Double-Blind Comparison of Addition of a Second Mood Stabilizer Versus an Antidepressant to an Initial Mood Stabilizer for Treatment of Patients With Bipolar Depression
Abstract
Objective: This study’s purpose was to clarify the appropriate treatment of bipolar depression by comparing the addition of an antidepressant versus a second mood stabilizer for inpatients being treated with lithium carbonate or divalproex sodium. Method: Twenty-seven patients were randomly assigned to groups that received double-blind treatment with paroxetine or a second mood stabilizer (lithium carbonate or divalproex sodium) for 6 weeks. Results: Both groups showed significant improvement in depressive symptoms during the 6-week trial. There were significantly more noncompleters in the group being treated with the two mood stabilizers than in the group being treated with a mood stabilizer and paroxetine. Conclusions: Both treatments appeared to be effective; however, the addition of an antidepressant may have greater clinical utility in the treatment of bipolar depression.
The treatment of bipolar disorder is a vastly understudied subject (1, 2). The morbidity of depression, common in bipolar disorder, and the substantial risk of suicide necessitate acute intervention (2). Little is known about the treatment of depressive episodes in patients with bipolar disorder and its impact on the course of the illness (1). In particular, controlled clinical trials comparing standard clinical treatments for depression in patients with bipolar disorder are lacking (3). It is a widely accepted clinical practice to add a second mood stabilizer or an antidepressant to the treatment regimens of patients with bipolar disorder who relapse into depression while being treated with a mood stabilizer under the assumption that these drugs may be of equivalent efficacy (4–6). This treatment has not been empirically tested in a controlled study. The objective of this study was, therefore, to clarify the appropriate treatment of bipolar depression by comparing the addition of an antidepressant versus a second mood stabilizer in depressed patients who were receiving lithium carbonate or divalproex sodium.
Method
The participants were 27 outpatients (nine men and 18 women) with bipolar disorder (type I, N=11; type II, N=16), diagnosed by means of the Structured Clinical Interview for Axis I DSM-IV Disorders—Patient Version (7), who experienced a major depressive episode while being treated with either lithium or divalproex. The patients were nonpsychotic and were free of comorbid alcohol or drug abuse (for at least 6 months), acute medical illness, or rapid cycling. The participants had been receiving a mood stabilizer for at least 3 months at therapeutic blood serum concentrations (mean lithium dose=1200 mg/day, SD=240 [0.8 mmol/liter, SD=0.2]; mean divalproex dose=1200 mg/day, SD=210 [570 mmol/liter, SD=71]). Most of the patients (21 of 27) were being treated with no other medications, except for five who were receiving hypnotics (chloral hydrate or zopiclone) and three who were receiving thyroid replacement therapy. Written informed consent was obtained from all subjects. At baseline, mood symptoms were rated by using the 17-item Hamilton Rating Scale for Depression (8) and the Young Mania Rating Scale (9). The patients’ level of functioning was measured with the Global Assessment of Functioning Scale (10). Patients with two consecutive weekly ratings on the Hamilton depression scale of 16 or greater were randomly assigned to receive a second mood stabilizer (lithium or divalproex) or an antidepressant (paroxetine) administered in a double-blind fashion for 6 weeks. All patients received two identical gel capsules twice a day that contained the medication. On the basis of clinical response, blood serum concentrations of the drug, and tolerance, the dose was titrated upward within the first 2 weeks of treatment by a physician who was not blind to the medication (I.P.-S.) within the first 2 weeks of treatment. Mood symptoms were rated weekly by a blind rater using the Hamilton depression scale, the Young Mania Rating Scale, and the Global Assessment of Functioning Scale.
Results
Sixteen patients (seven men and nine women; mean age=40 years, SD=12) received a second mood stabilizer, and eleven patients (two men and nine women; mean age=41 years, SD=12) received paroxetine. When they entered the study, 19 patients were being treated with lithium and eight patients were being treated with divalproex. All subjects who received paroxetine completed the 6-week trial. In the group receiving two mood stabilizers, six patients did not complete the trial because of intolerance (N=2), noncompliance (N=2), an unrelated medical complication (N=1), or the emergence of a mixed state (N=1), and the completion rate in this group was significantly lower than in the paroxetine group (χ2=7.43, df=1, p<0.01). The time point at which each patient withdrew is indicated in figure 1. The mean doses (and blood serum concentrations of mood stabilizers) achieved for the trial medications were as follows: paroxetine, mean dose=36 mg/day, SD=12; lithium, mean dose=1300 mg/day, SD=200 (mean blood level=0.9 mmol/liter, SD=0.2); divalproex, mean dose=1200 mg/day, SD=300 (mean blood level=510 mmol/liter, SD=150).
Weekly ratings were analyzed by using a 2×7 (treatment group by duration of treatment) mixed analysis of variance, followed by planned contrasts comparing measures from each week against baseline measures. Missing data for noncompleters were estimated by carrying forward the last observation (the data were reanalyzed by using estimations based on both linear and nonlinear regression models, yielding identical results in all analyses). The error degrees of freedom in the analyses were reduced to account for the imputed data points. There was a main effect (Figure 1) for duration of treatment for Hamilton depression scale scores (F=24.04, df=6, 122, p<0.001), but there was no group main effect or group-by-duration of treatment interaction. Planned contrasts against baseline measures revealed significant main effects for the duration of treatment for all six weekly ratings (p<0.001 in all cases). There was also a significant main effect of the duration of treatment on Global Assessment of Functioning Scale scores (F=15.34, df=6, 122, p<0.001). There were no significant differences in Young Mania Rating Scale scores in either treatment group.
