Body Dysmorphic Disorder: Clinical Overview and Relationship to Obsessive-Compulsive Disorder
Abstract
Body dysmorphic disorder (BDD), characterized by a distressing or impairing preoccupation with nonexistent or slight defects in appearance, is associated with markedly poor quality of life and high rates of suicidality. Onset of BDD is usually in childhood or adolescence and, unless appropriately treated, tends to be chronic. The first-line pharmacologic approach for both delusional and non-delusional BDD is serotonin reuptake inhibitors (SRIs), often at high doses. SRI augmentation and switching strategies can be effective. The first-line psychotherapy is cognitive-behavioral therapy (CBT) tailored to BDD’s unique clinical features. Cosmetic treatment (such as surgery or dermatologic treatment), although received by a majority of patients with BDD, is not recommended. BDD has many similarities to obsessive-compulsive disorder (OCD) and appears closely related to OCD but also has some important differences. This article, which updates a 2015 article on BDD that we published in this journal, provides a clinically focused overview of BDD and its relationship to OCD.
Body dysmorphic disorder (BDD), a psychiatric disorder involving a distressing or impairing, obsessive focus on perceived flaws in one’s appearance, has many similarities to obsessive-compulsive disorder (OCD); thus, it is classified with OCD in the category of “obsessive-compulsive and related disorders” in the DSM-5 (1). However, BDD has some important differences from OCD, some of which have treatment implications. In this article, we provide a clinically focused overview of BDD and discuss similarities and differences between BDD and OCD.
BDD is more common than OCD, with a point (current) prevalence of nearly 2%–3% of the general population (2). BDD typically causes substantial impairment in psychosocial functioning on par with, or perhaps even more severe than, that typically seen in OCD (3). Despite its prevalence and severity, BDD usually goes unrecognized in clinical settings (2). Sometimes patients are misdiagnosed as having OCD. Yet, BDD is not simply OCD. Although BDD has much in common with OCD, and they are often comorbid conditions, BDD and OCD have several important differences and are distinct disorders. For example, BDD is characterized by more frequent suicidality, higher comorbidity with major depressive disorder and substance use disorders, and poorer insight, which can make it more difficult to engage and retain patients in treatment (4).
BDD was first described in the 1800s and subsequently by the legendary psychopathologists Emil Kraepelin and Pierre Janet (5). Despite this historical tradition and BDD’s severity, systematic scientific research on BDD has been done only in recent decades, and it still lags behind that of most severe psychiatric disorders. Yet, understanding of BDD, including what constitutes effective treatment, has increased dramatically. Box 1 provides a summary of some key clinical aspects of BDD.
BOX 1. Some key facts about body dysmorphic disorder (BDD)
Core features of BDD are distressing or impairing preoccupation with nonexistent or slight defects or flaws in appearance; the preoccupation triggers excessive repetitive behaviors that attempt to check, fix, hide, or obtain reassurance about the perceived bodily deformities (e.g., mirror checking, comparing with others, excessive grooming, reassurance seeking, and skin picking).
BDD is more common than obsessive-compulsive disorder (OCD), with a current prevalence of nearly 2%–3% of the population.
BDD affects nearly as many men as women.
Many patients with BDD express suicidal ideation. Rates of suicidal ideation, suicide attempts, and completed suicide appear markedly elevated and higher than a range of other severe psychiatric disorders, including OCD.
Reluctance to spontaneously reveal appearance concerns is common; thus, patients with BDD may be misdiagnosed as having major depressive disorder, social anxiety disorder, OCD, agoraphobia, and other disorders. Clinicians need to specifically inquire about BDD symptoms.
A selective serotonin reuptake inhibitor is the recommended first-line medication for BDD, even if appearance beliefs are delusional in nature.
Serotonin reuptake inhibitor (SRI) doses and trial durations are similar to those used for OCD; higher doses and a longer treatment trial are recommended than those typically used for depression and most other disorders.
Second-generation antipsychotics may be helpful in addition to an SRI but are not recommended as monotherapy (including for delusional BDD). They are especially appealing for patients with severe depression, hostility or aggression, agitation, or concerning levels of suicidality. Other augmentation strategies (e.g., buspirone, glutamate modulators) can also be helpful.
Cognitive-behavioral therapy that is specifically tailored to BDD is the psychosocial treatment of choice. Simply treating BDD as if it were OCD is not recommended. Because the treatment can be complex and challenging, use of an evidence-based, BDD-specific treatment manual can be helpful.
Cosmetic treatment (e.g., surgical, dermatologic), is not recommended for BDD. It rarely improves symptoms and may make them worse.
Definition and Core Clinical Features of BDD
Diagnostic Criteria for BDD in DSM-5
Criterion A.
Individuals with BDD are preoccupied with one or more perceived defects or flaws in their appearance; the perceived defects, however, are not observable or appear only slight to other people (1). Patients often describe disliked areas as looking “ugly”; other descriptors include “unattractive,” “deformed,” “defective,” “abnormal,” or “hideous.” In reality, the disliked body areas look normal to other people (6). The appearance preoccupations usually occur for ≥1 hour per day; the average is 3–8 hours per day (7). They are intrusive, distressing, unwanted, and usually difficult to resist and control (7).
The skin (usually facial skin) is the most frequently disliked body area (e.g., perceived acne, scarring, color, wrinkles), followed by hair (e.g., thinning hair or excessive facial hair) and nose (often size or shape). However, any body area may be the focus of preoccupation (e.g., teeth, eyes, mouth, jaw, ears, head size or shape, breasts, thighs, stomach, legs, hands, genitals, or body build) (8, 9). The number of areas of excessive concern ranges from one part to virtually the entire body, with an average of five to seven areas of concern over the course of the disorder (8, 9). More than 25% of patients have at least one concern involving asymmetry (e.g., uneven hair or asymmetrical nostrils), which should be diagnosed as BDD rather than OCD (10).
Criterion B.
The appearance preoccupations trigger excessive repetitive behaviors that focus on checking, fixing, hiding, or obtaining reassurance about the perceived flaws (11). These behaviors intend to (but often do not) alleviate emotional distress caused by the appearance preoccupations (11). Virtually all patients perform one or more of these repetitive behaviors at some point during the course of the disorder (11). BDD repetitive behaviors have many similarities to OCD compulsions; they are commonly referred to as compulsions or rituals. Like OCD rituals, BDD repetitive behaviors are typically difficult to resist or control, are distressing, and usually occur for an hour or more per day (average of 3–8 hours per day) (7). However, unlike OCD, some BDD rituals, such as mirror checking, often amplify distress (11, 12).
Table 1 lists common repetitive BDD behaviors, which may be clues to the presence of BDD (8, 9). This list is not exhaustive; patients may engage in other repetitive behaviors, such as compulsively shopping for hair products, taking “selfies,” videotaping their “receding” hairline, or searching online for information about surgery. A more recent ritual that has emerged during the COVID-19 pandemic is scrutinizing one’s own appearance, or comparing one’s appearance with that of other people, on videoconferencing platforms such as Zoom. Most of these behaviors are observable, but some (most notably, comparing with others) are mental rituals and thus cannot be observed by others.
| Lifetime | |
|---|---|
| Behavior or compulsion | rate (%) |
| Comparing disliked body parts with the same areas on others (e.g., in person, online, on television) | 88 |
| Checking disliked body areas in mirrors or other reflecting surfaces | 87 |
| Grooming (e.g., applying makeup; cutting, styling, shaving, or removing head hair, facial hair, or body hair) | 59 |
| Seeking reassurance about the perceived defects or questioning others about how they look (e.g., “Can’t you see this on my face?”) | 54 |
| Touching the disliked areas to check their appearance | 52 |
| Changing clothes (e.g., to camouflage disliked areas or find an outfit that distracts others from the “defects”) | 46 |
| Dieting (e.g., to make a “wide” face narrower) | 39 |
| Skin picking to improve perceived skin flaws | 38 |
| Tanning (e.g., to darken “pale” skin) | 25 |
| Excessive exercising | 21 |
| Excessive weightlifting | 18 |
TABLE 1. Common repetitive behaviors (i.e., rituals, compulsions) among patients with body dysmorphic disordera
More than 90% of patients camouflage their perceived defects, hiding them with such things as a hat, their hair or hands, makeup, clothes, or body position (8, 9). The goal of camouflaging is to avoid or escape unpleasant feelings or prevent a feared event, such as being ridiculed because of the perceived appearance flaws. In this sense, camouflaging is a safety behavior; however, camouflaging can be done repeatedly (e.g., repeatedly rearranging one’s bangs to hide a supposedly high forehead) and thus may fulfill DSM-5 criterion B.
Criteria C and D.
The appearance preoccupations and resulting repetitive behaviors must cause clinically significant distress or impairment in social, occupational, or other important areas of functioning (criterion C) (1). Appearance preoccupations that focus on perceived excessive body fat or weight and that qualify for an eating disorder diagnosis should be diagnosed as an eating disorder rather than BDD (criterion D) (1).
BDD Specifiers in DSM-5: Muscle Dysmorphia, Insight, and Panic Attacks
With muscle dysmorphia.
This specifier identifies individuals (usually men) who are preoccupied with the inaccurate belief that their body build is too small or insufficiently lean and muscular (13). Some men with the muscle dysmorphia form of BDD are unusually muscular because they use potentially dangerous anabolic steroids or excessively lift weights (13). This specifier is also used when patients have non–muscle-focused appearance preoccupations in addition to preoccupations involving insufficient body size and muscularity.
Insight.
This specifier indicates level of insight regarding BDD beliefs (a typical belief is “I look ugly”) (1):
With good or fair insight: the person recognizes that their belief about their appearance is definitely or probably not true or that it may or may not be true.
With poor insight: the person thinks that their BDD belief probably is true.
With absent insight/delusional beliefs: the person is completely convinced that their BDD belief is true.
