatments fail, the patient and family must be informed of the relevant facts in a manner that fosters the treatment alliance and continued partnership in decision-making (34, 35). A useful approach is to let the patient know up front that there is bad news to be delivered, and to assess their readiness to engage in the discussion. For example, in the case of an MRI scan that newly reveals evidence for metastatic spread in a cancer patient previously thought to be in remission, one might say, “I’m sorry to say that I have some bad news from the results of your MRI scan. Would you like to talk about that now?” In situations where the news to be delivered is less unequivocally “bad,” one might choose more neutral language that does not preemptively interpret the patient’s response to the information provided; for example, “I have the results of your MRI scan. Would you like to talk about that now?” | ||||
| 2. | Eliciting history: It is important to continually reassess the patient’s symptomatic status, to inform therapeutic and palliative interventions. Ask directly about pain, as described more below. Make specific inquiries about other relevant physical and emotional symptoms (also see below), as well as functional status and interpersonal needs. Again, geriatric psychiatrists have well-developed skills at eliciting the relationship of physical and emotional skills to functional abilities and resource needs (interpersonal and instrumental). A number of factors may make symptom elicitation difficult. For example, some patients may be reluctant to acknowledge symptoms out of ignorance that these can be alleviated, or out of fear that such disclosure will lead to the discovery of more “bad news;” thus the above-noted educational and alliance-building approaches are crucial for forming the context within which symptoms may be elicited. As another example, the high prevalence of cognitive deficits—from delirium or dementia—among dying patients may require careful attention to the patient interview, and involvement of family or other caregivers, to elicit relevant symptom history. | ||||
| 3. | Decision-making: Inevitably, the patient and family will have to make decisions about care options. These may include decisions about whether to continue or pursue disease-focused treatments that may have some potential to extend life or slow the disease process, combined with a high potential to increase suffering. Decisions about emergency interventions, including mechanical ventilation and cardiopulmonary resuscitation, are too often made in crisis rather than ahead of time. Patients, families, and providers sometimes collude in avoiding discussion of these emotionally-laden topics during times of clinical stability, yet it is just such times that provide the opportunity for thoughtful conversations. The physician needs to bring these topics up, perhaps introducing the discussion with process comments; for example, “I’m glad that you are feeling well today. I do think that at some point we should talk about the kinds of treatment options you might have if your illness worsens down the road, including what kinds of treatments you might want or not want. Would you like to talk more about this today?” As the discussion continues, the physician should frame the decision-making by providing needed information in a manner that is accurate and complete enough to facilitate the patient’s arriving at a decision consistent with his or her value system. Indeed, there is evidence that ill older patients can make health-utility decisions balancing competing considerations (36). At the same time, choice of words and frame are crucial. For example, patients are more likely to choose palliative approaches over life-extending care if they have a more accurate understanding of their prognosis, or of the (often low) likelihood of meaningful recovery after cardiopulmonary resuscitation (37, 38). | ||||
In the context of a strong therapeutic alliance, patients and families may ask difficult questions (“How long will I live, Doc?”), or may assert wishes as facts that contradict our best professional estimates (“I’m going to beat the odds and live to see my grandson graduate from high school next year.”). Useful responses often will incorporate elements of conveying empathy for the process underlying the patient’s statement (e.g., “That graduation is pretty important to you; it’s hard to think that your heart condition might prevent you from being there.”), and statement or re-statement of the relevant facts along with factual acknowledgement of our professional uncertainty (“Unfortunately most people with your stage of heart disease do not live more than 3 to 6 months, although there are rare exceptions.”). Use of “I wish” statements can be a useful way to communicate empathy along with the prognostic facts (“I wish I could tell you that you were likely to live that long.”), and “if” comments may help facilitate needed planning discussions (“We can hope for the best, but if it turns out to be the case that you die before the graduation, how would you like to have spent your last months with your grandson?”) (24, 39). Skills at conveying empathy and aiding future planning are, of course, part of the repertoire of mental health clinicians (as well as well-trained practitioners from other specialties), and those with geriatrics expertise are experienced at such work in the context of medical comorbidity and disability.
Relieving suffering
Distressing symptoms in dying patients may occur as part of dysfunction in any organ system. Some may represent underlying medical emergencies, to which decisions must be made as to the level of interventions attempted. Common symptoms most relevant to psychiatry include the following:
Pain. Physical pain is among the most common symptoms in dying patients, and among the most feared by patients and families. The control of pain is a crucial topic, one that requires more space and has received it elsewhere (40–43). Geriatric psychiatrists, in common with colleagues in consultation psychiatry as well as other subspecialties, should have expertise in the assessment and management of pain. Some basic tenets of pain control are listed here.
| 1. | The goal is to eliminate pain. For most patients, it is possible to achieve this goal fully, or at the least to reduce pain to tolerable levels without intolerable side effects. There are some patients who do not want to have their pain eliminated. In this event, careful discussion should elicit whether this wish arises out of, for example, (actual or feared) side effects of analgesics, depression-related hopelessness, or the patient’s religious or other cultural values precluding acceptance of pain treatment; in the latter case, exploration with the patient and family can determine whether there is any flexibility within the value/belief system. | ||||
| 2. | Pain must be routinely assessed. This is best done by direct inquiry, asking the patient to rate pain on a scale of 0–10 (or by using an equivalent visual-analog scale when verbal communication is difficult); such inquiries may be included in routine nursing assessments as the “fifth vital sign.” It must be recognized that there is no substitute for such direct inquiries of pain ratings by the patient. Observer ratings frequently under-rate pain, partly because chronic pain may manifest as depression, irritability, or other nonspecific symptoms rather than as the visible discomfort we expect to see in acute pain. Because depression so commonly exists concurrently with pain, and may color perceptions of the pain or treatment options, depression must be assessed as well; see below. | ||||
| 3. | In many cases, optimal pain treatment may include “disease-focused” therapies, whether or not the use of such therapies can be expected to improve survival or overall disease progression. Common examples include anti-anginal drugs for angina pain, radiation therapy for bony carcinomatous metastases, or glucocorticoids to reduce pain associated with inflammation and edema. | ||||
| 4. | Analgesic drugs are a mainstay of pain control. A systematic approach to drug choice should be applied, using a fixed-dose regimen to provide around-the-clock relief along with prn (or “rescue”) medications for break-through pain. The regimen should be reevaluated frequently and increased (in frequency or dosage) if break-through pain is present more than rarely. The World Health Organization analgesic ladder should be used to choose drug class on the basis of severity of pain (41, 44). Options for Step 1, mild pain, include acetaminophen and nonsteroidal antiinflammatory drugs (including aspirin and the COX-2 inhibitors). For Step 2, moderate pain, opioids such as oxycodone or codeine are given, typically in combination with a Step 1 drug. For Step 3, severe pain, opioids such as morphine are the treatment of choice. Route of administration will depend on the drug, the patient’s ability to swallow or absorb an oral dose, and convenience of access to other routes (e.g., nasogastric or direct enteral access, permanent intravenous ports). For some drugs and clinical situations, continuous administration, for example, a subcutaneous morphine drip, with patient control of the dose may be most appropriate. | ||||
Other drugs may provide analgesia for certain types of pain. For severe bone pain due to metastatic cancer, co-administration of non-steroidal anti-inflammatory drugs (NSAIDs) along with opioids is often effective (45–47). There is some empirical evidence to support the use of other adjuvant drugs in refractory bone pain; these include corticosteroids, biphosphonates, and calcitonin (48–52). For neuropathic pain syndromes refractory to opioids alone or co-administered with NSAIDs, empirical evidence best supports the use of tricyclic antidepressants (53, 54). There is limited evidence for the efficacy of selective serotonin reuptake inhibitors (SSRIs), and even more limited data for other antidepressants, including venlafaxine, bupropion, and mirtazapine (54–58). The use of anticonvulsants is commonly recommended as well, although the empirical evidence for indications other than trigeminal neuralgia, and for agents other than carbamazepine, is limited at best (59). Many other agents have been recommended for neuropathic pain, each with very limited evidence, especially as applied to terminal-illness contexts (60–65).
