Psychopharmacology and Experimental Therapeutics for Bipolar Depression
Abstract
Bipolar disorder is a chronic illness that affects 2%–4% of U.S. adults during their lifetime. The course of bipolar disorder is commonly characterized by prolonged periods of depression interspersed with manic-hypomanic episodes. Management of depression among patients with bipolar disorder is challenging because of the limited number of medications currently approved by the Food and Drug Administration, the high proportion of patients who do not respond to these medications, and the metabolic and other side effects associated with long-term use of these medications. In addition to reviewing the clinical options available to patients with bipolar depression and their treatment providers, this article presents an evidence-based management approach and discusses the off-label uses of currently available treatments and experimental therapeutics under development.
Bipolar disorder affects 2–4% of U.S. adults during their lifetime (1, 2). Therapeutic management of bipolar disorder, as depicted in Figure 1, includes shorter term acute-phase treatment of a depressive, manic-hypomanic, or mixed episode and longer-term maintenance-phase treatments that minimize the symptomatic and side effect burden and reduce the likelihood of future episodes. The chronic course of bipolar disorder has been characterized in long-term studies such as the National Institute of Mental Health Collaborative Depression Study, which prospectively followed patients with bipolar I disorder and bipolar II disorder for up to 20 years (3). In this study, patients reported significant depressive or manic-hypomanic symptoms for approximately half of all follow-up weeks; weeks with depressive symptoms were three times more likely than weeks with manic symptoms among patients with bipolar I disorder and 37 times more likely than hypomanic symptoms among patients with bipolar II disorder (3).
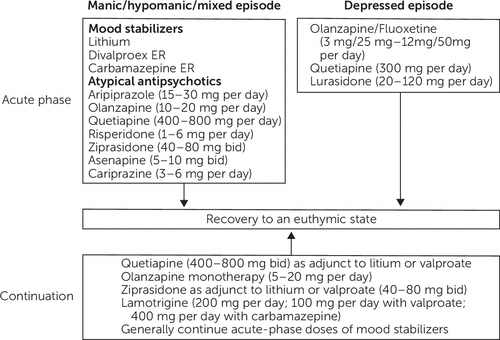
FIGURE 1. Food and Drug Administration–approved treatment for bipolar disorder
Similar to these findings, other studies have also reported that depressive symptoms are more prevalent than manic-hypomanic symptoms among patients with bipolar disorder (4). Adding to the burden of depression in bipolar disorder, recovery after a major depressive episode is 1.7–4.5 times less likely than it is after a manic or hypomanic episode (5). Management of bipolar depression is limited because only three medications (olanzapine-fluoxetine combination, quetiapine, and lurasidone) approved by the Food and Drug Administration (FDA) are available, whereas 10 FDA-approved medications (lithium, divalproex sodium, carbamazepine ER, risperidone, ziprasidone, aripiprazole, asenapine, cariprazine, olanzapine, and quetiapine) are available for manic-hypomanic episodes (Figure 1).
Taken together, these findings suggest that depression in patients with bipolar disorder is quite prevalent, is associated with worse long-term course, and is difficult to treat. In this article, we present our approach to managing bipolar depression and briefly review current FDA-approved and off-label treatment options, as well as experimental therapeutics for bipolar depression that are currently being investigated.
Approach to Management of Bipolar Depression
Diagnostic Evaluation
Differential diagnoses for bipolar depression include other mood disorders and personality disorder. More than half of patients with major depressive disorder endorse one or more subthreshold hypomanic symptoms, and the presence of these symptoms is associated with a lower likelihood of response to currently available treatments (6). Thus, a detailed history of prior symptomatic episodes and response to treatments may help in differentiating treatment-resistant major depressive disorder from bipolar depression. Among personality disorders, the affective instability and prominent mood symptoms associated with borderline personality disorder may often result in misdiagnosis of bipolar depression. Use of brief screening instruments for personality disorder, such as the Standardized Assessment of Personality—Abbreviated Scale (7), may aid clinicians in this regard. The presence of comorbid psychiatric disorders, including substance use disorder and cardiovascular and metabolic disorders (8, 9), may further limit the management of bipolar depression with currently available treatments. Thus, we recommend the use of structured or semistructured diagnostic instruments (such as the self-report Mood Disorders Questionnaire [10] or the clinician-administered Mini International Neuropsychiatric Interview [11]) to ascertain the diagnosis of bipolar depression and establish the presence of any comorbid psychiatric disorders (using a scale such as the Psychiatric Diagnostic Screening Questionnaire [12]). A detailed medical history, thorough physical, and routine lab work-up are also indicated to rule out medical conditions that may be mimicking or aggravating depressive symptoms, such as thyroid abnormalities, metabolic disorders, and obstructive sleep apnea (13, 14).