These levels of insight are similar to DSM-5 insight levels for OCD and hoarding disorder. As we noted in our 2015 article in this journal (14), this specifier conveys several clinically relevant points:
Individuals who are completely convinced that their BDD belief is true (i.e., that they truly are ugly, deformed, or abnormal looking) should be diagnosed as having “BDD with absent insight/delusional beliefs” rather than a psychotic disorder.
Because delusional BDD and nondelusional BDD appear to be the same disorder, the core treatment approaches are very similar for both forms of BDD.
Specifying level of insight allows identification of patients with poor or absent insight who may be reluctant to accept a psychiatric diagnosis and treatment (instead believing that they actually look deformed and need cosmetic treatment). Greater emphasis on motivational interviewing and development of a good therapeutic alliance may be needed to successfully engage and retain such patients in treatment.
Figure 1 shows level of insight among participants with BDD versus OCD from a study that directly compared the two disorders (15). An example of an inaccurate belief in BDD is “I look ugly”; an example of an inaccurate belief in OCD is “If I don’t check the stove 30 times, the house will burn down.” In both disorders, insight spans a full range, from excellent to absent insight/delusional beliefs. However, in BDD, insight regarding the perceived appearance defects is usually absent or poor. In contrast, about 85% of individuals with OCD have excellent, good, or fair insight into the beliefs that underlie their obsessions. Individuals with BDD are also less likely than those with OCD to recognize that their disorder-related beliefs have a psychiatric or psychological cause rather than being true (Figure 2). Thus, patients with BDD may be more difficult to engage and retain in treatment than those with OCD. A likely explanation for the typically poor or absent insight in BDD is that these individuals appear to have aberrations in visual processing (16, 17) and actually see themselves differently than other people see them.
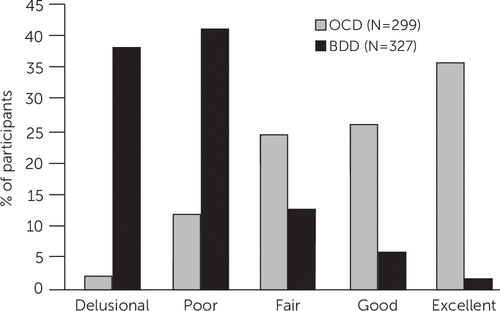
FIGURE 1. Level of insight among participants with body dysmorphic disorder (BDD) versus obsessive-compulsive disorder (OCD)a
a Based on the Brown Assessment of Beliefs Scale (73).
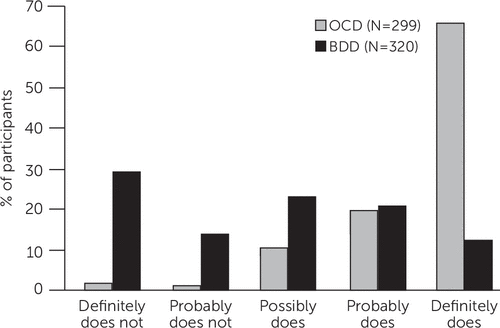
FIGURE 2. Participants’ view of whether their body dysmorphic disorder (BDD) or obsessive-compulsive disorder (OCD) beliefs have a psychiatric or psychological cause (as opposed to being true)a
With panic attacks.
As discussed in our 2015 article (14), the DSM-5 panic attack specifier is not specific to BDD; it may be used for any disorder characterized by disorder-triggered panic attacks (as opposed to panic attacks that “come out of the blue,” as in panic disorder) (1). Nearly 30% of individuals with BDD experience panic attacks that are triggered by BDD symptoms (e.g., when looking at perceived defects in the mirror; when feeling that others are scrutinizing their appearance “flaws”; or when they are in a place with bright lights, which they believe make their perceived flaws more visible) (18).
Although not designated by a specifier in DSM-5, BDD by proxy is a form of BDD in which an individual has distressing or impairing preoccupations with perceived defects in another person’s appearance (11). For example, a parent may be preoccupied with her toddler’s “pushed in” nose and not let the child leave the house or attend family gatherings because it would be too embarrassing for others to see his nose.
Key Associated Features
Many patients feel embarrassed and ashamed by their supposed physical deformities (11). BDD is also associated with high levels of rejection sensitivity, social anxiety and avoidance, anxiety, depressed mood, neuroticism, perceived stress, and hostility as well as low levels of self-esteem, extraversion, and assertiveness. Other negative emotions include guilt (e.g., after cosmetic surgery with which the patient is dissatisfied), disgust, and grief (e.g., over years lost to BDD symptoms) (19).
A majority of those with BDD experience BDD-related ideas or delusions of reference, inaccurately believing that others take special notice of them in a negative way because of their “defects” (e.g., stare at, talk about, or mock them) (8, 15). These experiences can exacerbate social avoidance and sometimes trigger anger and hostility toward others.
Social anxiety and avoidance are common symptoms of BDD (19). Social avoidance is typically due to embarrassment, shame, and anxiety about the perceived appearance flaws being seen by other people. Our clinical impression is that mask wearing during the COVID-19 pandemic can make it easier for some people with BDD to be around others, although a goal of treatment is to stop relying on camouflaging strategies (however, patients should be encouraged to follow CDC recommendations regarding mask wearing). In a recent prospective longitudinal observational study of BDD, over 3 years of follow-up, higher global social avoidance (due to BDD or any other source) predicted poorer overall functioning in several domains of global functioning (20).
Epidemiology
In nationwide epidemiologic studies, BDD is somewhat more common than OCD, with a point (current) prevalence of 1.7%–2.9% (2). Two of these studies were published subsequent to publication of the prior version of this article (14), and one of these more recent studies used DSM-5 diagnostic criteria. In these studies, BDD was slightly more common in women than men; in addition, compared with those without BDD, individuals with BDD had less education, lower income, and more unemployment and sick days. They were also more far likely to report suicidal ideation and suicide attempts because of appearance concerns (2).
BDD is even more common in clinical settings, with a prevalence of 13%–16% among general psychiatric adult inpatients in the United States (2). An inpatient study found that BDD was more common than OCD, schizophrenia, posttraumatic stress disorder (PTSD), eating disorders, and several other psychiatric disorders; patients with BDD had significantly lower scores on the Global Assessment of Functioning scale and twice the rate of suicide attempts as those without BDD (21). Among adolescent psychiatric inpatients, 7%–14% had current BDD (21, 22). In a 2016 systematic review and estimated weighted prevalence study, BDD’s prevalence was 13.2% in general cosmetic surgery settings, 20.1% in rhinoplasty surgery settings, 11.2% in orthognathic surgery settings, 5.2% in orthodontics and cosmetic dentistry settings, and 11.3% in dermatology outpatient settings (23).
Since the earlier version of this article was published in 2015 (14), several studies have examined BDD’s prevalence and clinical correlates in veteran and military samples. BDD, especially the muscle dysmorphia form, appears very common in these individuals. In a U.S. Department of Veterans Affairs primary care behavioral health clinic, 11% of veterans had current BDD; 25% of these veterans indicated that they had muscle dysmorphia concerns (24). In addition, a study of U.S. military personnel showed that 13% of men and 22% of women had BDD, with 13% of men and 4% of women reporting symptoms of muscle dysmorphia (25).
Age at Onset and Course of Illness
As stated in our 2015 article (14), two-thirds of people with BDD experience BDD onset before age 18. The most common age at onset is 12–13 (26), which suggests that physical changes and emotional, social, and developmental challenges associated with puberty may play a role in BDD’s onset for some individuals. However, BDD can onset as early as age 4 and as late as the 40s (26). Those with onset during childhood or adolescence (younger than age 18) are more likely to have attempted suicide and been psychiatrically hospitalized, and they also have more comorbidity (26).
In a prospective longitudinal observational study of BDD’s course of illness, BDD tended to be chronic (27). In the course of follow-up, which ranged up to 4 years, the cumulative probability of full remission (symptom free for at least 8 consecutive weeks) was only 0.20. The probability of full or partial remission (not meeting full diagnostic criteria for BDD for at least 8 consecutive weeks) was only 0.55. A lower likelihood of remission was predicted by being an adult, greater BDD severity at intake into the study, and longer lifetime duration of BDD.
Participants who partially or fully remitted during the follow-up period had a fairly high cumulative probability of subsequent full relapse of 0.42. The probability of subsequent full or partial relapse was 0.63, which was predicted by more severe BDD at study intake and earlier age at BDD onset. Most study participants received treatment in the community, but few received treatment that is considered adequate for BDD (27).
BDD Among Youths
BDD is particularly concerning among youths. As stated in our 2015 article (14), an inpatient study found that youths with BDD, compared with youths without significant body image concerns, had more severe anxiety and depression and significantly higher scores on a standardized measure of suicide risk (22). Moreover, in a recent large study in two independent twin samples (N=6,027 and N=3,454), BDD was associated with a substantial risk of suicidal ideation and behaviors in late adolescence and early adulthood (28).
As stated in our 2015 article (14), most clinical features of BDD appear similar among youths and adults. However, youths have poorer insight regarding their perceived appearance defects and are more likely than adults to have attempted suicide (44% vs. 24%) (29). At a trend level, youths have more severe BDD than adults and are more likely to have been psychiatrically hospitalized (43% vs. 24%) (29).
A substantial proportion of youths with BDD refuse to attend school because they feel too ugly to be seen, and 18%–22% drop out of school primarily because of BDD symptoms (29, 30). Because BDD often persists unless appropriately treated, it is important to identify and treat youths with BDD, especially those who attempt suicide or refuse to attend school. Untreated BDD among youths often impedes accomplishment of developmental tasks and transitions, such as completing school, dating, and developing social competence. These deficits not uncommonly persist well into adulthood and may even be lifelong.
