Subsyndromal Delirium in Older People: A Systematic Review of Frequency, Risk Factors, Course and Outcomes
Abstract
Objective
To determine the frequency, risk factors, course and outcomes of subsyndromal delirium (SSD) in older people by systematically reviewing evidence on these topics.
Methods
Subsyndromal delirium was defined as the presence of one or more symptoms of delirium, not meeting criteria for delirium and not progressing to delirium. MEDLINE, EMBASE, PsycINFO and the Web of Science were searched for potentially relevant articles published from 1996 to June 2011. The bibliographies of relevant articles were searched for additional references. Twelve studies met the inclusion criteria. The validity of included studies was assessed according to Evidence-Based Medicine criteria. Information about the study population and methods, age, gender, proportion with dementia, diagnostic criteria, period and frequency of observation, and the topics above was systematically abstracted, tabulated and synthesized using standard meta-analysis techniques.
Results
The combined prevalence of SSD was 23% (95% CI, 9–42%); the combined incidence was 13% (95% CI, 6–23%). Risk factors were similar to those for delirium. Episodes lasted up to 133 days and were often recurrent. Outcomes were poor and often intermediate between those of older people with or without delirium. Of note, there was significant unexplained heterogeneity in the results of studies of prevalence, incidence and some risk factors.
Conclusions
SSD in older people may be a frequent and clinically important condition that falls on a continuum between no symptoms and full delirium. Because of significant unexplained heterogeneity in the results of studies of SSD, however, the results of this review must be interpreted cautiously. Further research is necessary. Copyright © 2012 John Wiley & Sons, Ltd.
(Reprinted with permission from International Journal of Geriatric Psychiatry 2013; 28:771–780)
Introduction
Delirium is a cognitive disorder characterized by acute onset, fluctuating course, altered level of consciousness, inattention, disorientation, memory impairment, disorganized thinking, and perceptual and motor disturbances (American Psychiatric Association, 2000). It occurs in hyperactive, hypoactive or mixed forms in up to 42% of older hospital inpatients (Siddiqi et al., 2006) and 70% of long-term care (LTC) residents (McCusker et al., 2011). In both settings, delirium is independently associated with poor outcomes (Siddiqi et al., 2006,Witlox et al., 2010, McCusker et al., 2010)
The diagnosis of delirium requires the coexistence of symptoms from multiple domains. It is common, however, for older people in different healthcare settings to display one or more symptoms of delirium without having the full syndrome (Rockwood, 1993, Kiely et al., 2003). This condition is known as subsyndromal delirium (SSD).
Subsyndromal delirium was described as early as 1517 by Guainerio (Diethelm, 1971). “Discussing delirium … he emphasized that a predelirious period … can be recognized which … may not lead to full delirium.” Almost five hundred years later, Lipowski (1990) described a “prodromal phase … in which patients had one or more symptoms of delirium (decreased concentration and ability to think, restlessness, anxiety, irritability, drowsiness, hypersensitivity to stimuli, nightmares) that never progressed to full DSM-defined delirium.” DSM-IV-TR recognizes “sub-syndromal presentations … with some but not all of the symptoms of delirium” and recommends that such presentations be coded as cognitive disorder not otherwise specified (American Psychiatric Association, 2000). More recently, the DSM-V Neurocognitive Disorders Workgroup has been discussing whether to add subsyndromal delirium as a subcategory of delirium in parallel with a new category, mild neurocognitive disorder (Jeste, 2010). Of note, neither DSM-IV-TR nor the DSM-V Workgroup distinguishes between subsyndromal presentations that do or do not progress to full delirium.
Because the frequency and significance of SSD in older people is unclear, the primary objective of this study was to determine the frequency, risk factors, course and outcomes of this condition. For the purpose of this review, SSD was defined as the presence of one or more symptoms of delirium, not meeting criteria for delirium and not progressing to delirium (Levkoff et al., 1996). The review process, modified from the one described by Oxman et al. (1994), involved systematic selection of articles, abstraction of data, assessment of study validity, and qualitative and quantitative synthesis of results.
Methods
Selection of Articles
The selection process involved four steps. First, three computer databases (MEDLINE, EMBASE and PsycINFO) and the Web of Science were searched for potentially relevant articles published from 1996 to June 2011 using the keywords “subsyndromal” or “subclinical” or “subthreshold” and “delirium”. Second, relevant articles (based on the title and abstract) were retrieved for more detailed evaluation. Third, the bibliographies of relevant articles were searched for additional references. Finally, all relevant articles were screened to meet the following six inclusion criteria: (1) original research published in English or French; (2) study population of 20 patients or more; (3) patients’ mean age 60 years or more; (4) used acceptable diagnostic criteria for SSD; (5) subjects with SSD were re-screened to exclude those progressing to delirium; and (6) yielded information about one or more of the topics of interest: prevalence, incidence, risk factors, course or outcomes of SSD. There were no attempts to acquire unpublished data.
Abstraction of Data
Information about the study site, study design, population, inclusion and exclusion criteria, sample size at baseline, age, gender, proportion with dementia, diagnostic criteria, period of observation, frequency of observation, type of statistical analysis and the topics of interest was systematically abstracted from each report.