Side effects may limit the tolerability of analgesics. Common problems with opioids include nausea, constipation or obstipation, and sedation or other neuropsychiatric symptoms (e.g., perceptual disturbances with or without the presence of full-fledged delirium). Sometimes switching to a different opioid allows achieving adequate analgesia with fewer side effects in a particular patient. Combination of opioids with other classes of analgesics may allow reduction in opioid dosage with still-adequate pain control. In some cases, additional drugs may be used successfully to counteract opioid side effects, for example, the common prophylactic use of laxatives to prevent uncomfortable constipation or the use of psychostimulants to reduce opioid-induced sedation (66, 67).
Non-pharmacological approaches to pain may provide benefit in selected individuals and are widely used, although the empirical evidence for their efficacy varies widely, depending on the condition and the modality (68, 69). These approaches range from invasive (e.g., anesthetic or surgical interventions) to noninvasive physical modalities (e.g., physical rehabilitation approaches, transcutaneous electrical nerve stimulation (TENS), acupuncture, massage) to cognitive/interpersonal approaches (e.g., meditation, guided imagery, relaxation techniques). Geriatric psychiatrists may use their skills in such psychotherapeutic options as guided by patient preferences.
Depression. It is well recognized that depressive symptoms and syndromes have clinically important bi-directional relationships with comorbid medical conditions. Indeed, medical illnesses are among the most consistently identified correlates of the presence and course of depression in later life, while, conversely, depression is a powerful predictor of functional outcomes and mortality in broad populations. as well as in many specific chronic medical diseases (70, 71). The clinician or researcher seeking to diagnose depression in dying patients may face confounds in symptom assessment familiar to geriatric psychiatrists, who have expertise working with depression in medically ill patients—namely, how to “count” neurovegetative symptoms (e.g., anergia, anorexia, weight loss, sleep or psychomotor disturbance) toward the syndromic diagnosis of depression, when such symptoms may be due to underlying medical disease even in the absence of depression. Although DSM-IV asks clinicians to take an “etiologic” approach in practice, it is often difficult to judge whether the medical disease or depression causes a particular symptom. Other diagnostic approaches have been described, including exclusive (i.e., eliminating potentially confounded neurovegetative symptoms), substitutive (replacing confounded neurovegetative symptoms with additional emotional or ideational symptoms), or inclusive (counting all symptoms toward the depressive syndrome, regardless of potential etiological confound) (72). For research purposes, a multi-institutional work group recommended the inclusive approach, based on reliability considerations as well as prevalence and prognostic validity studies that fail to confer a clear advantage for one approach over another (73). However, many clinicians working with dying patients continue to recommend an essentially exclusive approach, that is, an emphasis on the emotional and ideational symptoms of depression (74). Even assessments of the latter symptoms are difficult in palliative care contexts, because it is not clear how to operationally define some ideational symptoms, such as hopelessness or recurrent thoughts of death, in persons facing impending death. Moreover, the conceptual, definitional, and therapeutic issues regarding minor and subsyndromal depressions, murky enough in broader patient populations (75–78), remain largely uncharted territory in dying patients.
Given such limitations, along with the difficulties in conducting psychopathological research in dying patients, the empirical research literature on depression in dying patients is relatively small, limited largely to cancer and HIV patient populations. Still, the findings are generally comparable to those seen in broader medical illness groups. The following are important “take-home” points about depression in dying patients:
| 1. | Depressive symptoms and syndromes are common in dying patients, although the range of estimated prevalence rates (approximately 15%–60%) likely reflects the heterogeneity of depressive conditions and their definitions as well as the patient populations (79–82). | ||||
| 2. | As in primary care and other broad settings, self-reported depression inventories, including the singleitem question “Are you depressed?”, have good operating characteristics as screens for diagnosable depressive disorders (83–85). | ||||
| 3. | Evidence from patients with cerebro- or cardiovascular disorders (86, 87) or cancer (88) suggest that depressive conditions in dying patients are multifactorial in origin, representing a combination of physiological effects of the disease process on brain functioning, premorbid diathesis toward depression, and current psychological and psychosocial factors. | ||||
| 4. | Depression is associated with poorer will to live and greater desire for a hastened death (89, 90). Together with extrapolations from data regarding functioning in a variety of medical populations (91, 92), these data strongly suggest that depression is associated with excess functional morbidity and poorer quality of life in dying patients, making it an important target for palliative care. | ||||
| 5. | Better treatment of chronic pain or other physical symptoms may result in improvement in concomitant depressive symptoms. Conversely, successful treatment of depression may reduce patient ratings of pain severity or pain-associated functional morbidity (93). | ||||
| 6. | Turning to depression treatments, to my knowledge there are no well-controlled trials of drugs or psychosocial treatments in terminally ill patients. Extrapolating from literature in chronically medically ill patients, depression in dying patients probably can respond to standard treatments (91, 94), with resultant improvement in “quality of life” such as subjective distress and functional morbidity, although response rates may be lower than that seen in healthier populations. In the absence of further empirical data, choice of antidepressant drug should be guided by side-effect profile in the context of the patient’s symptoms and comorbidities. Also, there may be a role for psycho-stimulants in targeting anergia, anhedonia, and abulia in patients who do not have a full major depressive syndrome, or in depressed patients with a very short life expectancy, in whom rapid symptomatic improvement is needed (95–99). Recently, the newer stimulant modafinil has been used (100), although it is not yet clear whether its putatively better side-effect profile, compared with older agents such as methylphenidate (on the basis of its brain regional selectivity) results in better efficacy due to greater tolerability of higher doses. | ||||
Suicidal ideation. The tenets of clinical suicidology as applied to older persons in general are of great relevance to dying patients (101), providing a crucial area for the expertise of the geriatric psychiatrist. Wishes or requests for hastened death, and attempted and completed suicide, are common in cancer patients (102, 103), and likely in other terminal populations, as well. Issues relevant to assisted suicide and euthanasia are discussed below. The above-noted concerns about distentangling “normal” hopelessness and worthlessness from pathological processes in those facing imminent death may complicate the clinical assessment of suicidality, and are problematic when attempting to determine prevalence rates and risk factors. Nonetheless, persistent wishes to die, and certainly specific suicidal ideation or plans, warrant careful clinical evaluation for modifiable contributing factors, which in terminal patients are likely to include depressive disorder, pain, agitation/distress as part of delirium or dementia, or social isolation (13).
Anxiety. Although empirical prevalence data are relatively lacking, it is well recognized that anxiety is a common and distressing symptom in dying patients (13, 14, 104). Anxiety may be comorbid with depression or delirium, or it may present alone as a primary condition or as secondary to medical illness or its treatments. Affective, ideational, or physiological manifestations of anxiety commonly accompany distressing physical symptoms such as pain, dyspnea, and vertigo. Anxiety in dying patients often occurs in a generalized anxiety pattern, although panic attacks or acute stress disorder-like symptoms (referable to the medical disease, its treatments, or to a feared mode of death) may be seen. Anxiety may contribute, along with depression, to a desire for a hastened death (105). Geriatric psychiatrists will draw on their expertise in assessing anxiety in the context of such comorbidities.