First-Line Treatment for Bipolar Depression
One of the three FDA-approved medications (olanzapine-fluoxetine combination, quetiapine, and lurasidone) should be considered as the first-line treatment for bipolar depression. Because no significant differences have been found in treatment outcomes among the first-step treatments (15), choice among these medications is often guided by the profile of anticipated side effects. Both olanzapine and quetiapine are associated with significant metabolic disturbance. However, their use may be preferred in patients with intractable insomnia because of their sedating side effects. Patients and their clinicians often must consider off-label use because of the limited efficacy of current FDA-approved treatments. For example, only 48.8% of patients receiving olanzapine attained remission during an eight-week-long acute-phase treatment (16). Similarly, remission rates with quetiapine and lurasidone ranged from 51.6% to 52.9% (17, 18) and 40% to 50% (19, 20) respectively.
Ongoing Assessments During the Course of Treatment
We recommend the use of a measurement-based care approach, which includes assessments of symptoms (depressive and manic-hypomanic) and side effect severity along with adherence (21), so that patients and clinicians can systematically evaluate the efficacy and tolerability of FDA-approved treatments. Repeated laboratory, anthropometric, and electrocardiographic measurements are also needed to establish the long-term safety of the currently approved FDA treatments.
Non–FDA-Approved (Off-Label) Treatment Options for Bipolar Depression
Atypical Antipsychotics
Aripiprazole, an atypical antipsychotic, was studied as a monotherapy for bipolar depression in two randomized controlled trials. No significant improvement was seen with aripiprazole, compared with placebo, during acute-phase treatment (22). A recent meta-analysis found that aripiprazole, along with ziprasidone (another atypical antipsychotic), was significantly less effective than lurasidone (15), suggesting that these medications should be avoided in patients with bipolar depression. Although asenapine has not been studied among patients with bipolar depression, it has been shown to be efficacious in reducing the severity of depression among people with mixed episodes (23). Among newer atypical antipsychotic medications, cariprazine at a dose of 1.5 mg/day, but not at doses of 0.75 mg/day and 3.0 mg/day, was found to be superior to placebo in reducing the severity of depression (24), warranting additional studies of this dose. In summary, the evidence for the efficacy of atypical antipsychotics is limited, except for those approved by the FDA for this indication (25).
Antidepressants
Use of antidepressant medications remains common among patients with bipolar depression (26). A large study of treatment-seeking patients with bipolar depression, the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, compared the utility of adding antidepressant medication (paroxetine or bupropion) with placebo. This trial found no difference in rates of either symptomatic improvement or treatment-emergent affective switch among patients with bipolar depression (N=366) treated with antidepressant or placebo (27). In a study of patients with bipolar II disorder who were treated with sertraline alone, lithium alone, or a combination of sertraline and lithium, rates of treatment-emergent manic or hypomanic switch or rates of improvement did not differ among the three treatment arms over a 16-week double-blind period (28). Similarly, in a study of patients with bipolar depression who had attained remission with the addition of an antidepressant medication to ongoing mood stabilizer medication, discontinuation of antidepressant medication was associated with shorter time to relapse of depressive symptoms over a one-year follow-up period compared with continuation of an antidepressant over the same period (29). Taken together, these findings suggest that a subgroup of patients with bipolar depression may benefit from acute- or continuation-phase treatment with antidepressants.
Ketamine
Ketamine, which targets glutamatergic neurotransmission by inhibiting N-methyl-D-aspartate (NMDA) receptors, has been shown to be effective for patients with bipolar depression (30). In the first study of racemic ketamine (delivered via intravenous [IV] dosing) in bipolar depression, hospitalized patients (N=18) with bipolar depression who had not responded to at least one antidepressant treatment and were stable on a four-week course of mood stabilizer (lithium or sodium valproate) at a therapeutic level were randomized to a cross-over study with an infusion of either ketamine or saline, with repeat infusion with the alternate medication after two weeks (31). In this study, after a single IV infusion of ketamine, patients had a rapid reduction in both clinician-rated and self-reported severity of depression within 40 minutes after the end of infusion, and it was maintained for three days after infusion (31). The response rate among those who received ketamine (71%) was much higher than among those who received placebo (6%). These findings were replicated in a separate cohort of patients with bipolar depression (N=15). In this cohort, 79% of patients responded to ketamine, whereas 0% responded to placebo (32).