Impairment in Psychosocial Functioning
BDD is associated with markedly impaired psychosocial functioning and very poor mental and general health–related quality of life, as noted in our 2015 article (14, 31). Scores on measures such as the 36-item Short Form Survey are typically several standard deviations (SDs) poorer than community norms and 0.4–0.7 SDs poorer than norms for depression (31). On the Social Adjustment Scale–Self-Report, scores for BDD are significantly worse than community norms (Cohen’s d ranging from 0.82 to 2.07) (31).
The previously noted prospective observational study of BDD found that psychosocial functioning remained consistently poor over a follow-up period of 1–3 years (mean±SD=2.7±0.9 years). The cumulative probability of attaining functional remission on the Global Assessment of Functioning scale (score>70 for at least 2 consecutive months) during the follow-up period was only 5.7% (32). On the Social and Occupational Functioning and Assessment Scale, the cumulative probability of attaining functional remission (score >70 for at least 2 consecutive months) was only 10.6%. More severe BDD symptoms predicted poorer functioning and quality of life.
Individuals with more severe impairment due to BDD often are completely socially isolated, quit their job or drop out of school, and may be housebound (sometimes for many years) to avoid being seen (8, 11). Nearly 40% of individuals with BDD have been psychiatrically hospitalized, and more than one-quarter of those with BDD attribute at least one hospitalization primarily to BDD (9).
Suicidality
As noted in our 2015 article (14), rates of suicidality are very high in BDD and higher than in OCD (4, 7, 33–35). In outpatient samples, about 80% of individuals with BDD have experienced suicidal ideation, and 24%–28% have attempted suicide (36, 37). In a nationwide epidemiologic study in Germany, 31% of participants with BDD reported thoughts about committing suicide specifically because of appearance concerns, and 22% had actually attempted suicide because of appearance concerns (38).
Several studies indicate that suicidality is more common in BDD than in a range of other psychiatric disorders. For example, in a study of U.S. veterans in a primary care behavioral health clinic, 58% of those with BDD had a history of a suicide attempt compared with 19% of veterans without BDD (24). In a study of psychiatric inpatients, those with BDD had twice as many suicide attempts as those without BDD (21). A 2016 systematic review and meta-analysis of 17 studies that compared BDD with other groups (e.g., healthy control participants as well as participants with OCD, eating disorders, or any anxiety disorder) reported that those with BDD were nearly four times more likely to have experienced suicidal ideation (pooled odds ratio [OR]=3.87) and 2.6 times more likely to have attempted suicide (pooled OR=2.57) (34). Furthermore, a 2019 study in a partial hospital setting (N=498) found that, after adjusting for age, gender, and other psychiatric disorders, BDD had a significant association with suicidal ideation (OR=6.62) and suicidal behaviors (OR=2.45) (35). These ORs were higher than for any other psychiatric disorders examined, including major depressive disorder, bipolar depression, PTSD, and OCD (for OCD, OR=0.78 for suicidal ideation, and OR=0.92 for suicidal behavior).
Greater BDD severity is an independent predictor of suicidal ideation and suicide attempts (36). Both earlier and more recent studies have found that the relationship between BDD and elevated suicidality is independent of comorbidity; however, certain comorbid conditions may further strengthen this relationship, such as comorbid major depressive disorder, PTSD, and a substance use disorder (33, 35, 36). Conversely, the presence of comorbid BDD may increase risk of suicidality among patients with other disorders. For example, in a study of inpatients with anorexia nervosa, those with comorbid BDD had triple the number of suicide attempts as those without BDD (39). Moreover, patients with comorbid BDD and OCD are more likely to attempt suicide than those with OCD or BDD (40).
Completed suicide in BDD has been only minimally studied, but the rate appears markedly elevated; it may be even higher than in bipolar disorder and major depressive disorder (41). In a retrospective 20-year study, most patients in two dermatology practices who committed suicide had acne or BDD (42).
Patients with BDD may feel suicidal because they feel hopeless about being “deformed,” feel that they cannot improve how they look, believe that other people reject them because they are “ugly,” feel socially isolated and mocked by others because of how they look (referential thinking), and believe they are unlovable and worthless because of their appearance. In addition, many have comorbid major depressive disorder and other risk factors for suicidality (8, 33). Suicide attempts among patients with BDD often have high potential lethality and intent; thus, they must be taken seriously (36).
Gender-Related Aspects of BDD
As stated in our 2015 article (14), BDD’s clinical features (e.g., demographic characteristics, body areas of concern, comorbidity, suicidality) have more similarities than differences in women and men (9, 43, 44). However, women appear more likely to be preoccupied with weight (being overweight), breasts, hips, legs, and “excessive” body hair. They are more likely to check mirrors, pick their skin, camouflage their bodies to hide disliked areas, and have a comorbid eating disorder. Men are more likely to be single; be preoccupied with insufficiently muscular body build (muscle dysmorphia), thinning hair, and genitals (often penis size); and have a comorbid substance use disorder. Men also appear somewhat more impaired in terms of psychosocial functioning (e.g., to be unemployed and receiving disability payments).
Major Depressive Disorder, Substance Use Disorders, and Other Comorbid Conditions
As noted in our 2015 article (14), in the largest samples of participants assessed with a diagnostic interview, about three-quarters of individuals with BDD have past or current major depressive disorder, the most common comorbid disorder (8, 45). BDD usually begins before major depressive disorder, and many patients attribute depressive symptoms as being caused by BDD (45). Nearly 40% of those with BDD have past or current social anxiety disorder, and about one-third have past or current OCD (8, 45).
A past or current substance use disorder occurs in 30%–50% of individuals with BDD, nearly 70% of whom attribute their substance use problem at least in part to the distress caused by BDD (8, 45, 46). We more recently published several articles that are consistent with this finding. In one of these studies, which examined motives for drinking alcohol, scores for drinking to cope with negative affect were 1.6 SD units higher in a BDD sample than published community scores; BDD scores were less elevated relative to community scores for enhancement of positive affect (1.1 SD units higher) and social motives (0.5 SD units) (47). In this study and our 2019 study of motives for drug use, coping motives were strongly associated with using substances because body image concerns were upsetting; moreover, they were also positively associated with attempted suicide (47, 48).
Substance use disorders appear to be even more common than the 30%–50% noted previously among men with the muscle dysmorphia form of BDD, and use of anabolic androgenic steroids to build muscle is a particular risk (13). Use of anabolic androgenic steroids may develop into an addiction and may have serious adverse physical and psychiatric effects, such as depressive symptoms when use is discontinued and aggressive behavior (“roid rage”) (49). These individuals may also use an array of prescription medications (usually bought on the Internet without a prescription) and dietary supplements, some of which contain anabolic androgenic steroids, to lose body fat or build muscle. A study of entry-level military personnel that was done subsequent to the publication of our 2015 article (14) found that individuals with muscle dysmorphia were 5.4 times more likely than those without muscle dysmorphia to use body-building supplements (25). These supplements can be dangerous and put individuals at risk for serious physical problems.
A Patient With BDD
Aaron, a 26-year-old White man who was single and unemployed and who lived with his parents, was brought by his parents for a psychiatric diagnostic evaluation. His parents believed that he had BDD; however, Aaron did not believe this assessment, and he was reluctant to come to the evaluation. He believed that a diagnosis of BDD did not apply to him because he truly was ugly. Aaron believed that his skull was too narrow (although measurements confirmed that it was within the normal range); he also believed that his nose was misshapen and too large. Aaron had consulted 12 surgeons across the country, requesting a cranioplasty to widen his skull. One surgeon agreed to perform the surgery, which Aaron had scheduled. He also planned to have a rhinoplasty in the near future. Although these body areas looked normal, Aaron was convinced that he looked “deformed.” He obsessed about these perceived defects for 8–10 hours a day and spent 6–8 hours a day checking the disliked areas in mirrors and comparing his appearance with others (often with celebrities online). He often asked his parents to confirm that he looked ugly and searched online for information about cosmetic surgery. He was unable to work and avoided virtually all social situations and relationships because he felt too ugly to be seen. He often expressed passive suicidal ideation because “life isn’t worth living if I look like a freak.”
Because Aaron’s symptoms were severe he was treated with both medication and cognitive-behavioral therapy (CBT) that was tailored to BDD and provided in an intensive outpatient setting. He was not willing to agree to forgo the scheduled cranioplasty, but he did agree to postpone it until after he received adequate treatment with evidence-based treatments for BDD. Aaron was treated with fluoxetine, reaching a dose of 80 mg/day after 6 weeks; this dose was well tolerated, and a lower dose had not improved any symptoms. After 12 weeks of fluoxetine treatment, the dose was gradually increased to 120 mg/day, which was well tolerated. During this time, Aaron also received intensive CBT for BDD 3 days per week. After 6 months on this treatment regimen, his BDD symptoms were in near remission, he was no longer depressed or suicidal, he was starting to look for a job, and he no longer had any desire to pursue cosmetic surgery.
Emerging Clues About BDD’s Etiology and Pathophysiology
Studies of BDD’s etiology and pathophysiology are more recent than many studies of its clinical features. Elucidation of BDD symptoms and common associated features, as well as the development of reliable screening and diagnostic measures, were needed before studies of BDD’s etiology and pathophysiology could be done. Some findings in this section were included in our 2015 article (14), whereas others are more recent.
Genetic Factors
Several genetics studies have been published since our 2015 article (14), which further establish the importance of genetic factors in the etiology of BDD. Large twin studies indicate that heritability of BDD (“dysmorphic concern,” which is similar to BDD) among adults is moderate (43%–44%), with the remaining contribution attributable to environmental factors (50). In three population-based twin cohorts at ages 15 (N=6,968), 18 (N=3,738), and 20–28 (N=4,671), heritability of body dysmorphic concerns was estimated at 49% at age 15, 39% at age 18, and 37% at ages 20–28 (the remaining variance was due to nonshared environment) (51).