Recommendations for treatment of anxiety are based largely on extrapolations from other populations, given that controlled trials in terminally ill patients are lacking. If rapid symptom control is needed because of acute distress or limited remaining life-span, benzodiazepines or (in the presence of delirium or significant dementia) antipsychotic medications are the treatments of choice, sometimes in combination with opioids or other sedatives (see below). Opioids may be particularly useful for anxiety in the context of dyspnea due to cardiac or pulmonary disease (106, 107). If time available permits, antidepressant medications may be the primary treatment for generalized anxiety or panic-pattern symptoms (whether or not they are judged to be secondary to medical illness), or if the anxiety is comorbid with significant depressive symptoms. The role of other agents, such as buspirone, in treating anxiety in dying patients has not been well defined. Similarly, cognitive-behavioral or supportive therapy for anxiety may be useful in patients who have sufficient time, cognitive capacity, and motivation for treatment, although, again, empirical studies in terminal patients are few and are not well controlled (108, 109).
Delirium. Unsurprisingly, dying patients commonly develop “acute brain failure;” that is, the fluctuating global cognitive dysfunction of delirium related to primary CNS disease or to the CNS sequelae of systemic diseases or medications (110, 111). Although cognitive impairment is the hallmark of the delirium syndrome, it is important to remember that other portions of the mental status examination may be prominently affected, for example, depression, anxiety, psychosis, or psychomotor disturbances. Often the manifestations of delirium considerably add to the patient’s morbidity and to distress for the family. The standard approach to delirum evaluation, namely to identify and treat the underlying causes, may not always apply to dying patients. The causes may not be remediable, or may be iatrogenic in the service of palliation, for example, opioids or glucocorticoids. In many cases, work-up to determine the underlying cause(s) will be precluded by patient/family decisions to pursue a purely palliative approach. In any case, one should re-evaluate the benefit/risk profile of all CNS-active medications, balancing the helpful effects of such drugs with their potential contribution to the state of delirium. Symptomatic management often targets distressing components of delirium, such as agitation, psychosis, or affective lability. Although there may be some role for simple environmental interventions (i.e., decreasing unnecessary stimuli when possible), the mainstay of therapy for these symptoms remains antipsychotic medications. The second-generation antipsychotic medications have been used, but their advantage (presumably in terms of side-effect profile) over older antipsychotic drugs in terminal (or indeed any) delirium is not yet clear (112). For intractable terminal delirium that produces severe or dangerous agitation or distress, other agents may be used alone or in combination with antipsychotics, including benzodiazepines, opioids, or anesthetic agents (see the section below on terminal sedation).
The geriatric psychiatrist is well versed in the assessment and management of delirium in the medically ill older patient. She or he also can serve a useful educational role for the family and sometimes for other medical providers, informing them about the cause of the distressing symptoms (e.g., that the emotional symptoms in delirium do not have a purely “psychological” origin and may not respond well to interpersonal interactions alone), and helping avoid the unnecessary and often counterproductive use of other psychotropic medications (e.g., hypnotics or antidepressants).
Role of supportive psychotherapy. In addition to the psychiatric disorder-specific indications for focused psychotherapies, other forms of time-limited psychotherapy may be quite useful for selected patients or their families (108, 109). Geriatric mental health specialists can draw on their knowledge of individual and family developmental issues as related to later life, as well as their specific psychotherapeutic skills. Particularly if contacts are begun earlier in the trajectory of an ultimately life-ending illness, individual psychotherapy may be used to facilitate life review, to help the patient set, prioritize, and achieve manageable goals in the time they have remaining, and, in some cases, to process longer-standing unresolved conflicts or dysfunctional patterns of thinking or interactions that affect the dying process. Some patients may come to view dying as an opportunity for psychological or spiritual growth, a possibility that physicians should suggest and support when possible (113).
Family dynamics, of course, are a clinically crucial context within which the dying process takes place, and may be quite prominent in their effects on the treatment alliance and on needed decision-making. As with the individual, clinicians should assess the family to inform an approach that capitalizes on the family’s strengths and shores up areas of weakness (30). The mnemonic “L-I-F-E” has been suggested to organize approaches to brief family assessment, including Life-cycle stresses, Individual roles within the family, Family relational processes, and Ethnic factors (114). Depending on the family’s needs, interventions may range from education and community referral to deeper interventions, such as restoring effective problem-solving, improving patterns of communication, or improving attachment and caregiving bonds (115). Family contacts also may be viewed as preventative: education and support may aid the grieving process even before the patient dies, optimally reducing the likelihood that bereavement will be complicated by depression or traumatic grief (116), and increasing the likelihood or rapidity with which family members seek treatment for complicated bereavement.
Other clinical issues
Capacity. Given the high prevalence of delirium or dementia in terminally ill patients, along with the prevalences of other conditions affecting the physical ability to communicate, it is not surprising that many dying patients at some point are not able to fully participate in making informed decisions about their care. Geriatric or consultation psychiatrists are often consulted when capacity determination is complex or difficult; for example, when subtle cognitive difficulties produce a state of “partial capacity,” or when it is difficult to determine whether a depressive disorder is present (i.e., to distinguish this from “normal” in the context of severe physical illness) and whether such a depressive disorder is clouding capacity. As always, determination of capacity applies only to the current clinical state; capacity often changes as the patient’s mental state improves or worsens over time. Also, capacity determination should be applied to a particular decision or action to be made; thus, for example, one assesses that a patient does not demonstrate capacity at the present time to make an informed decision about X (specific medical intervention). To demonstrate capacity, patients should show the four key components of capacity (117, 118): based on an understanding of the facts of their condition and the options before them, and appreciation of the significance of these facts; the patient must convey an expressed choice based on reasoned consideration of the situation in a manner consistent with his or her personal and cultural preferences and values. Many have argued for a “sliding-scale” approach to capacity determinations (117, 119, 120), that is, that the least-stringent capacity standard should be applied to those medical decisions that are of low danger and that are most clearly in the patient’s best interest, whereas the most stringent standard of capacity should be applied to those decisions that are “very dangerous and fly in the face of both professional and public rationality” (119). Because of this, geriatric psychiatrists are also more likely to be consulted for capacity determination when the implications of a capacity determination are particularly profound (e.g., decisions about initiating or discontinuing life-sustaining treatment).
It also should be noted that consultations for capacity determination often are requested in the context of complex issues with the patient, the family, or the negotiation of an appropriate treatment plan. The geriatric psychiatrist should be mindful of such broader issues; while being sensitive to the boundaries of the consultee’s initial request, the so-called “competency consult” may lead to additional opportunities to be of help to the patient, family, or treatment team.