Since these initial publications, open-label studies of adults and adolescents with bipolar depression (33, 34) have reported on the therapeutic benefits of ketamine. In addition, ketamine has also been shown to rapidly reduce suicidal ideation among patients with bipolar depression (35). Reflecting these studies showing ketamine’s efficacy, practitioners in the community are already using it with patients with bipolar depression (36). The recently concluded phase-three studies of intranasal s-ketamine (also known as esketamine, one of two enantiomers [R,S] of the racimate) offer the potential for the availability to clinicians of an easy-to-administer ketamine formulation (37). Although double-blind studies have shown that the rate of manic or hypomanic switch did not differ between patients receiving ketamine and those receiving saline (31), caution regarding treatment-emergent affective switch is indicated in view of case reports after clinical ketamine infusion (38).
Medications Targeting Glutamatergic Neurotransmission (Other Than Ketamine)
Riluzole has been studied among patients with bipolar depression. Two separate open-label studies showed a significant reduction in the severity of depression with riluzole in the 50- to 200-mg dose range (39, 40). However, a double-blind randomized placebo-controlled study with 94 patients with bipolar depression showed no significant improvement with 200 mg/day of riluzole over eight weeks compared with placebo (Payne et al., unpublished data, reported by http://www.stanleyresearch.org/03T-457). Lamotrigine, which modulates glutamate release and has been approved by the FDA for maintenance-phase treatment of bipolar disorder in preventing depressive relapse, was not more efficacious than placebo in four out of five studies of patients with bipolar depression (41). Memantine, an NMDA receptor antagonist, has also been studied among patients with bipolar depression. In two randomized controlled studies, memantine showed no significant reduction compared with placebo (42, 43). D-cycloserine, which binds to the glycine site of NMDA receptor, has been shown to prolong the antidepressant effect of ketamine (44).
Other Medications
A randomized controlled trial compared modafinil with placebo as an adjunctive treatment to mood stabilizer for six weeks (N=85) and found a significantly greater reduction in depression severity with modafinil (100–200 mg) than with placebo from baseline to week six (45). In addition, remission and response rates were significantly higher with modafinil, with no significant differences in treatment-emergent manic or hypomanic symptoms (45). Pramipexole, a dopamine agonist, was studied with a small group of patients (N=22) with bipolar depression (46). In this study, eight of 12 patients receiving pramipexole attained 50% or more reduction in symptoms (response) with six weeks of treatment (46). These findings were replicated with a separate group of patients with bipolar depression (47). Medications with no significant benefits over placebo in bipolar depression include ethyl-eicosapentanoic acid (48), mifepristone (49), levothyroxine 300 mcg/day (reported by Bauer et al.; http:/www.stanleyresearch.org/02T-238), ropinirole (reported by Grunhaus et al.; http://www.stanleyresearch.org/02T-123), levetiracetam (50), zonisamide (reported by Blumenthal et al.; http://www.stanleyresearch.org/07TGF-1163), tianeptine (reported by Kapczinski et al.; http://www.stanleyresearch.org/07TGF-1148), 4-aminopyridine (reported by Suppes et al.; http://www.stanleyresearch.org/04T-619), pregnenolone (reported by Brown et al.; http://www.stanleyresearch.org/09T-1280), and acetyl-l-carnitine plus alpha-lipoic acid (51).
Neurostimulation
Electroconvulsive therapy (ECT) remains the most effective treatment for treatment-refractory bipolar depression, with remission rates of 57%−63% (52). Repetitive transcranial magnetic stimulation (TMS), among other neurostimulation treatments, has been studied among patients with bipolar depression. Repetitive TMS using a figure-of-eight coil has not been shown to be effective with patients with bipolar depression (53). However, a recently reported small study of patients with bipolar depression reported that TMS using an H-coil (deep TMS) was more effective than sham (54). As reported in a recent review, TMS is well tolerated by patients with bipolar depression, but findings supporting its efficacy are limited (55). A small study showed that transcranial direct current stimulation (tDCS) resulted in a reduction in depressive symptoms among patients with bipolar depression that was similar in magnitude to that among patients with major depressive disorder (56). However, a larger study (N=120) of tDCS among patients with unipolar and bipolar depression showed that active tDCS was inferior to sham in reducing severity of depressive symptoms (Loo et al., unpublished study, http://www.stanleyresearch.org/11T-005). Similar outcomes were reported for deep brain stimulation of subcallosal cingulate among treatment-refractory patients with bipolar and unipolar depression (57).