Regarding BDD’s relationship to OCD, as stated in our 2015 article (14), family studies indicate that BDD is more common in first-degree relatives of OCD probands than control probands, suggesting shared etiology (genetic or environmental) with OCD (52, 53). In a study of 5,409 female twins that used multivariate twin modeling methods, one latent factor loaded on all obsessive-compulsive and related disorders, particularly on OCD, BDD, and hoarding disorder (54). However, disorder-specific genetic factors were also evident for OCD, BDD, and hoarding disorder. Nonshared environmental risk factors were evident among these disorders as well. These findings are consistent with other research data indicating that BDD and OCD are related disorders with some shared features but are also distinct from each other. Interested readers may wish to read the article in this issue on genetics of OCD (55).
Neurobiological Factors
Visual-processing studies (e.g., using functional magnetic resonance imaging) suggest that individuals with BDD actually see things differently than those without BDD; they exhibit a bias for encoding and analyzing details of faces and nonface objects such as houses (16, 17, 50). Holistic visual processing, which emphasizes a global and more integrated view of objects, appears disrupted; thus, details of the face and body override the “big picture,” gestalt view of the whole. Small eye-tracking studies similarly suggest a hyper-focus on specific features or details instead of the bigger picture (50).
Available data also indicate abnormalities in executive functioning, emotion recognition, attention, and neurocognition (56). In addition, patients with BDD, compared with healthy control groups, show relative hyperactivity in left orbitofrontal cortex and bilateral head of the caudate when viewing their own face versus a familiar face, which may reflect obsessional preoccupation while viewing their own face (50). Although this study did not directly compare BDD with OCD, this activation pattern is characteristic of OCD.
Diffusion tensor imaging studies of brain structure and white matter integrity have resulted in varied findings (50). One study found widespread compromised white matter (reduced organization), whereas another study did not, although statistical power was limited. The latter study, however, found significant correlations between poorer BDD-related insight and fiber disorganization in white matter tracts that connect visual with emotion and memory processing systems.
Information-Processing Biases
Individuals with BDD have a bias toward interpreting neutral faces as contemptuous and angry; they also tend to misinterpret ambiguous scenarios as threatening (56). These findings are consistent with the frequent occurrence of ideas and delusions of reference in BDD.
Psychological and Social-Environmental Factors
Twin studies have found that BDD has unique disorder-specific environmental risk factors that are not shared by other obsessive-compulsive and related disorders (51, 54). A history of teasing is one possible risk factor (57). Studies also suggest high rates of childhood neglect or abuse (57). A study that compared BDD with OCD found that a history of emotional, physical, and sexual abuse was reported by a higher proportion of patients with BDD than patients with OCD (58). It is likely that sociocultural influences regarding the importance of appearance also play a role (57).
Evolutionary Perspective
As we noted in our 2015 article (14), an evolutionary perspective may also be relevant to BDD (e.g., a desire to attract mates or avoid social rejection) (59). For example, in animals, greater symmetry of body parts or the absence of facial defects (such as skin lesions) may signal reproductive health and fitness or absence of disease. Another example is that compulsive grooming in BDD has notable similarities to compulsive grooming behaviors in animals, such as acral lick syndrome in dogs and compulsive feather plucking in birds.
BDD’s Relationship to OCD and Other Disorders: Similarities and Differences
BDD has received far less investigation than OCD; nonetheless, data are emerging on their similarities and differences, and numerous studies have directly compared them across various domains (4). Replication studies and additional direct comparison studies are needed. This section and Table 2 briefly summarize key findings.
| Similarities | Differences |
|---|---|
| Unwanted, distressing obsessions and preoccupations | Different focus of obsessions, core beliefs, and compulsions (appearance focused in BDD) |
| Distressing repetitive behaviors (rituals, compulsions) that aim to reduce anxiety or distress | Some BDD repetitive behaviors (such as mirror checking) appear less likely to reduce anxiety and may increase anxiety and distress |
| Content of some obsessions and rituals, such as symmetry concerns, checking, and reassurance seeking | Poorer insight as well as more ideas and delusions of reference in BDD (and more paranoid personality disorder) |
| Similar BDD-YBOCS and Y-BOCS scores for individual scale items | More frequent comorbid major depressive disorder and substance use disorders in BDD |
| Most demographic features | More frequent suicidality in BDD |
| Often severe functional impairment and poor quality of life | Functional impairment may be more severe in BDD, particularly in education, employment, and other domains |
| High levels of perfectionism, high neuroticism, low extraversion | Possibly more childhood emotional and sexual abuse in BDD |
| Course of illness (often chronic) if not appropriately treated | Differences in translational studies (e.g., greater anger recognition bias and greater frequency of threatening interpretations of ambiguous social and appearance-related information in BDD) |
| Familiality | Poorer facial affect perception in BDD |
| Overlapping genetic vulnerability | Disorder-specific genetic effects and environmental influences |
| Abnormalities in frontostriatal systems, including hyperactivity on fMRI in orbitofrontal cortex and head of the caudate | More intensive strategies (e.g., motivational interviewing) may be needed to engage and retain patients with BDD in treatment |
| CBT as first-line psychosocial treatment (cognitive restructuring, ritual prevention, and exposure) | Differences in CBT: in BDD, more complex and often longer treatment, with greater focus on cognitive techniques and behavioral experiments; inclusion of perceptual retraining; if needed, use of habit reversal training for skin picking, hair plucking and pulling, and use of strategies to address desire for or use of cosmetic treatment; greater need to address depressive symptoms |
| SRIs as first-line pharmacotherapy (high doses often needed) |
TABLE 2. Some similarities and differences between BDD and OCDa
BDD is widely considered one of the disorders that is most closely related to OCD on the basis of similarities in a variety of domains (4). Examples are similar demographic and phenomenological features; in addition, similarities include often-chronic course, familiality, genetic overlap (see Genetic Factors section), and other domains (Table 2). An important similarity is the frequent need for similarly high doses of serotonin reuptake inhibitors (SRIs) as a first-line treatment (discussed more later).
However, direct comparison studies suggest that BDD and OCD also have some notable differences and are not identical disorders (4) (Table 2). Some clinically important differences include the following:
In BDD, insight is usually poorer, and ideas and delusions of reference are more common (as discussed previously; Figures 1 and 2) (15, 60).
People with BDD appear more likely to have lifetime suicidal ideation and to attempt suicide than people with OCD (7, 33–35). A large recent study in a partial hospital setting (35) as well as a 2016 meta-analysis of suicidality in BDD (34) were done subsequent to the publication of our 2015 article (14) and confirmed earlier findings.
Higher rates of comorbid major depressive disorder and substance use disorder have been found among people with BDD (40).
Compared with patients with OCD, those with BDD have significantly poorer facial affect perception and an anger recognition bias (61). Those with BDD are also more likely to interpret appearance-related and social scenarios as threatening (56).
Although CBT for BDD and OCD both include exposure and response (ritual) prevention, as noted in our 2015 article (14), CBT for BDD is more complex and often lengthier than CBT for OCD. It usually requires a greater focus on motivational interviewing, cognitive approaches, and incorporation of behavioral experiments into exposure exercises. CBT for BDD also includes elements that are not relevant to CBT for OCD, such as perceptual retraining that targets visual-processing abnormalities, habit reversal training for BDD-related skin picking and hair plucking and pulling (if present), and interventions for seeking of cosmetic treatment (Table 1) (62).
Although BDD and OCD are both associated with substantial functional impairment, individuals with BDD may have more impairment in some domains (3).
In a prospective longitudinal study, BDD symptoms persisted in a sizable proportion of participants who remitted from comorbid OCD (63). This finding suggests that BDD is not simply a symptom of OCD.
As noted in our 2015 article (14), BDD also has similarities with other disorders, such as social anxiety disorder, with which BDD shares prominent shame, fear of embarrassment and humiliation, rejection sensitivity, and social anxiety and avoidance (19). BDD shares distorted body image and appearance preoccupations with eating disorders, and there are some overlaps (as well as differences) in visual-processing abnormalities (64). BDD also has some features in common with depressive disorders (such as low self-esteem) and psychotic disorders (such as delusional beliefs). However, BDD differs in important ways from these other disorders (discussed more later). For example, a study of visual processing, published subsequent to our 2015 article (14), directly compared individuals with BDD with those with anorexia nervosa using functional magnetic resonance imaging and EEG. This study found that the two groups had some similarities in visual-processing abnormalities; however, more abnormalities were found among patients with BDD than patients with anorexia nervosa (64). Previous direct comparison studies found that compared with anorexia nervosa and bulimia nervosa, BDD is characterized by poorer insight, more negative self-evaluation and self-worth, poorer functioning and quality of life because of appearance concerns, and more avoidance of activities (64–67).
How to Assess Patients for BDD
As we discussed in our 2015 article (14), BDD is common but is usually undiagnosed in mental health settings (2). Patients typically do not spontaneously reveal their appearance concerns because they are too embarrassed, fear the clinician will negatively judge them (e.g., consider them vain) or not understand their concerns, or do not know that psychiatric treatment may be helpful for appearance concerns (68). Furthermore, because insight is usually absent or poor, many patients believe that the BDD diagnosis does not apply to them (Figures 1 and 2). To detect BDD, clinicians usually need to ask patients about BDD symptoms.
Keeping in mind that BDD is often comorbid with OCD can be helpful. Although the rate of BDD among patients with OCD varies across studies, taken together, studies suggest that about 15%–20% of individuals with OCD have comorbid BDD (69).
In most cases, diagnosing BDD is straightforward, especially when using questions such as those in Box 2 as a guide. The most complex differential is with eating disorders when patients present with concerns that they weigh too much or that parts of their body are too fat (see the Eating Disorders section). Additional differential diagnosis issues are discussed in the next section.