Advance care planning. Given that, at some point, many dying patients will lose the capacity to participate in decisions about their care, it is highly desirable that patients express their preferences in advance while they are able to do so. Patients may specify their wishes about future medical care in broad, general terms, or may specifically request that particular interventions be used or not used in their care. Mechanisms for doing so include specific physician’s orders at the request of the patient (e.g., “Do Not Resuscitate” or “Do Not Intubate” orders), living wills (i.e., documents in which the patient requests or forbids specific interventions in advance), the verbal expression of care preferences to family or providers, or the appointment of a healthcare proxy or equivalent, such as durable medical power of attorney. Physicians should broach the topic of advance care directives with their patients relatively early in their illness’ trajectory, as there is evidence that doing so increases the likelihood that a patient’s wishes will be followed, avoiding unwanted interventions or the preservation of life in a condition that the patient had previously deemed not worth sustaining (19, 23). Because of the heterogeneity of patients’ general values about death and dying and the difficulty in translating such values into specific healthcare preferences, attempts to elicit preferences from patients should include inquiries about specific healthcare choices as much as possible (121). Even so, advance statements of healthcare preferences cannot take into account the myriad variables that might affect assessment of the desirability of a particular option or life state. Thus, advance statements about who should speak on the patient’s behalf should capacity be lost—that is, a healthcare proxy or equivalent—are the most flexible and useful. Geriatric psychiatrists working with patients with potentially life-threatening illnesses should encourage their patients to have such discussions with their primary care provider or relevant specialist, and, for some patients and families, may play a facilitative role in helping the patient think through the available options in the context of their values.
Withholding or stopping life-sustaining treatments. Most patients and families facing death from terminal illness at some point will be faced with a decision as to whether to begin treatments such as mechanical ventilation, cardiopulmonary resuscitation, or artificial administration of fluids and food, or whether to discontinue such treatments after they have begun. As already noted, these concerns often are incorporated in the content of advance directives. Physicians can help the decision-making by providing frank assessments of the likelihood that these interventions will lead to meaningful recovery, and whether interventions such as ventilation will be short-lived or indefinite. Patient and family decisions can then weigh these facts in the context of how they define “quality of life” or dignity. Sometimes religious or cultural values require the individual to accept all possible life-sustaining treatments. It must be recognized that, although most experts believe there is no ethical distinction between stopping and not initiating a treatment, for the persons directly involved, stopping treatment often feels like “pulling the switch” or otherwise “causing” the patient’s death (122). Physicians can helpfully reframe such decisions as allowing the disease to take its natural course toward death, and as not continuing treatments that merely prolong suffering. In so doing, one must be clear about one’s own goals in providing or framing information. We must use our best judgment, based on assessment of the clinical situation, the needs of the patient and family, and relevant ethical considerations, to decide in each case what is the best proportion of “neutral” information-sharing versus purposeful shaping of information and language to achieve particular ends (such as guiding a family to make a specific decision, or easing their suffering in the decision-making process).
The withholding of artificial hydration and nutrition often contributes to a more comfortable death with gradual loss of consciousness, particularly with assertive supportive care (e.g., oral care) (123). Also, there is a lack of evidence that tube-feeding lengthens or improves life in terminally ill patients (124–126). Education about these issues is essential, because many patients and families expect that “starving to death” is an uncomfortable process. In most states in the U.S., discontinuation of life-sustaining treatments, including artificial hydration and nutrition, is viewed like any other medical treatment, and usual guidelines about the use of advance directives or substituted judgment of a healthcare proxy apply (127). However, some states have explicit legislation regarding specific interventions. For example, in New York, decisions to forgo life-sustaining treatment in a patient currently lacking capacity to make this decision must include some evidence that this decision is consistent with the patient’s specific previously expressed wishes.
Terminal sedation. Although proper palliative care, in most cases, can eliminate physical suffering or reduce it to tolerable levels, there are some patients for whom our best efforts cannot produce an acceptable quality of life (122). Fear of such suffering may be part of what drives requests for assisted suicide or euthanasia (see next section). An approach acceptable to many clinicians, patients, and families, even those for whom assisted suicide is not viewed as acceptable, is the use of terminal sedation; that is, the use of medications to produce an unconscious state in which the patient ultimately dies from the underlying disease (128–130). Several classes of medications may be used for this purpose, most commonly including benzodiazepines (e.g., continuously infused midazaolam), opioids, and anesthetics such as propofol. To state the obvious, terminal sedation should be used as an option of last resort, after careful consideration with the patient and family. For many patients, however, preparatory education about the availability of terminal sedation substantially allays fears of suffering or physician abandonment in the final days of life. Thus, terminal sedation should be mentioned as an option far more frequently, and earlier in the illness course, than it is actually used.
Geriatric psychiatrists can play important roles regarding decisions to withhold life-sustaining treatments or to initiate terminal sedation. These may include clarifying the patient’s or family’s values (including the effects of religious beliefs on perceptions of these options, and negotiation when disagreements within the family make it difficult to achieve consensus), and providing expert opinions about psychiatric symptoms’ coloration of the patient’s decision-making and the likelihood that such symptoms can be reversed or ameliorated.
Physician-assisted suicide and euthanasia. Physician-assisted suicide (PAS) is defined as a person killing him- or herself by using medications or a device provided by a physician for that explicit purpose. Euthanasia is defined as the physician-ordered intentional killing of a patient, typically by a lethal dose of a medication administered by the physician or designee, such as a nurse. Most clinicians and ethicists agree that both are to be distinguished from the death of a terminally ill patient that is hastened by the side effects of opioids or other medications used to control pain or suffering, where the explicit goal is to reduce suffering rather than to cause death; this distinction is known as the “doctrine of double effect” (2, 131, 132) and is increasingly recognized by the legal system as a legitimate component of proper palliative care (127), although some have questioned the validity of this distinction, grounded as it is on the complex and often ambiguous concept of intentionality (133).
The public and professional debate about the desirability of PAS or euthanasia has been highly visible and often hotly contested. A variety of clinical, ethical, religious, and legal arguments have been mounted both in favor of and against legalizing such practices, as discussed elsewhere (134, 135). Descriptions of these practices, for example, euthanasia in the Netherlands, or PAS in Oregon, often offer differing interpretations of the available data, depending on the observer’s bias (134, 136–140). At this time, euthanasia is illegal throughout the United States. Oregon has legally sanctioned PAS in terminally ill patients after meeting certain prerequisites intended to prevent abuses such as the use of PAS to cause death from suicidal depression. Clinicians, including thoughtful proponents of PAS, recognize that proper evaluation of requests for PAS may lead to a range of appropriate responses, including the use of reassuring assertions of the tenet of physician non-abandonment, or by identification and treatment of remediable suffering from pain, depression, or other symptoms (141–143). As with many areas of palliative care, there is much empirical research needed that could inform the ongoing debate about the proper clinical and legal paths to take, and geriatric psychiatrists’ expertise is well suited to address crucial unanswered questions (144).
End-of-life care in patients with dementia
Neurodegenerative dementias, including those due to Alzheimer disease and cerebrovascular disorders, are substantial contributors to death among elderly patients (145, 146). The clinical approaches to diagnosis and management of these conditions—including treatments of distressing non-cognitive neuropsychiatric symptoms, an important component of “palliative care” in dementia—are, of course, core skills of the geriatric psychiatrist, and have been reviewed elsewhere (147). The diagnosis of dementia provides an opportunity to (indeed, mandates that we) engage in ongoing end-of-life discussions with the patient and family, and to begin doing so early in the course of a disease that often will lead to death over a lengthy and relatively predictable trajectory. Elicitation of values and care preferences for many will facilitate the transition to a predominantly palliative approach as the disease progresses; as with other chronic diseases, there is evidence that currently such decisions often are not being made optimally (148), and, of course, in dementia, there are the added complexities of progressive cognitive impairment affecting decisional capacity (149).
Future directions
This article has noted repeatedly how the clinical skills of geriatric psychiatrists, encompassing the numerous domains discussed in this review, are quite relevant to the care of dying patients. It is my hope that our subspecialty, together with colleagues from those of allied disciplines, will take a leading role in working with our own patients as they face life-threatening illnesses, and an active approach working with our medical colleagues in consultative or collaborative roles.