Treatments Targeting Circadian Rhythm
Disruption of circadian rhythms among patients with bipolar disorder is well recognized and may play a pathophysiological role (58). Thus, bright light therapy has been proposed for treatment of patients with bipolar depression. A recent double-blind randomized placebo-controlled study of 46 patients with bipolar depression (59) found a significantly higher likelihood of remission with bright light therapy (68.2%) compared with control placebo red light (22.2%). In this study, patients started with 15-minute sessions of 7000-lux bright white light between 12:00 p.m. and 2:30 p.m., with the duration of light increased every week to the target dose of 60 minutes per daily session by week four. Patients were maintained on stable doses of their mood stabilizers throughout the study (59). Previous reports have shown the efficacy of light therapy combined with total sleep deprivation among patients with treatment-refractory bipolar depression (60). However, use of total sleep deprivation (61) and light therapy (62) with patients with bipolar disorder can provoke manic symptoms, restricting their use among patients with bipolar depression.
Experimental Therapeutics for Bipolar Depression
Medications Targeting Monoamine Neurotransmission
Lumateperone (also known as ITI-007) is an atypical antipsychotic that is an antagonist of the serotonin 2A receptor and a partial agonist of the presynaptic dopamine 2 receptor. It is currently in phase-three trials as monotherapy (ClinicalTrials.gov identifier NCT02600494) and as an adjunctive therapy to lithium and valproate (NCT02600507). Vortioxetine, an FDA-approved serotonin modulator antidepressant, is also being studied in a phase-two study (NCT03598868) as adjunctive treatment for bipolar depression. Another medication targeting monoamine neurotransmission is the compound JNJ-18038683, which is a 5-HT7 antagonist and is being evaluated for its effect on improving depression and cognitive symptoms among patients with bipolar disorder (NCT02466685).
Glutamatergic Medications
An ongoing study is testing the efficacy of repeated infusions of ketamine with ECT in patients with treatment-refractory bipolar depression and major depressive disorder (NCT03674671). Strategies to prolong the antidepressant effect of ketamine infusion include a six-week course of D-cycloserine and lurasidone after a single infusion of ketamine (being developed as NRX-101; NCT03402152).
Anti-Inflammatory Medications
Minocycline has been shown to reduce depressive symptoms in open-label studies of bipolar depression (63). The combination of minocycline and aspirin was shown to have a rate of response 2.9 times higher than the placebo condition, but this difference was not statistically significant (64). Two ongoing studies are testing the efficacy of minocycline in combination with N-acetyl cysteine (NCT02719392) and celecoxib (NCT02703363), respectively. Because probiotics have been shown to reduce rehospitalization among patients with bipolar disorder after a manic episode (65), ongoing studies are testing their efficacy after patients have been hospitalized for bipolar depression (NCT03349528). Other anti-inflammatory treatments for bipolar depression currently being studied include fecal microbiota transplant (NCT03279224), celecoxib (NCT01479829, NCT02726659), and infliximab (NCT02363738).
Neurostimulation
Ongoing studies are also testing the efficacy of novel neurostimulation modalities. One of the most promising among modalities is magnetic seizure therapy (MST), which has been shown to have efficacy similar to ECT among patients with major depressive disorder, but without the cognitive side effects seen with ECT (66). A triple-blinded (participant, provider, and outcome assessor) randomized study plans to enroll 100 patients with treatment-refractory bipolar depression to test the efficacy and tolerability of MST versus ECT (NCT03641300). Low-frequency magnetic stimulation is another novel neurostimulation intervention for bipolar depression that is currently being studied (NCT02707276, NCT03484494). Of note, low-frequency magnetic stimulation was not superior to sham in a recently reported study of patients with treatment-resistant depression (67).
Other Treatments
Other treatments currently being investigated for bipolar depression include bezafibrate (a lipid-lowering medication; NCT02481245), nitrous oxide (a noninflammable gas commonly used as an anesthetic drug in dental practices; NCT02757521), trehalose (a naturally occurring disaccharide that is commonly used as a food additive; NCT02800161), creatine monohydrate (an amino acid that is often used as a supplement; NCT01655030), and cannabidiol (a cannabis plant extract that has fewer psychoactive effects than tetrahydrocannabinol; NCT03310593). In addition, there is an ongoing study with patients with bipolar depression of a novel compound manufactured by Sunovion called SEP-4199 (NCT03543410).