BOX 2. Diagnostic questions for body dysmorphic disorder
DSM-5 criterion A: Preoccupation with perceived defects or flaws in appearance that are not observable or appear only slight to others:
“Are you very worried about your appearance in any way?” or “Are you unhappy with how you look?”
“Can you tell me about your concern?” or “What don’t you like about how you look?”
“How much time would you estimate that you spend each day thinking about your appearance, if you add up all the time you spend?” or “Do these concerns preoccupy you?”
DSM-5 criterion B: Repetitive behaviors in response to appearance concerns:
“Is there anything you feel an urge to do over and over again in response to your appearance concerns?” Give examples of repetitive behaviors.
DSM-5 criterion C: Clinically significant distress or impairment in functioning:
“How much distress do these concerns cause you?” Ask about anxiety, social anxiety, depression, feelings of panic, shame, and suicidal thinking.
“Do these concerns interfere with your life or cause problems for you in any way?” Ask about effects on work, school, other aspects of role functioning (e.g., caring for children), relationships, intimacy, family and social activities, household tasks, and other types of interference.
DSM-5 criterion D: Concerns not better explained by an eating disorder:
Ask diagnostic questions for anorexia nervosa, bulimia nervosa, and binge eating disorder if body image concerns focus on weighing too much or an excessively large or fat body, stomach, thighs, arms, or other areas typically disliked in eating disorders.
Muscle dysmorphia specifier:
“Are you preoccupied with the idea that your body build is too small or that you’re not muscular enough?”
Insight specifier:
Elicit a global belief about all of the perceived defect(s): “What word would you use to describe how bad your [fill in disliked areas] look?” Optional: “Some people use words like unattractive, ugly, or deformed.” (The global belief must be inaccurate. Do not use beliefs that are true, such as “I don't look perfect,” or “I want to look better.”)
“How convinced are you that these body areas look [fill in patient’s global descriptor]?”
Several measures may be helpful for screening, diagnosis, and treatment. The Body Dysmorphic Disorder Questionnaire (which has a dermatology version) screens for BDD (69). Diagnostic measures (semistructured clinician- or rater-administered measures) include the BDD Diagnostic Module and Structured Clinical Interview for DSM-5 (69, 70) The Yale-Brown Obsessive-Compulsive Scale Modified for Body Dysmorphic Disorder is the most widely used measure of BDD severity (71, 72). This rater-administered measure should be used only with individuals diagnosed as having BDD. The Brown Assessment of Beliefs Scale is a widely used measure of insight in BDD; it can also be used to assess insight in OCD, olfactory reference disorder, hypochondriasis, eating disorders, and other disorders (73, 74). These measures have strong psychometric properties.
How to Differentiate BDD From Disorders With Which It Is Often Confused
As we discussed in our 2015 article (14), treatment of BDD differs from that of other disorders; thus, it is important to differentiate BDD from them.
OCD
Obsessions that focus on perceived defects in one’s physical appearance, including symmetry concerns, should be diagnosed as BDD, not OCD.
Excoriation (Skin-Picking) Disorder
When problematic skin picking is done in response to concerns about perceived skin blemishes or other skin flaws, and the intent of picking is to improve the skin’s appearance, BDD should be diagnosed rather than excoriation (skin-picking) disorder.
Trichotillomania (Hair-Pulling Disorder)
When problematic hair plucking or pulling is triggered by concerns that one’s hair is ugly or looks abnormal (e.g., “excessive” facial hair or “asymmetrical” eyebrows), and the plucking intends to improve one’s appearance, BDD should be diagnosed rather than trichotillomania (hair-pulling disorder).
Major Depressive Disorder
Depressive symptoms are common in BDD; they appear to often be secondary to the distress and impairment that BDD causes. BDD should be diagnosed in individuals with depression if diagnostic criteria for BDD are met.
Social Anxiety Disorder
Many patients with BDD fear being rejected and humiliated because of how they look; thus, social anxiety and social avoidance are very common in BDD (19). When these symptoms are attributable to concerns about one’s physical appearance, BDD should be diagnosed rather than social anxiety disorder.
Agoraphobia
Some people with BDD avoid public places or are housebound because they feel too ugly to be seen or fear that others will stare at them or mock them because of how they look. Such avoidance should be diagnosed as BDD, not agoraphobia.
Generalized Anxiety Disorder
Patients with BDD have excessive anxiety and worry about their appearance. Such anxiety and worry should not be attributed to generalized anxiety disorder.
Eating Disorders
As specified by DSM-5 criterion D, individuals who have preoccupations with excessive weight or body fat that qualify for an eating disorder diagnosis should be diagnosed as having an eating disorder rather than BDD. However, when eating disorder symptoms do not meet full diagnostic criteria for an eating disorder, it may be difficult to determine whether BDD or “other specified feeding and eating disorder” is the more accurate diagnosis; careful questioning and clinical judgment are needed to make the correct diagnosis. BDD and eating disorders can also be comorbid (e.g., when distressing or impairing preoccupation with perceived facial scarring coexists with excessive concern about being too fat in a person with anorexia nervosa).
Psychotic Disorders
When appearance beliefs among patients with BDD are delusional in nature, patients should be diagnosed as having BDD, absent-insight specifier. BDD may be characterized by appearance-related delusions of reference but not by other psychotic symptoms, disorganized speech or behavior, or negative symptoms.
Gender Dysphoria
BDD should not be diagnosed if diagnostic criteria for gender dysphoria are met and appearance preoccupations focus only on genitals, secondary sex characteristics, or other aspects of appearance that reflect one’s natal sex, and if other diagnostic criteria for gender dysphoria are met.
Olfactory Reference Disorder (Olfactory Reference Syndrome)
This disorder is characterized by distressing or impairing preoccupation with emitting a foul body odor when no odor is clearly present. DSM-5 classifies this condition as an “other specified obsessive-compulsive and related disorder.” The ICD-11 includes olfactory reference disorder as a separate disorder in the chapter on obsessive-compulsive or related disorders.
Clearly Noticeable Physical Defects
When defects (e.g., because of an accident or congenital anomaly) are clearly observable to others and cause distressing or impairing preoccupations, patients should be diagnosed as having “other specified obsessive-compulsive and related disorder.” However, if skin picking due to BDD concerns causes noticeable skin lesions or scarring, BDD should be diagnosed even if such flaws are clearly noticeable.
Normal Appearance Concerns
BDD is characterized by time-consuming and excessive appearance-related preoccupations and other previously noted symptoms. Unlike typical appearance concerns, BDD causes clinically significant distress or impairment in functioning (usually both).
Treatment of BDD
Treatment Challenges and How to Address Them
As discussed in our 2015 article, because poor or absent insight is so common in BDD, it can be difficult to engage and retain individuals with this disorder in psychiatric treatment. Before proceeding with implementation of medication or CBT, it is important to do the following:
Strive to build rapport, trust, and a strong therapeutic alliance by being nonjudgmental and expressing empathy for the patient’s suffering.
Provide psychoeducation about BDD.
It usually is not helpful to try to talk patients, especially those with delusional beliefs, out of their appearance concerns. Rather, the clinician can note that people with BDD have a distorted and negative view of how they look, which differs markedly from the view that others have of them. Clinicians can note that BDD is characterized by aberrations in brain functioning: their brain is “too good” at seeing detail and has trouble seeing “the big picture,” so details achieve too much prominence in what they see. It is also possible that staring at perceived defects in the mirror at close range for long periods of time may contribute to their distorted view.
For patients who are considering cosmetic treatment, discuss the likelihood that the outcome will be poor. Cosmetic treatment can make BDD symptoms worse and may trigger legal action or even violent behavior toward clinicians who provide such treatment (75–77).
Convey that psychiatric treatment is likely to be helpful, and encourage the patient to try it.
Address misconceptions about recommended treatment (e.g., that SRIs will be poorly tolerated or that CBT exposures will be too difficult).
When patients resist treatment, focus on their poor functioning and the potential for recommended treatments to alleviate their dysfunction and distress.
When patients are reluctant to try medication or CBT, try using motivational-interviewing techniques (62).
Assess and monitor suicidal ideation. Treat patients who are highly suicidal with both medication and therapy. For patients who are more highly suicidal, consider incorporating cognitive-behavioral approaches for suicidality into treatment (78).
Consider partial hospital or inpatient care for patients who are more severely ill or suicidal while keeping in mind that patients may resist such care because they feel so anxious being seen by other people.
Involve supportive family members if clinically appropriate. At the very least, it can be helpful for them to understand the diagnosis, recommended treatment, and the treatment rationale as well as to support the patient and recommended treatment.
Surgical, Dermatologic, Dental, and Other Cosmetic Treatment
Most individuals with BDD seek and receive cosmetic surgery (most often rhinoplasty, followed by breast augmentation), dermatologic treatment (such as topical acne agents and isotretinoin), dental treatment, and other types of cosmetic treatment for BDD concerns (75, 76).
Most patients with BDD are dissatisfied with cosmetic treatment and find that it does not improve BDD symptoms; indeed, symptoms may worsen (11, 75, 76). In a survey of cosmetic surgeons, 43% of respondents reported that following surgery, patients with BDD were even more preoccupied with the treated “defects,” and only 1% were free of their preoccupation (77). The concern switched to another body part in 39% of cases. Forty percent reported that a dissatisfied patient with BDD had threatened the surgeon legally or physically. Occasionally, dissatisfied patients murder the physician or commit suicide (5, 11, 69). A recent rhinoplasty practice guideline from the American Academy of Otolaryngology states that BDD symptoms and complaints may worsen following surgery and that BDD is a contraindication to elective rhinoplasty (79).
Pharmacotherapy
Recommendations regarding first-line pharmacotherapy for BDD (SRIs, often at high doses) are similar to those in our 2015 article (14). However, some recommendations for SRI augmentation strategies have changed since that article was published.