The time is also ripe for applying the empirical research tools of geriatric psychiatry to patients with life-threatening illnesses. Indeed, geriatric psychiatry has emerged in recent years as a leader of psychiatry research (150), in aspects such as: inclusion and assessment of psychiatric symptoms and syndromes in the context of complex medical and neurological comorbidities; identification and study of “spectrum disorders,” such as minor or subsyndromal depression; and assessment and treatment of complex behavioral symptoms that cut across the psychopathological categories established primarily in younger and medically healthier patients; for example, agitation in dementia. Experience gained from such approaches is directly relevant to the many areas in palliative care requiring further study. Such work must range from basic descriptive psychopathology and epidemiology (rarely used to date with dying patients other than cancer or HIV populations), to testing theoretical models by which biological, psychological, or psychosocial factors affect the dying process, to appropriate controlled trials testing the feasibility and effectiveness of psychopharmacological and psychotherapeutic interventions to target the above-described distressing symptoms, all with the goals of improving the quality of the final months of life.
| Physical and emotional symptoms |
| Support of function and autonomy |
| Advance care planning |
| Limitation of unwanted or futile aggressive care near death |
| Patient and family satisfaction |
| Global quality of life |
| Family burden (including bereavement) |
| Survival time prognostication |
| Provider continuity and skill |
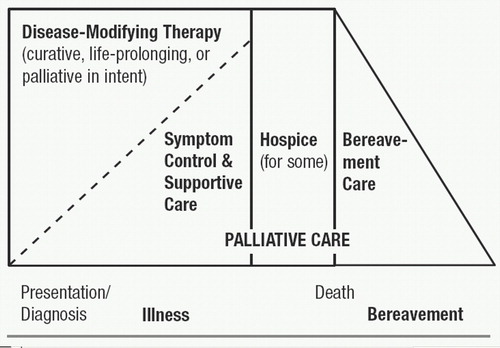
Figure 1. Continuum of Care
Source: EPEC (Education for Physicians on End-of-Life Care) Participant’s Handbook. The Robert Wood Johnson Foundation/American Medical Association, © The EPEC Project, 1999
1 Smith R: A good death. an important aim for health services and for us all. BMJ 2000; 320:129–130Crossref, Google Scholar
2 Quill TE, Brody RV: “You promised me I wouldn’t die like this!” a bad death as a medical emergency. Arch Intern Med 1995; 155:1250–1254Crossref, Google Scholar
3 Neuberger J: Dying Well: A Guide To Enabling A Good Death. Cheshire, UK, Hochland and Hochland, 1999Google Scholar
4 Blackburn A: Care of dying patients in hospital: why care of the dying is still poor. BMJ 1994; 309:1579Crossref, Google Scholar
5 Institute of Medicine Committee on Care at the End of Life: A profile on death and dying in America, in Approaching Death: Improving Care At The End Of Life. Edited by Field MJ, Cassel CK. Washington, DC, National Academy Press, 1997, pp 33–49Google Scholar
6 Clark D: Between hope and acceptance: the medicalisation of dying. BMJ 2002; 324:905–907Crossref, Google Scholar
7 Seidlitz L, Duberstein PR, Cox C, et al: Attitudes of older people toward suicide and assisted suicide: an analysis of Gallup Poll findings. J Am Geriatr Soc 1995; 43:995–998Google Scholar
8 Lunney JR, Lynn J, Hogan C: Profiles of Medicare decedents. J Am Geriatr Soc 2002; 50:1108–1112Crossref, Google Scholar
9 Hogan C, Lynn J, Gabel J, et al: Medicare beneficiaries’ costs and use of care in the last year of life. Washington, DC, Medicare Payment Advisory Commission, 2000 (http://www.medicaring.org/educate/download/medpac.doc)Google Scholar
10 Lynn J: Measuring quality of care at the end of life: a statement of principles. J Am Geriatr Soc 1997; 45:526–527Crossref, Google Scholar
11 Tilden VP, Tolle S, Drach L, et al: Measurement of quality of care and quality of life at the end of life. Gerontologist 2002; 42(special issue III):71–80Crossref, Google Scholar
12 Wenger NS, Rosenfeld K: Quality indicators for end-of-life care in vulnerable elders. Ann Intern Med 2001; 135:677–685Crossref, Google Scholar
13 Breitbart W, Chochinov HM, Passik S: Psychiatric aspects of palliative care, in Oxford Textbook Of Palliative Medicine, 2nd Edition. Edited by Doyle D, Hanks GWC, MacDonald N. New York, Oxford University Press, 1998, pp 933–954Google Scholar
14 Chochinov HM: Psychiatry and terminal illness. Can J Psychiatry 2000; 45:143–150Crossref, Google Scholar
15 Shuster JL, Breitbart W, Chochinov HM, et al: Psychiatric aspects of excellent end-of-life care. Psychosomatics 1999; 40:1–4Crossref, Google Scholar
16 Doyle D, Hanks GWC, MacDonald N (eds): Oxford Textbook Of Palliative Medicine, 2nd Edition. New York, Oxford University Press, 1998Google Scholar
17 Snyder L, Quill TE (eds): Physician’s Guide To End-Of-Life Care. Philadelphia, PA, American College of Physicians/American Society of Internal Medicine, 2001Google Scholar
18 Kinzbrunner BM, Weinreb NJ, Policzer JS (eds): 20 Common Problems In End-Of-Life Care. New York, McGraw-Hill, 2002Google Scholar
19 Larson DG, Tobin DR: End-of-life conversations: evolving practice and theory. JAMA 2000; 284:1573–1578Crossref, Google Scholar
20 Covinsky KE, Eng C, Lui LY, et al: The last two years of life: functional trajectories of frail older people. J Am Geriatr Soc 2003; 51:492–498Crossref, Google Scholar
21 Dickinson GE, Field D: Teaching end-of-life issues: current status in United Kingdom and United States medical schools. AmJ Hosp Palliat Care 2002; 19:181–186Crossref, Google Scholar
22 Ury WA, Rahn M, Tolentino V: Can a pain-management and palliative-care curriculum improve the opioid prescribing practices of medical residents? J Gen Intern Med 2002; 17:625–631Crossref, Google Scholar
23 Wenger NS, Phillips RS, Teno JM, et al: Physician understanding of patient resuscitation preferences: insights and clinical applications. J Am Geriatr Soc 2000; 48(5suppl):S44–S51Google Scholar
24 Lo B, Quill T, Tulsky J: Discussing palliative care with patients. Ann Intern Med 1999; 130:744–749Crossref, Google Scholar
25 Fried TR, Bradley EH, Towle VR, et al: Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002; 346:1061–1066Crossref, Google Scholar
26 Quill TE: Initiating end-of-life discussions with seriously ill patients: addressing the “elephant in the room. ” JAMA 2000; 284:2502–2507Crossref, Google Scholar
27 Cassel EJ: The Nature Of Suffering And The Goals Of Medicine. New York, Oxford University Press, 1991Google Scholar
28 Quill TE, Cassel CK: Nonabandonment: a central obligation for physicians. Ann Intern Med 1995; 122:368–374Crossref, Google Scholar
29 Kagawa-Singer M, Blackhall LJ: Negotiating cross-cultural issues at the end of life: “You got to go where he lives.” JAMA 2001; 286:2993–3001Crossref, Google Scholar
30 Rosen EJ: Families Facing Death: A Guide For Healthcare Professionals And Volunteers. San Francisco, CA, Jossey-Bass, Inc., 1998Google Scholar