Conclusion
Depression in bipolar disorder is prevalent, disabling, and often difficult to treat. Although only three medications are currently approved for treatment of bipolar depression, patients and their clinicians may consider off-label uses of several promising treatments. Several ongoing studies are testing the efficacy of novel interventions. In addition to the identification of novel treatments, identification of clinical and biological markers to personalize treatment selection for patients with bipolar depression is urgently needed.
1 : Epidemiology of DSM-5 bipolar I disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions—III. J Psychiatr Res 2017; 84:310–317 Crossref, Google Scholar
2 : Prevalence and correlates of DSM-5 bipolar and related disorders and hyperthymic personality in the community. J Affect Disord 2014; 167:198–205 Crossref, Google Scholar
3 : Long-term symptomatic status of bipolar I vs. bipolar II disorders. Int J Neuropsychopharmacol 2003; 6:127–137 Crossref, Google Scholar
4 : Sub-syndromal and syndromal symptoms in the longitudinal course of bipolar disorder. Br J Psychiatry 2006; 189:118–123 Crossref, Google Scholar
5 : Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry 2010; 67:339–347 Crossref, Google Scholar
6 : Do baseline sub-threshold hypomanic symptoms affect acute-phase antidepressant outcome in outpatients with major depressive disorder? Preliminary findings from the randomized CO-MED trial. Neuropsychopharmacology 2018; 43:2197–2203 Crossref, Google Scholar
7 : Standardised Assessment of Personality-Abbreviated Scale (SAPAS): preliminary validation of a brief screen for personality disorder. Br J Psychiatry 2003; 183:228–232 Crossref, Google Scholar
8 : Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 2007; 64:543–552 Crossref, Google Scholar
9 : The increasing medical burden in bipolar disorder. JAMA 2005; 293:2528–2530 Crossref, Google Scholar
10 : Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2000; 157:1873–1875 Crossref, Google Scholar
11 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(Suppl 20):22–33, quiz 34–57Google Scholar
12 : A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Arch Gen Psychiatry 2001; 58:787–794Crossref, Google Scholar
13 : Bipolar disorders. Nat Rev Dis Primers 2018; 4:18008 Crossref, Google Scholar
14 MacKinnon DD: Depression in Adults. BMJ Best Practice. 2018. https://bestpractice.bmj.com/topics/en-gb/55/pdf/55.pdf. Accessed Feb 15, 2019Google Scholar
15 : Lurasidone compared to other atypical antipsychotic monotherapies for bipolar depression: a systematic review and network meta-analysis. World J Biol Psychiatry 2018; 19:586–601 Crossref, Google Scholar
16 : Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003; 60:1079–1088Crossref, Google Scholar
17 : Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study). J Clin Psychopharmacol 2006; 26:600–609 Crossref, Google Scholar
18 : A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005; 162:1351–1360 Crossref, Google Scholar
19 : Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 2014; 171:160–168 Crossref, Google Scholar
20 : Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 2014; 171:169–177 Crossref, Google Scholar
21 : Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163:28–40 Crossref, Google Scholar
22 : A clinical review of aripiprazole in bipolar depression and maintenance therapy of bipolar disorder. J Affect Disord 2011; 128(Suppl 1):S21–S28 Crossref, Google Scholar
23 : Asenapine for bipolar disorder. Neuropsychiatr Dis Treat 2015; 11:3007–3017 Google Scholar
24 : An 8-week randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of cariprazine in patients with bipolar I depression. Am J Psychiatry 2016; 173:271–281 Crossref, Google Scholar
25 : Comparative efficacy and acceptability of drug treatments for bipolar depression: a multiple-treatments meta-analysis. Acta Psychiatr Scand 2014; 130:452–469 Crossref, Google Scholar
26 : Pharmacological treatment patterns at study entry for the first 500 STEP-BD participants. Psychiatr Serv 2006; 57:660–665 Crossref, Google Scholar
27 : Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356:1711–1722Crossref, Google Scholar
28 : Switch rates during acute treatment for bipolar II depression with lithium, sertraline, or the two combined: a randomized double-blind comparison. Am J Psychiatry 2017; 174:266–276 Crossref, Google Scholar
29 : Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003; 160:1252–1262 Crossref, Google Scholar
30 : Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database Syst Rev 2015; 9:
31 : A randomized add-on trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 2010; 67:793–802 Crossref, Google Scholar
32 : Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 2012; 71:939–946 Crossref, Google Scholar