SRI efficacy.
No medications are approved for BDD by the U.S. Food and Drug Administration (FDA) because no pharmaceutical companies have sought this indication. However, SRIs, at adequately high doses, are the first-line somatic treatment for BDD (11, 80, 81). A placebo-controlled trial with fluoxetine; a blinded, crossover trial of the SRI clomipramine versus the non-SRI antidepressant desipramine; and five methodologically rigorous, open-label SRI trials (two with fluvoxamine, one with citalopram, and two with escitalopram; N=15–100) found that an adequately dosed SRI usually improves BDD-related preoccupations, repetitive behaviors, distress, and impairment in functioning as well as associated features such as depression, anxiety, anger-hostility, and quality of life (80–83). SRIs also decrease suicidal ideation and protect against suicidality worsening among patients with BDD (80, 84). No studies have directly compared the efficacy of different SRIs for BDD, but a prospective series from a clinical practice (N=90) found similar response rates for each type of SRI (85).
In the only relapse prevention study of BDD (86), responders to initial treatment with the SRI escitalopram were randomly assigned to continue treatment with escitalopram or switch to placebo for 6 months. In this 2016 study, we found that continuation-phase escitalopram delayed time to relapse, and fewer participants treated with escitalopram relapsed than did participants treated with placebo (86). In addition, more than one-third of patients who continued treatment with escitalopram for these additional 6 months following the acute treatment phase further improved (86).
SRIs appear more efficacious than non-SRI antidepressants or other psychotropic medication, although data are limited (80, 83). SRI monotherapy is as efficacious for patients with delusional BDD beliefs as for those with nondelusional beliefs; thus, an SRI, rather than antipsychotic monotherapy, is recommended for patients with the absent insight/delusional beliefs specifier (80, 81).
SRI dosing.
As discussed in our 2015 article (14), a critical consideration is that that SRI doses often need to be in the range used for OCD and higher than those typically used for many other disorders, such as depression (11, 80). Patients often improve, or further improve, when the dose of an ineffective SRI is raised. Further improvement may also occur when the maximum SRI dose recommended by the manufacturer is exceeded (11, 80). However, 250 mg/day should not be exceeded for clomipramine, and the revised dosing limit for citalopram might be considered firmer than other selective serotonin reuptake inhibitor (SSRI) dosing limits. Because this dose is often too low for BDD, citalopram is no longer recommended in most cases.
Dose-finding studies have not been done in BDD. Mean±SD daily doses, and typical maximum doses, that the first author has used in her clinical practice are as follows: escitalopram, 29±12 mg in one sample and 45±15 mg in another sample (maximum dose=60 mg); fluoxetine, 67±24 mg (maximum dose=120 mg); fluvoxamine, 308±49 mg (maximum dose=450 mg); sertraline, 202±46 mg (maximum dose=400 mg); paroxetine, 55±13 mg (maximum dose=100 mg); clomipramine, 203±53 mg (maximum dose=250 mg); and citalopram, 66±36 mg (40 mg/day is the current dosing limit for patients younger than age 60) (11, 80). These maximum doses are consistent with those in the American Psychiatric Association’s practice guideline for OCD (87). Somewhat lower initial and maximum doses should be considered for youths and older adults; doses exceeding FDA maximum doses are not recommended for preteens.
An electrocardiogram is recommended at 40 mg/day and higher of escitalopram, can be considered at high doses of other SSRIs, and should be obtained when prescribing clomipramine.
SRI trial duration.
Patients should receive an SRI trial of 12–16 weeks to determine response. The FDA maximum dose (but 30 mg/day for escitalopram) should be reached and used for at least 4 of those weeks (11, 80) if a lower dose has not led to meaningful improvement and the SRI is well tolerated. The mean time for SRI response is 4–9 weeks (11, 80).
SRI augmentation and switching.
No studies have rigorously compared next-step options if the above approach yields less than full remission. The first author’s preferred approach is to gradually raise the SSRI dose above the FDA limit, if tolerated (excluding clomipramine and citalopram) (80). This approach often leads to further improvement.
Alternative options are to augment the SRI or switch to another SRI. Augmentation is generally preferred if the SRI is well tolerated and has led to at least partial improvement in symptoms. In the only controlled augmentation study in BDD, the typical neuroleptic pimozide was not more efficacious than placebo in augmenting fluoxetine (88) (subsequent to this study, this medication combination became contraindicated). However, clinical consensus indicates that second-generation antipsychotics can be helpful as SRI augmentation strategies, and this approach is especially appealing when symptoms such as depression, suicidality, agitation, or hostility are more severe (80, 81). Aripiprazole (2–15 mg/day) may be especially helpful and relatively well tolerated (80, 81). Among patients who are more severely ill and more highly suicidal, it may be helpful to add an atypical neuroleptic to an SRI before a full SRI trial has been completed (80).
Data from case series suggest that buspirone (mean dose of 50–60 mg/day) may be effective as an SRI augmenter (89). This option may be more appealing when anxiety is more severe and depression is milder. Although studies are lacking, glutamate modulators such as memantine and the nutraceutical N-acetylcysteine, which have a good side effect profile, have shown some promise as SRI augmenting agents for OCD; clinical experience suggests that they may also be helpful for BDD. Doses typically used by the first author are 1,200–1,800 mg twice per day for N-acetylcysteine and 10 mg twice per day for memantine. Both medications are started at lower doses and gradually titrated upward.
Adding clomipramine to an SSRI (or vice versa) is an option that the first author does not use as often as in the past, given the availability of the newer and often better tolerated augmentation alternatives already discussed. It is important to keep in mind that SSRIs, especially 2D6 inhibitors, can substantially and unpredictably increase clomipramine levels; this development can be problematic given clomipramine’s low therapeutic index and potential side effects, such as cardiac effects, that are characteristic of tricyclic antidepressants. Serial clomipramine levels and electrocardiograms should be obtained if this medication combination is used.
One study found that 43% of patients who did not respond to an initial adequate SRI trial did respond to at least one subsequent adequate SRI trial (85). This finding makes sense because SRIs differ from one another, including in terms of secondary binding properties that may be important to an individual patient’s response or possible side effects. In the first author’s clinical experience, use of newer augmentation agents can increase this response rate. If several SSRI trials have not been successful, clomipramine is a good alternative.
Non-SRI monotherapy.
Venlafaxine and levetiracetam improved BDD symptoms in small open-label trials; placebo-controlled trials are needed (90, 91). Clinical experience suggests that other serotonergic medications such as duloxetine (up to 120 mg/day) and vilazodone (up to 60 mg/day) can improve BDD symptoms (80). However, given the limited evidence base, serotonin-norepinephrine reuptake inhibitors (SNRIs) and other non-SRIs are not generally recommended as first-line treatments for BDD (11, 80).
Electroconvulsive therapy (ECT) studies in BDD are lacking. Case series data suggest that ECT is usually not effective, although ECT can be considered for patients who are highly suicidal or those with severe comorbid major depressive disorder (especially those who have been refractory to SRIs) (11, 80). No studies have reported on the efficacy of deep brain stimulation or transcranial magnetic stimulation for BDD. However, several case reports have reported improvement in BDD with various neurosurgical approaches; moreover, a successful outcome was reported in one case report using deep brain stimulation that targeted the ventral capsule and ventral striatum, a brain area that is often targeted by deep brain stimulation for OCD (80, 92, 93).
CBT
Recommendations regarding CBT are similar to those in our 2015 article (14). However, since that article was published, additional important studies on CBT for BDD have been published that have, for example, examined response to CBT versus supportive psychotherapy, examined remission rates with CBT, and elucidated predictors of response to CBT.
CBT efficacy studies.
CBT that is tailored to BDD’s unique clinical features is the best-studied psychotherapy for BDD and has been shown to be effective for most patients (11, 94). CBT that is specific for BDD, rather than CBT for other disorders, should be used. CBT for BDD has some similarities to CBT for OCD as well as important differences.
Initial studies found that CBT for BDD was more efficacious than a waitlist control (94). A subsequent study demonstrated greater improvement with CBT than with anxiety management (95), and more recent studies have shown that CBT is more consistently efficacious than supportive psychotherapy (96, 97). In a 2021 report, we found that full or partial remission rates from BDD were high following CBT for BDD and higher than following supportive psychotherapy (98). Gains with CBT are usually maintained at 1 month, 3 months, and 6 months posttreatment.
BDD can be challenging to treat with CBT, especially when patients are more severely ill and functionally impaired. It can be helpful to use a BDD-specific CBT treatment manual, which provides detailed guidance for the therapist. Two CBT treatment manuals with published evidence of their efficacy are available for adults (62, 99). No empirically based treatment manual is available for children and adolescents, although the previously noted Wilhelm, Phillips, and Steketee (62) manual for adults can be successfully adapted for use in adolescents, as Greenberg et al. have done (100).
Components of CBT for BDD.
The treatment developed by the first author and her colleagues has the following components (62):
Foundation for treatment: Because BDD can be challenging to treat and insight is typically poor, more extensive initial groundwork is often needed than when treating other disorders. The first three or four sessions are devoted to developing a good understanding of the patient’s symptoms, providing psychoeducation, and building an individualized cognitive-behavioral model of the patient’s symptoms to clarify the connection between their various BDD symptoms and the rationale for CBT techniques. Meaningful treatment goals are set. Motivational-interviewing techniques are often needed during initial sessions and later in treatment to enhance motivation for treatment.
Cognitive restructuring: Patients learn to identify and evaluate negative appearance–related thoughts and beliefs and to identify cognitive errors, such as fortune telling, mind reading, and all-or-nothing thinking. They learn to develop more accurate and helpful appearance-related beliefs.
Exposures: Exposure helps patients gradually face avoided situations, which are usually social situations. Behavioral experiments, in which patients design and carry out experiments to test the accuracy of their beliefs, are done during exposures.