31 Lo B, Ruston D, Kates LW, et al: Discussing religious and spiritual issues at the end of life. a practical guide for physicians. JAMA 2002; 287:749–754Crossref, Google Scholar
32 Chochinov HM: Dignity-conserving care: a new model for palliative care. JAMA 2002; 287:2253–2260Crossref, Google Scholar
33 Chochinov HM, Hack T, McClement X, et al: Dignity in the terminally ill: a developing empirical model. Soc Sci Med 2002; 54:433–443Crossref, Google Scholar
34 Nguyen VD, Weinreb NJ: How to inform the patient: conveying bad news, in 20 Common Problems In End-Of-Life Care. Edited by Kinzbrunner BM, Weinreb NJ, Policzer JS. New York, McGraw-Hill, 2002, pp 47–56Google Scholar
35 Quill TE, Townsend P: Bad news: delivery, dialogue, and dilemmas. Arch Intern Med 1991; 151:463–468Crossref, Google Scholar
36 Tsevat J, Dawson NV, Wu AW, et al: Health values of hospitalized patients 80 years or older. JAMA 1998; 279:371–375Crossref, Google Scholar
37 Murphy DJ, Burrows B, Santilli S, et al: The influence of the probability of survival on patients’ preferences regarding cardiopulmonary resuscitation. N Engl J Med 330:545–549Crossref, Google Scholar
38 Weeks JC, Cook EF, O’Day SJ, et al: Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998; 279:1709–1714Crossref, Google Scholar
39 Quill TE, Arnold RM, Platt F: “I wish things were different:” expressing wishes in response to loss, futility, and unrealistic hopes. Ann Intern Med 2001; 135:551–555Crossref, Google Scholar
40 Salerno E, Willens JS (eds): Pain Management Handbook, An Interdisciplinary Approach. St. Louis, Mosby, 1996Google Scholar
41 World Health Organization Expert Committee: Cancer Pain Relief And Palliative Care. Geneva, WHO, 1990Google Scholar
42 Bruera E, Portenoy R (eds): Cancer Pain: Assessment And Management. Cambridge, UK, Cambridge University Press, 2003Google Scholar
43 Lynn J, Schuster JL, Kabcenell A: Improving Care For The End Of Life. A Sourcebook For Health Care Managers And Clinicians. Oxford, UK, Oxford University Press, 2000, pp 37–58Google Scholar
44 Abrahm JL: Management of pain and spinal cord compression in patients with advanced cancer, in Physician’s Guide To End-Of-Life Care. Edited by Snyder L, Quill TE. Philadelphia, PA, American College of Physicians/American Society of Internal Medicine, 2001Google Scholar
45 Dahl JL: Effective pain management in terminal care. Clin Geriatr Med 1996; 12:279–300Crossref, Google Scholar
46 Weinreb NJ, Kinzbrunner B, Clark M: Pain management, in 20 Common Problems In End-Of-Life Care. Edited by Kinzbrunner BM, Weinreb NJ, Policzer JS. New York, McGraw-Hill, 2002, pp 91–145Google Scholar
47 Rawlins MD: Non-opioid analgesics, in Oxford Textbook Of Palliative Medicine, 2nd Edition. Edited by Doyle D, Hanks GWC, MacDonald N. New York, Oxford University Press, 1998, pp 355–360Google Scholar
48 Enck RE: Understanding and managing bone metastases. Am J Hosp Palliat Care 1991; 8:3–4Google Scholar
49 Hanks GW: The pharmacological treatment of bone pain. Cancer Surv 1988; 7:87–101Google Scholar
50 Watanabe S, Bruera E: Corticosteroids as adjuvant analgesics. J Pain Symptom Manage 1994; 9:442–445Crossref, Google Scholar
51 Major PP, Cook R: Efficacy of biphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am J Clin Oncol 2002; 25(suppl1):S10–S18Crossref, Google Scholar
52 Mystakidou K, Befon S, Hondros K, et al: Continuous subcutaneous administration of high-dose salmon calcitonin in bone metastasis: pain control and beta-endorphin plasma levels. J Pain Symptom Manage 1999; 18:323–330Crossref, Google Scholar
53 Sindrup SH, Jensen TS: Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain 1999; 83:389–400Crossref, Google Scholar
54 McQuay HJ, Tramer M, Nye BA, et al: A systematic review of antidepressants in neuropathic pain. Pain 1996; 68:217–227Crossref, Google Scholar
55 Semenchuk MR, Sherman S, Davis B: Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology 2001; 57:1583–1588Crossref, Google Scholar
56 Tasmuth T, Hartel B, Kalso E: Venlafaxine in neuropathic pain following treatment of breast cancer. Eur J Pain 2002; 6:17–24Crossref, Google Scholar
57 Brannon GE, Stone KD: The use of mirtazapine in a patient with chronic pain. J Pain Symptom Manage 1999; 18:382–385Crossref, Google Scholar
58 Theobald DE, Kirsh KL, Holtsclaw E, et al: An open-label, crossover trial of mirtazapine (15 and 30 mg) in cancer patients with pain and other distressing symptoms. J Pain Symptom Manage 2002; 23:442–447Crossref, Google Scholar
59 Wiffen P, Collins S, McQuay H, et al: Anticonvulsant drugs for acute and chronic pain. Cochrane Database of Systematic Reviews, 2003Google Scholar
60 Uhle EI, Becker R, Gatscher S, et al: Continuous intrathecal clonidine administration for the treatment of neuropathic pain. Stereotact Funct Neurosurg 2000; 75:167–175Crossref, Google Scholar
61 Tanelian DL, Brose WG: Neuropathic pain can be relieved by drugs that are use-dependent sodium channel-blockers: lidocaine, carbamazepine, and mexiletine. Anesthesiology 1991; 74:949–951Crossref, Google Scholar
62 Fromm GH: Baclofen as an adjuvant analgesic. J Pain Symptom Manage 1994; 9:500–509Crossref, Google Scholar
63 Pud D, Eisenberg E, Spitzer A, et al: The NMDA receptor-antagonist amantadine reduces surgical neuropathic pain in cancer patients: a double blind, randomized, placebo-controlled trial. Pain 1998; 75:349–354Crossref, Google Scholar
64 Felsby S, Nielson J, Arendt-Nielson L, et al: NMDA receptor blockade in chronic neuropathic pain: a comparison of ketamine and magnesium chloride. Pain 1995; 64:283Crossref, Google Scholar
65 Rummans TA: Nonopioid agents for treatment of acute and subacute pain. Mayo Clin Proc 1994; 69:481–490Crossref, Google Scholar
66 Bruera E, Breineis C, Paterson AH, et al: Use of methylphenidate as an adjuvant to narcotic analgesics in patients with advanced cancer. J Pain Symptom Manage 1989; 4:3–6Crossref, Google Scholar
67 Wilwerding MB, Loprinzi CL, Mailliard JA, et al: A randomized, crossover evaluation of methylphenidate in cancer patients receiving strong narcotics. Support Care Cancer 1995; 3:135–138Crossref, Google Scholar
68 Courts NF: Nonpharmacologic approaches to pain, in Pain Management Handbook: An Interdisciplinary Approach. Edited by Salerno E, Willens JS. St. Louis, MO, Mosby, 1996Google Scholar
69 Urba SG: Nonpharmacologic pain management in terminal care. Clin Geriatr Med 1996; 12:301–311Crossref, Google Scholar
70 Caine ED, Lyness JM, King DA: Reconsidering depression in the elderly. Am J Geriatr Psychiatry 1993; 1:4–20Crossref, Google Scholar
71 Katz IR: On the inseparability of mental and physical health in aged persons: lessons from depression and medical comorbidity. Am J Geriatr Psychiatry 1996; 4:1–16Google Scholar