33 : Clinical experience using intranasal ketamine in the longitudinal treatment of juvenile bipolar disorder with fear of harm phenotype. J Affect Disord 2018; 225:545–551 Crossref, Google Scholar
34 : A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar Disord 2015; 17:438–443Crossref, Google Scholar
35 : Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord 2017; 19:176–183 Crossref, Google Scholar
36 : A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry 2017; 174:695–696Crossref, Google Scholar
37 : S111. Randomized, double-blind study of flexibly-dosed intranasal esketamine plus oral antidepressant vs. active control in treatment-resistant depression. Biol Psychiatry 2018; 83(9, Supplement):S390Crossref, Google Scholar
38 : Possible affective switch associated with intravenous ketamine treatment in a patient with bipolar I disorder. Biol Psychiatry 2016; 79:e71–e72Crossref, Google Scholar
39 : Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology 2010; 35:834–846 Crossref, Google Scholar
40 : An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 2005; 57:430–432 Crossref, Google Scholar
41 : Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord 2008; 10:323–333 Crossref, Google Scholar
42 : Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: a double-blind, randomized, placebo-controlled trial. Bipolar Disord 2012; 14:64–70 Crossref, Google Scholar
43 : The effects of add-on low-dose memantine on cytokine levels in bipolar II depression: a 12-week double-blind, randomized controlled trial. J Clin Psychopharmacol 2014; 34:337–343 Crossref, Google Scholar
44 : Single-dose ketamine followed by daily D-cycloserine in treatment-resistant bipolar depression. J Clin Psychiatry 2015; 76:737–738Crossref, Google Scholar
45 : A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007; 164:1242–1249Crossref, Google Scholar
46 : Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry 2004; 161:564–566 Crossref, Google Scholar
47 : Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry 2004; 56:54–60 Crossref, Google Scholar
48 : Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry 2006; 60:1020–1022 Crossref, Google Scholar
49 : A randomized trial to examine the effect of mifepristone on neuropsychological performance and mood in patients with bipolar depression. Biol Psychiatry 2012; 72:943–949 Crossref, Google Scholar
50 : Levetiracetam in the management of bipolar depression: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2011; 72:744–750 Crossref, Google Scholar
51 : A placebo-controlled trial of acetyl-l-carnitine and α-lipoic acid in the treatment of bipolar depression. J Clin Psychopharmacol 2013; 33:627–635 Crossref, Google Scholar
52 : Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord 2009; 118:55–59 Crossref, Google Scholar
53 : A negative double-blind controlled trial of sequential bilateral rTMS in the treatment of bipolar depression. J Affect Disord 2016; 198:158–162 Crossref, Google Scholar
54 : Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology 2017; 42:2593–2601 Crossref, Google Scholar
55 : Clinical repetitive transcranial magnetic stimulation for posttraumatic stress disorder, generalized anxiety disorder, and bipolar disorder. Psychiatr Clin North Am 2018; 41:433–446 Crossref, Google Scholar
56 : Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:96–101 Crossref, Google Scholar
57 : Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 2012; 69:150–158 Crossref, Google Scholar
58 : Circadian rhythms and sleep in bipolar disorder. Bipolar Disord 2010; 12:459–472 Crossref, Google Scholar
59 : Adjunctive bright light therapy for bipolar depression: a randomized double-blind placebo-controlled trial. Am J Psychiatry 2018; 175:131–139 Crossref, Google Scholar
60 : Combined total sleep deprivation and light therapy in the treatment of drug-resistant bipolar depression: acute response and long-term remission rates. J Clin Psychiatry 2005; 66:1535–1540 Crossref, Google Scholar
61 : Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Res 1999; 86:267–270Crossref, Google Scholar
62 : Light therapy for bipolar disorder: a case series in women. Bipolar Disord 2007; 9:918–927 Crossref, Google Scholar
63 : A pilot study of minocycline for the treatment of bipolar depression: effects on cortical glutathione and oxidative stress in vivo. J Affect Disord 2018; 230:56–64 Crossref, Google Scholar
64 : Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry 2018; 8:27 Crossref, Google Scholar
65 : Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: a randomized controlled trial. Bipolar Disord 2018; 20:614–621 Crossref, Google Scholar
66 : Magnetic seizure therapy of major depression. Arch Gen Psychiatry 2001; 58:303–305 Crossref, Google Scholar
67 : Double-blind, proof-of-concept (POC) trial of low-field magnetic stimulation (LFMS) augmentation of antidepressant therapy in treatment-resistant depression (TRD). Brain Stimul 2018; 11:75–84 Crossref, Google Scholar