Ritual (response) prevention: This training helps patients cut down on and eventually stop repetitive behaviors, such as mirror checking, excessive grooming, and comparing with others.
Perceptual retraining: This exercise includes mindfulness skills and helps patients to develop a more holistic, rather than a detail-oriented, view of their appearance. Patients look in the mirror and, from head to toe, describe each part of their body (not just disliked areas) with neutral (not negative) language. This exercise takes only 5–10 minutes per day. It does not involve staring at disliked areas.
Advanced cognitive strategies: These strategies address negative core beliefs; common core beliefs in BDD include a belief that one is worthless, unlovable, or will always be alone. Self-esteem and self-compassion are fostered.
Habit reversal training: This training is used for BDD-related skin picking or hair picking and plucking if present.
Depression treatment: This treatment focuses on activity scheduling and behavioral activation as well as cognitive restructuring; it can be used for inactive patients or those with more severe depression. This approach is recommended early in treatment for patients with too much free time, which can fill up with BDD obsessions and rituals.
Cosmetic treatment: Various cognitive and behavioral strategies are used to target desire for, seeking of, or current receipt of cosmetic treatment for BDD. For example, cognitive restructuring can address unrealistic beliefs about potential benefits of cosmetic treatment, and ritual prevention can help patients stop seeking information about cosmetic procedures online.
Body shape and weight concerns: This module addresses muscle dysmorphia and concerns with being overweight or fat, if present. As an example, for an exposure exercise, a patient could go out to lunch with friends, if previously avoided, while collecting evidence for her prediction that her friends will stare at her “fat” stomach at least 20 times during the meal.
Relapse prevention: At the end of treatment, patients prepare to terminate formal treatment and to continue to implement learned strategies.
Examples of approaches that are not recommended include staring in mirrors (which reinforces the ritual of mirror checking and may potentially worsen perceptual distortions), listening to audiotapes that say the patient is ugly, or creating obvious “flaws” such as painting bright red spots on one’s face before going out in public. These approaches can worsen BDD symptoms and are not consistent with the goal of helping patients develop a more accurate view of their appearance.
Session number and frequency.
Session number and frequency varies substantially in published reports, from 12 weekly hour-long sessions to 12 weeks of daily 90-minute sessions (94). The duration of CBT should be tailored to each patient. Often, at least 6 months of weekly hour-long treatment is needed. Patients who are more severely ill usually need more intensive or longer treatment. Completion of daily structured homework assignments is essential. After formal treatment ends, patients should continue to practice CBT skills on their own; booster sessions with the therapist can be done as needed. Patients who have not met developmental milestones or who have been unemployed or socially isolated because of BDD may need vocational or social skills training after CBT.
Predictors of therapy response.
Our recent secondary analysis of the only study (97) of therapist-delivered CBT versus supportive psychotherapy for BDD (N=120) found that greater treatment credibility predicted better outcomes, which suggests that fostering credibility during therapy may maximize gains. Improvement was not impeded by more severe BDD, more severe depressive symptoms, or poorer BDD-related insight (101).
Combined Pharmacotherapy and CBT
The efficacy of combined treatment versus monotherapy has not been studied; however, combined treatment is especially recommended for patients who are more severely ill (80). For those who are too ill or depressed to participate in CBT, initial improvement with medication may make CBT more feasible (80).
Family Therapy
Family sessions or family therapy that provides education, support, and cognitive-behavioral strategies for family members to use can be a helpful adjunctive approach (102).
Approaches for Treatment-Refractory BDD
Many, if not most, patients who appear treatment refractory have not received adequate treatment. Common problems include inadequate SRI doses, too brief an SRI trial, use of non-SRIs as monotherapy, or poor medication adherence. Most patients have not received CBT from a CBT-trained therapist using an evidence-based treatment manual with good homework compliance. Patients who are treatment refractory should receive both medication and CBT; in addition, intensive outpatient, partial hospital, or residential treatment that focuses on BDD can also be considered.
Conclusions and Future Directions
Because BDD remains an understudied disorder, virtually all aspects of BDD need further study. Treatment research is especially needed, including studies of children and adolescents as well as studies of non-CBT psychotherapies, treatment augmentation strategies, non-SRI medications, and neuromodulation. Studies that more precisely tailor treatment to an individual’s specific symptoms are also needed. Some aspects of BDD, such as suicidality and hostility or aggression, are clinically important and particularly understudied. It is hoped that research on BDD’s pathophysiology will continue to advance and lead to improved understanding and treatment of BDD. Further investigation of the relationship between BDD and OCD, as well as other disorders, is also needed. The need for this work is pressing given the substantial morbidity and mortality associated with BDD.
1 Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA,
2 :
3 : A comparison of quality of life and psychosocial functioning in obsessive-compulsive disorder and body dysmorphic disorder. Ann Clin Psychiatry 2007; 19:181–186Crossref, Google Scholar
4 :
5 : Body dysmorphic disorder: the distress of imagined ugliness. Am J Psychiatry 1991; 148:1138–1149Crossref, Google Scholar
6 : Body dysmorphic disorder: 30 cases of imagined ugliness. Am J Psychiatry 1993; 150:302–308Crossref, Google Scholar
7 : A comparison study of body dysmorphic disorder and obsessive-compulsive disorder. J Clin Psychiatry 1998; 59:568–575Crossref, Google Scholar
8 : Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosomatics 2005; 46:317–325Crossref, Google Scholar
9 : Gender differences in body dysmorphic disorder. J Nerv Ment Dis 1997; 185:570–577Crossref, Google Scholar
10 : Symmetry concerns as a symptom of body dysmorphic disorder. J Obsessive Compuls Relat Disord 2013; 2:292–298Crossref, Google Scholar
11 : Understanding Body Dysmorphic Disorder: An Essential Guide. New York, Oxford University Press, 2009Google Scholar
12 : Mirror, mirror on the wall, who is the ugliest of them all? The psychopathology of mirror gazing in body dysmorphic disorder. Behav Res Ther 2001; 39:1381–1393Crossref, Google Scholar
13 : Clinical features of muscle dysmorphia among males with body dysmorphic disorder. Body Image 2005; 2:395–400Crossref, Google Scholar
14 : Body dysmorphic disorder: clinical aspects and relationship to OCD. Focus 2015; 13:162–174Link, Google Scholar
15 : A comparison of insight in body dysmorphic disorder and obsessive-compulsive disorder. J Psychiatr Res 2012; 46:1293–1299Crossref, Google Scholar
16 : Visual information processing of faces in body dysmorphic disorder. Arch Gen Psychiatry 2007; 64:1417–1425Crossref, Google Scholar
17 : Abnormalities of object visual processing in body dysmorphic disorder. Psychol Med 2011; 41:2385–2397Crossref, Google Scholar
18 : Cued panic attacks in body dysmorphic disorder. J Psychiatr Pract 2013; 19:194–203Crossref, Google Scholar
19 :
20 : Social avoidance as a predictor of psychosocial functioning in body dysmorphic disorder: a prospective longitudinal analysis. Cognit Ther Res 2020; 44:557–566Crossref, Google Scholar
21 : Prevalence and clinical features of body dysmorphic disorder in adolescent and adult psychiatric inpatients. J Clin Psychiatry 2001; 62:517–522Crossref, Google Scholar
22 : Body dysmorphic disorder and other clinically significant body image concerns in adolescent psychiatric inpatients: prevalence and clinical characteristics. Child Psychiatry Hum Dev 2006; 36:369–382Crossref, Google Scholar
23 : Body dysmorphic disorder in different settings: a systematic review and estimated weighted prevalence. Body Image 2016; 18:168–186Crossref, Google Scholar
24 : The prevalence of body dysmorphic disorder and its clinical correlates in a VA primary care behavioral health clinic. Psychiatry Res 2015; 228:162–165Crossref, Google Scholar
25 : Prevalence of body dysmorphic disorder and muscle dysmorphia among entry-level military personnel. Mil Med 2016; 181:494–501Crossref, Google Scholar
26 : Age at onset and clinical correlates in body dysmorphic disorder. Compr Psychiatry 2013; 54:893–903Crossref, Google Scholar
27 : A 4-year prospective observational follow-up study of course and predictors of course in body dysmorphic disorder. Psychol Med 2013; 43:1109–1117Crossref, Google Scholar
28 : The association between body dysmorphic symptoms and suicidality among adolescents and young adults: a genetically informative study. Psychol Med (Epub ahead of print, Sep 17, 2020) Google Scholar
29 : Clinical features of body dysmorphic disorder in adolescents and adults. Psychiatry Res 2006; 141:305–314Crossref, Google Scholar
30 : Thirty-three cases of body dysmorphic disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999; 38:453–459Crossref, Google Scholar
31 :
32 : Functional impairment in body dysmorphic disorder: a prospective, follow-up study. J Psychiatr Res 2008; 42:701–707Crossref, Google Scholar