72 Koenig HG, George LK, Peterson BL, et al: Depression in medically ill, hospitalized older adults: prevalence, characteristics, and course of symptoms according to six diagnostic schemes. Am J Psychiatry 1997; 154:1376–1383Crossref, Google Scholar
73 Lyness JM, Bruce ML, Koenig HG, et al: Depression and medical illness in late life: report of a symposium. J Am Geriatr Soc 1996; 44:198–203Crossref, Google Scholar
74 Block SD: Assessing and managing depression in the terminally ill patient. Ann Intern Med 2000; 132:209–218Crossref, Google Scholar
75 Judd LL, Akiskal HS, Paulus MP: The role and significance of subsyndromal depressive symptoms (SSD) in unipolar major depressive disorder. J Affect Disord 1997; 45:5–18Crossref, Google Scholar
76 Kendler KS, Gardner CO Jr: Boundaries of major depression: an evaluation of DSM-IV criteria. Am J Psychiatry 1998; 155:172–177Google Scholar
77 Lyness JM, King DA, Cox C, et al: The importance of subsyndromal depression in older primary care patients: prevalence and associated functional disability. J Am Geriatr Soc 1999; 47:647–652Crossref, Google Scholar
78 Flint AJ: The complexity and challenge of non-major depression in late life. Am J Geriatr Psychiatry 2002; 10:229–232Crossref, Google Scholar
79 Bukberg J, Penman D, Holland JC: Depression in hospitalized cancer patients. Psychosom Med 1984; 46:199–212Crossref, Google Scholar
80 Chochinov HM, Wilson KG, Enns M, et al: Prevalence of depression in the terminally ill: effects of diagnostic criteria and symptom threshold judgments. Am J Psychiatry 1994; 151:537–540Crossref, Google Scholar
81 Kathol RG, Mutgi A, Williams J, et al: Diagnosis of major depression in cancer patients according to four sets of criteria. Am J Psychiatry 1990; 147:1021–1024Crossref, Google Scholar
82 Derogatis LR, Marrow GR, Fetting J, et al: The prevalence of psychiatric disorders among cancer patients. JAMA 1983; 249:751–757Crossref, Google Scholar
83 Lloyd-Williams M, Friedman T, Rudd N: Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manage 2000; 20:259–265Crossref, Google Scholar
84 Lloyd-Williams M: Is it appropriate to screen palliative care patients for depression? Am J Hosp Palliat Care 2002; 19:112–114Crossref, Google Scholar
85 Chochinov HM, Wilson KG, Enns M, et al: “Are you depressed?” screening for depression in the terminally ill. Am J Psychiatry 1997; 154:674–676Crossref, Google Scholar
86 Ramasubbu R: Relationship between depression and cerebrovascular disease: conceptual issues. J Affect Disord 2000; 57:1–11Crossref, Google Scholar
87 Lyness JM, Caine ED: Vascular disease and depression: models of the interplay between psychopathology and medical comorbidity, in Physical Illness and Depression in Older Adults: A Handbook of Theory, Research, and Practice. Edited by Williamson GM, Shaffer DR, Parmelee PA. New York, Kluwer Academic/Plenum, 2000, pp 31–49Google Scholar
88 Ballenger JC, Davidson JR, Lecrubrier Y, et al: Consensus statement on depression, anxiety, and oncology. J Clin Psychiatry 2001; 62(suppl8):64–67Google Scholar
89 Chochinov HM, Tataryn D, Clinch JJ, et al: Will to live in the terminally ill. Lancet 1999; 354:816–819Crossref, Google Scholar
90 Breitbart W, Rosenfeld B, Pessin H, et al: Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA 2000; 284:2907–2911Crossref, Google Scholar
91 Lebowitz BD, Pearson JL, Schneider LS, et al: Diagnosis and treatment of depression in later life: Consensus Statement Update. JAMA 1997; 278:1186–1190Crossref, Google Scholar
92 Lyness JM, Caine ED, King DA, et al: Depressive disorders and symptoms in older primary care patients. one-year outcomes. Am J Geriatr Psychiatry 2002; 10:275–282Crossref, Google Scholar
93 Cole BE: The psychiatric management of end-of-life pain and associated psychiatric comorbidity. Curr Pain Headache Rep 2003; 7:89–97Crossref, Google Scholar
94 Krishnan KR, DeLong M, Kraemer H, et al: Comorbidity of depression with other medical diseases in the elderly. Biol Psychiatry 2002; 52:559–588Crossref, Google Scholar
95 Satel SL, Nelson CJ: Stimulants in the treatment of depression: a critical overview. J Clin Psychiatry 1989; 50:241–249Google Scholar
96 Burns MM, Eisendrath SJ: Dextroamphetamine treatment for depression in terminally ill patients. Psychosomatics 1994; 35:80–82Crossref, Google Scholar
97 Homsi J, Walsh D, Nelson KA: Methylphenidate for depression in hospice practice: a case series. Am J Hosp Palliat Care 2000; 17:393–398Crossref, Google Scholar
98 Sarhill N, Walsh D, Nelson KA, et al: Methylphenidate for fatigue in advanced cancer: a prospective open-label pilot study. Am J Hosp Palliat Care 2001; 18:187–192Crossref, Google Scholar
99 Macleod AD: Methylphenidate in terminal depression. J Pain Symptom Manage 1998; 16:193–198Crossref, Google Scholar
100 Cox JM, Pappagallo M: Modafinil: a gift to portmanteau. Am J Hosp Pallliat Care 2001; 18:408–410Crossref, Google Scholar
101 Conwell Y, Pearson JL: Theme issue: suicidal behaviors in older adults. Am J Geriat Psychiatry 2002; 10:359–361Crossref, Google Scholar
102 Chochinov HM, Wilson KG, Enns M, et al: Depression, hopelessness, and suicidal ideation in the terminally ill. Psychosomatics 1998; 39:366–370Crossref, Google Scholar
103 Bolund C: Suicide and cancer, II: medical and care factors in suicide by cancer patients in Sweden, 1973–1976. J Psychosoc Oncol 1985; 3:17–30Crossref, Google Scholar
104 Holland JC: Anxiety and cancer: the patient and family. J Clin Psychiatry 1989; 50:20–25Google Scholar
105 Kelly B, Burnett P, Pelusi D, et al: Factors associated with the wish to hasten death: a study of patients with terminal illness. Psychol Med 2003; 33:75–81Crossref, Google Scholar
106 Johnson TA, McDonagh TA, Harkness A, et al: Morphine for the relief of breathlessness in patients with chronic heart failure: a pilot study. Eur J Heart Fail 2002; 4:753–756Crossref, Google Scholar
107 Bruera E, MacMillan K, Pither J, et al: Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage 1990; 5:341–344Crossref, Google Scholar
108 Zuehlke TE, Watkins JT: The use of psychotherapy with dying patients: an exploratory study. J Clin Psychol 1975; 31:729–732Crossref, Google Scholar
109 Massie MJ, Holland JC, Straker N: Psychotherapeutic interventions, in Handbook of Psycho-Oncology: Psychological Care of the Patient With Cancer. Edited by Holland JC, Rowland JH. New York, Oxford University Press, 1989, pp 455–469Google Scholar
110 Lawlor PG, Bruera ED: Delirium in patients with advanced cancer. Hematol Oncol Clin N Am 2002; 16:701–714Crossref, Google Scholar
111 Casarett DJ, Inouye SK: Diagnosis and management of delirium near the end of life. Ann Intern Med 2001; 135:32–40Crossref, Google Scholar