Discussion
The addition of a second mood stabilizer or an antidepressant to the treatment regimens for these patients with bipolar depression resulted in a significant reduction in depression scores and increase in the level of functioning in a 6-week trial for patients with bipolar disorder who experienced depression while receiving lithium or divalproex. More patients who were randomly assigned to a second mood stabilizer than to paroxetine failed to complete the trial. This implies that the addition of an antidepressant may be more effective in the acute treatment of bipolar depression. Furthermore, the addition of paroxetine was not associated with the emergence of manic symptoms in this 6-week period, as was reported in a previous study (11).
These preliminary results need to be interpreted with caution. First, the group size was small. Second, we cannot rule out the possibility that the addition of either drug was no better than no additional treatment because of the lack of a placebo group. Third, the present study was limited to 6 weeks’ duration. The rates of a later emergence of manic symptoms or a relapse into depression in either group are presently unknown and require longer follow-up periods. Fourth, our study group included outpatients with moderately severe nonpsychotic depression and patients with type I and II bipolar disorder, which possibly limits the generalizability of these results to patients with more severe depression. Finally, because of the small group size, the subjects who entered the study while receiving lithium or divalproex were not analyzed separately. Although these drug combinations may not have equivalent effects, there were no apparent differences across subjects who entered the study while receiving these two drugs.
In conclusion, these preliminary results suggest that the usual practices for treating bipolar depression—adding another mood stabilizer and adding an antidepressant—are both effective. These results confirm the short-term efficacy of adding an antidepressant to a mood stabilizer in treating bipolar depression. Moreover, the finding that adding lithium to valproate is effective in treating bipolar depression is consistent with the early literature on the antidepressant effects of lithium in bipolar disorder (12) and with an open trial demonstrating the antidepressant effects of divalproex sodium in the treatment of unipolar depression (13).
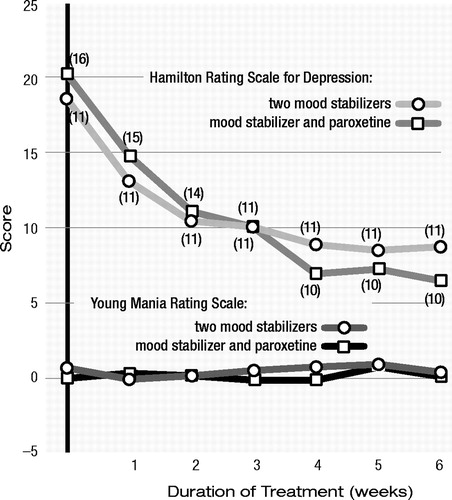
Figure 1. Mean Scores for Depression and Mania of Subjects With Bipolar Depression Who Received a Second Mood Stabilizer or Paroxetine in Addition to an Initial Mood Stabilizera
a Parenthetical numbers indicate numbers of remaining subjects, but the data points include imputed (last observation carried forward data on dropouts.
1 American Psychiatric Association: Practice Guideline for the Treatment of Patients With Bipolar Disorder. Am J Psychiatry 1994; 151(Dec suppl)Google Scholar
2 Goodwin FK, Jamison KR: Manic Depressive Illness. New York, Oxford University Press, 1990Google Scholar
3 Zornberg GL, Pope HG Jr: Treatment of depression in bipolar disorder: new directions for research. J Clin Psychopharmacol 1993; 13:397–408Crossref, Google Scholar
4 Quitkin FM, Kane J, Rifkin A, Ramos-Lorenzi JR, Nayak DV: Prophylactic lithium carbonate with and without imipramine for bipolar 1 patients: a double-blind study. Arch Gen Psychiatry 1981; 38:902–907Crossref, Google Scholar
5 Himmelhoch JM, Thase ME, Mallinger AG, Houck P: Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991; 148:910–916Crossref, Google Scholar
6 Prien RF, Klett CJ, Caffey EM Jr: Lithium carbonate and imipramine in prevention of affective episodes: a comparison in recurrent affective illness. Arch Gen Psychiatry 1973; 29:420–425Crossref, Google Scholar
7 First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for Axis I DSM-IV Disorders—Patient Version (SCID-I/P). New York, New York State Psychiatric Institute, Biometrics Research, 1994Google Scholar
8 Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Google Scholar
9 Young RC, Biggs JT, Ziegler VE: A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Google Scholar
10 First MB, Spitzer RL, Gibbon M, Williams JBW: DSM-IV Axis 5: Global Assessment of Functioning Scale. New York, New York State Psychiatric Institute, Biometrics Research, 1994Google Scholar
11 Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A, Ackerman L: Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995; 152:1130–1138Crossref, Google Scholar
12 Goodwin FK, Murphy DL, Dunner DL, Bunney WE Jr: Lithium response in unipolar versus bipolar depression. Am J Psychiatry 1972; 129:44–47Crossref, Google Scholar
13 Davis LL, Kabel D, Patel D, Choate AD, Foslien-Nash C, Gurguis GN, Kramer GL, Petty F: Valproate as an antidepressant in major depressive disorder. Psychopharmacol Bull 1996; 32:647–652Google Scholar