33 : Suicidality and aggressive behavior in body dysmorphic disorder; in Body Dysmorphic Disorder: Advances in Research and Clinical Practice. Edited by Phillips KA. New York, Oxford University Press, 2017Google Scholar
34 : Suicidality in body dysmorphic disorder (BDD): a systematic review with meta-analysis. Clin Psychol Rev 2016; 49:55–66Crossref, Google Scholar
35 : Body dysmorphic disorder and major depressive episode have comorbidity-independent associations with suicidality in an acute psychiatric setting. J Affect Disord 2019; 259:266–270Crossref, Google Scholar
36 : Suicidal ideation and suicide attempts in body dysmorphic disorder. J Clin Psychiatry 2005 2005; 66:717–725Crossref, Google Scholar
37 : Body dysmorphic disorder. A survey of fifty cases. Br J Psychiatry 1996; 169:196–201Crossref, Google Scholar
38 : Updates on the prevalence of body dysmorphic disorder: a population-based survey. Psychiatry Res 2010; 178:171–175Crossref, Google Scholar
39 : Body dysmorphic disorder in patients with anorexia nervosa: prevalence, clinical features, and delusionality of body image. Int J Eat Disord 2002; 32:291–300Crossref, Google Scholar
40 : Obsessive-compulsive disorder versus body dysmorphic disorder: a comparison study of two possibly related disorders. Depress Anxiety 2007; 24:399–409Crossref, Google Scholar
41 : Suicidality in body dysmorphic disorder: a prospective study. Am J Psychiatry 2006; 163:1280–1282Crossref, Google Scholar
42 : Suicide in dermatological patients. Br J Dermatol 1997; 137:246–250Crossref, Google Scholar
43 : Gender similarities and differences in 200 individuals with body dysmorphic disorder. Compr Psychiatry 2006; 47:77–87Crossref, Google Scholar
44 : Gender-related differences in body dysmorphic disorder (dysmorphophobia). J Nerv Ment Dis 1997; 185:578–582Crossref, Google Scholar
45 : Axis I comorbidity in body dysmorphic disorder. Compr Psychiatry 2003; 44:270–276Crossref, Google Scholar
46 : Substance use disorders in individuals with body dysmorphic disorder. J Clin Psychiatry 2005; 66:309–316, quiz 404–405Crossref, Google Scholar
47 : Motives to drink alcohol among individuals with body dysmorphic disorder. J Obsessive Compuls Relat Disord 2017; 12:52–57Crossref, Google Scholar
48 : Motives for illicit drug use among individuals with body dysmorphic disorder. J Psychiatr Pract 2019; 25:427–436Crossref, Google Scholar
49 : Anabolic-androgenic steroid use and body image in men: a growing concern for clinicians. Psychother Psychosom 2020; 89:65–73Crossref, Google Scholar
50 :
51 : Prevalence and heritability of body dysmorphic symptoms in adolescents and young adults: a population-based nationwide twin study. Psychol Med 2018; 48:2740–2747Crossref, Google Scholar
52 : Is obsessive-compulsive disorder an anxiety disorder, and what, if any, are spectrum conditions? A family study perspective. Psychol Med 2012; 42:1–13Crossref, Google Scholar
53 : The relationship of obsessive-compulsive disorder to possible spectrum disorders: results from a family study. Biol Psychiatry 2000; 48:287–293Crossref, Google Scholar
54 : The structure of genetic and environmental risk factors for dimensional representations of DSM-5 obsessive-compulsive spectrum disorders. JAMA Psychiatry 2014; 71:182–189Crossref, Google Scholar
55 : What have we learned about the genetics of obsessive-compulsive and related disorders in recent years? Focus 2021; 19:384–391 [this issue]Link, Google Scholar
56 :
57 :
58 : Rates of abuse in body dysmorphic disorder and obsessive-compulsive disorder. Body Image 2006; 3:189–193Crossref, Google Scholar
59 :
60 : Insight in obsessive compulsive disorder and body dysmorphic disorder. Compr Psychiatry 2004; 45:10–15Crossref, Google Scholar
61 : Facial affect recognition in body dysmorphic disorder versus obsessive-compulsive disorder: an eye-tracking study. J Anxiety Disord 2015; 35:49–59Crossref, Google Scholar
62 : Cognitive-Behavioral Therapy for Body Dysmorphic Disorder: A Treatment Manual. New York, Guilford Press, 2013Google Scholar
63 : Associations in the longitudinal course of body dysmorphic disorder with major depression, obsessive-compulsive disorder, and social phobia. J Psychiatr Res 2006; 40:360–369Crossref, Google Scholar
64 : Anorexia nervosa and body dysmorphic disorder are associated with abnormalities in processing visual information. Psychol Med 2015; 45:2111–2122Crossref, Google Scholar
65 : Multidimensional body image comparisons among patients with eating disorders, body dysmorphic disorder, and clinical controls: a multisite study. Body Image 2009; 6:155–163Crossref, Google Scholar
66 : A comparison of eating disorders and body dysmorphic disorder on body image and psychological adjustment. J Psychosom Res 1998; 44:441–449Crossref, Google Scholar
67 : Body image, emotions and thought control strategies in body dysmorphic disorder compared to eating disorders and healthy controls. J Psychosom Res 2012; 72:321–327Crossref, Google Scholar
68 : Prevalence and clinical characteristics of body dysmorphic disorder in an adult inpatient setting. Gen Hosp Psychiatry 2008; 30:67–72Crossref, Google Scholar
69 : The Broken Mirror: Understanding and Treating Body Dysmorphic Disorder. New York, Oxford University Press, 2005Google Scholar
70 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York,
71 : A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull 1997; 33:17–22Google Scholar
72 : Psychometric evaluation of the Yale-Brown Obsessive-Compulsive Scale Modified for Body Dysmorphic Disorder (BDD-YBOCS). J Obsessive Compuls Relat Disord 2014; 3:205–208Crossref, Google Scholar
73 : The Brown Assessment of Beliefs Scale: reliability and validity. Am J Psychiatry 1998; 155:102–108Crossref, Google Scholar
74 : Psychometric evaluation of the Brown Assessment of Beliefs Scale in body dysmorphic disorder. J Nerv Ment Dis 2013; 201:640–643Crossref, Google Scholar
75 : Surgical and nonpsychiatric medical treatment of patients with body dysmorphic disorder. Psychosomatics 2001; 42:504–510Crossref, Google Scholar
76 : Nonpsychiatric medical treatment of body dysmorphic disorder. Psychosomatics 2005; 46:549–555Crossref, Google Scholar
77 : Awareness and identification of body dysmorphic disorder by aesthetic surgeons: results of a survey of American Society for Aesthetic Plastic Surgery members. Aesthet Surg J 2002; 22:531–535Crossref, Google Scholar
78 : Cognitive Therapy for Suicidal Patients: Scientific and Clinical Applications. Washington, DC,
79 : Clinical practice guideline: improving nasal form and function after rhinoplasty. Otolaryngol Head Neck Surg 2017; 156(Suppl 2):S1–S30Crossref, Google Scholar
80 :
81 : Body dysmorphic disorder: a treatment synthesis and consensus on behalf of the International College of Obsessive-Compulsive Spectrum Disorders and the Obsessive Compulsive and Related Disorders Network of the European College of Neuropsychopharmacology. Int Clin Psychopharmacol 2021; 36:61–75Crossref, Google Scholar
82 : A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry 2002; 59:381–388Crossref, Google Scholar
83 : Clomipramine vs desipramine crossover trial in body dysmorphic disorder: selective efficacy of a serotonin reuptake inhibitor in imagined ugliness. Arch Gen Psychiatry 1999; 56:1033–1039Crossref, Google Scholar
84 : Suicidality in a placebo-controlled fluoxetine study of body dysmorphic disorder. Int Clin Psychopharmacol 2009; 24:26–28Crossref, Google Scholar
85 : Effectiveness of pharmacotherapy for body dysmorphic disorder: a chart-review study. J Clin Psychiatry 2001; 62:721–727Crossref, Google Scholar
86 : Pharmacotherapy relapse prevention in body dysmorphic disorder: a double-blind, placebo-controlled trial. Am J Psychiatry 2016; 173:887–895Crossref, Google Scholar
87 : Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry 2007; 164(Suppl):5–53Google Scholar
88 : Placebo-controlled study of pimozide augmentation of fluoxetine in body dysmorphic disorder. Am J Psychiatry 2005; 162:377–379Crossref, Google Scholar
89 : An open study of buspirone augmentation of serotonin-reuptake inhibitors in body dysmorphic disorder. Psychopharmacol Bull 1996; 32:175–180Google Scholar
90 : A prospective pilot study of levetiracetam for body dysmorphic disorder. CNS Spectr 2009; 14:252–260Crossref, Google Scholar
91 : An open-label trial of venlafaxine in body dysmorphic disorder. CNS Spectr 2008; 13:138–144Crossref, Google Scholar
92 : Deep brain stimulation of the ventral capsule/ventral striatum reproducibly improves symptoms of body dysmorphic disorder. Brain Stimul 2016; 9:957–959Crossref, Google Scholar
93 : Harmonizing the neurobiology and treatment of obsessive-compulsive disorder. Am J Psychiatry 2021; 178:17–29Crossref, Google Scholar
94 :
95 : Efficacy of cognitive behaviour therapy versus anxiety management for body dysmorphic disorder: a randomised controlled trial. Psychother Psychosom 2014; 83:341–353Crossref, Google Scholar
96 : Therapist guided Internet based cognitive behavioural therapy for body dysmorphic disorder: single blind randomised controlled trial. BMJ 2016; 352:i241Crossref, Google Scholar
97 : Efficacy and posttreatment effects of therapist-delivered cognitive behavioral therapy vs supportive psychotherapy for adults with body dysmorphic disorder: a randomized clinical trial. JAMA Psychiatry 2019; 76:363–373Crossref, Google Scholar
98 Rates of remission, sustained remission, and recurrence in a randomized controlled trial of cognitive behavioral therapy versus supportive psychotherapy for body dysmorphic disorder. Depress Anxiety (Epub ahead of print, Mar 16, 2021)Google Scholar
99 : Body Dysmorphic Disorder: A Treatment Manual. West Sussex, England, Wiley-Blackwell, 2010Crossref, Google Scholar
100 : Cognitive-behavioral therapy for adolescent body dysmorphic disorder: a pilot study. Behav Ther 2016; 47:213–224Crossref, Google Scholar
101 : Predictors and moderators of symptom change during cognitive-behavioral therapy or supportive psychotherapy for body dysmorphic disorder. J Affect Disord 2021; 287:34–40Crossref, Google Scholar
102 :