112 Schwartz TL, Masand PS: The role of atypical antipsychotics in the treatment of delirium. Psychosomatics 2002; 43:171–174Crossref, Google Scholar
113 Block SD: Psychological considerations, growth, and transcendence at the end of life. the art of the possible. JAMA 2001; 285:2898–2905Crossref, Google Scholar
114 King DA, Kim SYH, Conwell Y: Family matters: a social system perspective on physician-assisted suicide and the older adult. Psychol Public Policy Law 2000; 6:434–451Crossref, Google Scholar
115 King DA, Shields CG, Wynne LG: Interventions and consultation with families of older adults, in Kaplan and Sadock’s Comprehensive Textbook Of Psychiatry, 7th Edition. Edited by Sadock BJ, Sadock VA. Philadelphia, PA, Lippincott Williams and Wilkins, 2000, pp 3122–3127Google Scholar
116 Prigerson HG, Shear MK, Jacobs SC, et al: Consensus criteria for traumatic grief. a preliminary empirical test. Br J Psychiatry 1999; 174:560–561Crossref, Google Scholar
117 Grisso T, Appelbaum PS: Assessing Competence to Consent to Treatment: A Guide for Physicians and Other Health Professionals. New York, Oxford University Press, 1998Google Scholar
118 Kim SYH, Karlawish JH, Caine ED: Current state of research on decision-making competence of cognitively impaired older persons. Am J Geriatr Psychiatry 2002; 10:151–165Crossref, Google Scholar
119 Drane JF: Competency to give an informed consent: a model for making clinical assessments. JAMA 1984; 252:925–927Crossref, Google Scholar
120 Buchanan AE, Brock DW: Competence and incompetence, in Deciding for Others: The Ethics of Surrogate Decision-Making. New York, Cambridge University Press, 1989, pp 17–86Google Scholar
121 Vig EK, Davenport NA, Pearlman RA: Good deaths, bad deaths, and preferences for the end of life: a qualitative study of geriatric outpatients. J Am Geriatr Soc 2002; 50:1541–1548Crossref, Google Scholar
122 Quill TE, Lo B, Brock DW: Palliative options of last resort. a comparison of voluntarily stopping eating and drinking, terminal sedation, physician-assisted suicide, and voluntary active euthanasia. JAMA 1997; 278:2099–2104Crossref, Google Scholar
123 Printz LA: Terminal dehydration, a compassionate treatment. Arch Intern Med 1992; 152:697–700Crossref, Google Scholar
124 McCann R: Lack of evidence about tube feeding: food for thought. JAMA 1999; 282:1380–1381Crossref, Google Scholar
125 Finucane TE, Christmas C, Travis K: Tube-feeding in patients with advanced dementia: a review of the evidence. JAMA 1999; 282:1365–1370Crossref, Google Scholar
126 Weissman DE: Feeding tubes at end-of-life: the lack of physician leadership. J Palliat Med 2000; 3:1–3Crossref, Google Scholar
127 Meisel A, Snyder L, Quill TE: Legal barriers to end-of-life care: myths, realities, and grains of truth, in Physician’s Guide To End-Of-Life Care. Edited by Snyder L, Quill TE. Philadelphia, PA, American College of Physicians/American Society of Internal Medicine, 2001Google Scholar
128 Cowan JD, Walsh D: Terminal sedation in palliative medicine: definition and review of the literature. Support Care Cancer 2001; 9:403–407Crossref, Google Scholar
129 Cherny NI, Portenoy RK: Sedation in the management of refractory symptoms: guidelines for evaluation and treatment. J Palliat Care 1994; 10:31–38Google Scholar
130 Quill TE, Byock IR: Responding to intractable terminal suffering: the role of terminal sedation and voluntary refusal of food and fluids. Ann Intern Med 2000; 132:408–414Crossref, Google Scholar
131 Council on Ethical and Judicial Affairs, American Medical Association: Decisions near the end of life. JAMA 1992; 276:2229–2233Google Scholar
132 Foley KM: Competent care for the dying instead of physician-assisted suicide. N Engl J Med 1997; 336:54–58Crossref, Google Scholar
133 Quill TE: The ambiguity of clinical intentions. N Engl J Med 1993; 329:1039–1040Crossref, Google Scholar
134 Foley K, Hendin H (eds): The Case Against Assisted Suicide. For The Right To End-Of-Life Care. Baltimore, MD, Johns Hopkins University Press, 2002Google Scholar
135 Quill TE, Cassel CK, Meier DK: Care of the hopelessly ill: proposed clinical criteria for physician-assisted suicide. N Engl J Med 1992; 327:1380–1384Crossref, Google Scholar
136 Lyness JM: book review of The Case Against Assisted Suicide. N Engl J Med 2002; 347:541Crossref, Google Scholar
137 Ganzini L, Nelson HD, Schmidt TA, et al: Physicians’ experiences with the Oregon Death With Dignity Act. N Engl J Med 2000; 342:557–563Crossref, Google Scholar
138 Steinbrook R: Physician-assisted suicide in Oregon: an uncertain future. N Engl J Med 2002; 346:460–464Crossref, Google Scholar
139 Veldink JH, Wokke JHJ, van der Wal G, et al: Euthanasia and physician-assisted suicide among patients with amyotrophic lateral sclerosis in the Netherlands. N Engl J Med 2002; 346:1638–1644Crossref, Google Scholar
140 Groenewoud JH, van der Heide A, Onwuteaka-Philipsen BD, et al: Clinical problems with the performance of euthanasia and physician-assisted suicide in the Netherlands. N Engl J Med 2000; 342:551–556Crossref, Google Scholar
141 Quill TE, Meier DE, Block SD, et al: The debate over physician-assisted suicide: empirical data and convergent views. Ann Intern Med 1998; 128:552–558Crossref, Google Scholar
142 Ganzini L, Lee MA, Heintz RT, et al: The effect of depression treatment on elderly patients’ preferences for life-sustaining medical therapy. Am J Psychiatry 1994; 151:1631–1636Crossref, Google Scholar
143 Block SD, Billings JA: Patient requests for euthanasia and assisted suicide in terminal illness. the role of the psychiatrist. Psychosomatics 1995; 36:445–457Crossref, Google Scholar
144 Conwell Y, Caine ED: Rational suicide and the right to die: reality and myth. N Engl J Med 1991; 325:1100–1103Crossref, Google Scholar
145 Hurley AC, Volicer L: Alzheimer Disease: “It’s okay, Mama, if you want to go, it’s okay”. JAMA 2002; 288:2324–2331Crossref, Google Scholar
146 Michel JP, Pautex S, Zekry D, et al: End-of-life care of persons with dementia. J Gerontol 2002; 57:M640–M644Crossref, Google Scholar
147 Grossberg GT, Desai AK: Management of Alzheimer’s disease. J Gerontol 2003; 58A:331–353Crossref, Google Scholar
148 Evers MM, Purohit D, Perl D, et al: Palliative and aggressive end-of-life care for patients with dementia. Psychiatr Serv 2002; 53:609–613Crossref, Google Scholar
149 Karlawish JHT, Quill T, Meier DE: A consensus-based approach to providing palliative care to patients who lack decision-making capacity. Ann Intern Med 1999; 130:835–840Crossref, Google Scholar
150 Charney DS, Reynolds CF III, Lewis L, et al: Depression and Bipolar Support Alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry 2003; 60:664–672Crossref, Google Scholar