Because subsyndromal symptoms not progressing to delirium may represent the end of a resolving episode of delirium, we recorded whether enrolled cases of SSD were prevalent or incident cases. Prevalent SSD was defined as a diagnosis of SSD at the time of a first assessment; incident SSD was defined as a diagnosis of SSD following one or more assessments with no symptoms of delirium.
Assessment of Validity
To determine validity, the methods of each study were assessed according to relevant sets of validity criteria. Each study was scored with respect to meeting (+) or not meeting (−) each of the criteria.
Data Synthesis
Qualitative
All abstracted information was tabulated. A qualitative meta-analysis was conducted by summarizing, comparing and contrasting abstracted data.
Quantitative
Standard meta-analysis techniques (Egger et al., 2001) were applied to different groups of studies and studies with usable data from two or more studies. Meta-analysis was used to calculate the combined estimate for three different objectives: (1) prevalence and incidence of SSD (proportion); (2) odds ratio (OR) of SSD associated with each risk factor; and (3) OR of SSD associated with each outcome. For all three meta-analyses, according to usual practice, we used a fixed effect model first, followed by a test of homogeneity. Depending on whether homogeneity was accepted or rejected, we used the fixed or the random effect model to compute the estimate (proportion or OR) and its 95% confidence interval (CI). The meta-analysis was conducted using the R software 2.13.0 (package: META (metaprop)) and STATA software 10.0 (package meta). Forest plots were drawn of individual and pooled estimates of prevalence and incidence, and ORs for risk factors and outcomes. Finally, when possible, we fitted meta-regression models to assess the impact of study variables on the results (Egger et al., 2001).
Results
Selection of Articles
The search strategy yielded 63 potentially relevant studies; 21 were retrieved for more detailed evaluation. Twelve studies met the inclusion criteria (Levkoff et al., 1996, Marcantonio et al., 2002, Cole et al., 2003, Bourdel-Marchasson et al., 2004, Liptzin et al., 2005, Ouimet et al., 2007, Tan et al., 2008, Leonard et al., 2009, Ceriana et al., 2010, Skrobik et al., 2010, Cole et al., 2011, Cole et al., 2012), including two different studies of the same cohort (Cole et al., 2011, Cole et al., 2012). Nine studies were excluded: two did not use acceptable diagnostic criteria, five did not re-screen subjects with SSD to exclude those progressing to delirium, one was a duplicate publication and one did not meet three of the inclusion criteria.
Data Synthesis
Overview of included studies
The 12 studies (Table 1) were conducted in North America (n = 9) or Europe (n = 3) and enrolled older patients admitted to medical (n = 6) or surgical (n = 3) inpatient units, palliative care units (n = 1) or LTC facilities (n = 2). Sample size ranged from 53 to 1025, median 234–250. Mean age ranged from 63 to 85 years, median 70 years. The proportion of women ranged from 0% to 79%, median 55–59%; the proportion with dementia ranged from 0% to 70%, median 49%. The period of observation ranged from 5 to 180 days, median 7–8 days. When the period of observation involved more than one contact, the frequency of contacts ranged from three times per day to weekly, median daily. Ten studies enrolled incident SSD (Levkoff et al., 1996, Marcantonio et al., 2002, Cole et al., 2003, Bourdel-Marchasson et al., 2004, Liptzin et al., 2005, Tan et al., 2008, Leonard et al., 2009, Ceriana et al., 2010, Cole et al., 2011, Cole et al., 2012), 5 enrolled prevalent SSD (Levkoff et al., 1996, Cole et al., 2003, Bourdel-Marchasson et al., 2004, Leonard et al., 2009, Ceriana et al., 2010), and two enrolled a mixture of prevalent and incident SSD (Ouimet et al., 2007, Skrobik et al., 2010).
| Author (year) | Country | Population | Exclusion criteria | Exclusion criteria | N | Mean age | Female % | Dementia % | Definition of SSD | Period of observation (days) | Frequency of observation | Prevalence % | Incidence/week % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Levkoff et al. (1996) | USA | MI | 65+ | – | 250 | – | 66 | 20 | 1 or more of clouding of consciousness, inattention, disorientation, perceptual disturbances | 7 | Daily | 12.6 | 27.6 | |
| Marcantonio et al. (2002) | USA | SI | 65+, hip fracture | Pathological fracture, delirium | 122 | 79 | 79 | 38.5 | 1 or more CAM core symptoms | 7–8 | Daily | – | 28.3 | |
| Cole et al., (2003) | Canada | MI | 65+ | Stroke, oncology, delirium | 164 | 83 | 71 | 53 | Prevalent SSD: 2 or more of; clouding of consciousness, inattention, disorientation, perceptual disturbances; incident SSD: 1 or more of clouding of consciousness, inattention, disorientation, perceptual disturbances | 7 | Every 2 days | 60.9 | 36.5 | |
| Bourdel et al. (2004) | France | GU | 75+, living at home | – | 427 | 85 | 55 | 52 | 1 or more CAM core symptoms | 15–25 | Every 3 days | 20.6 | 4.9 | |
| Liptzin et al., (2005) | USA | SI | 50+, elective orthopedic surgery | Dementia or delirium prior to surgery | 80 | 67 | 57 | 0 | 1 or more of clouding of consciousness, inattention, disorientation, perceptual disturbances | 14 | Daily for 4 days, then at 14 days | – | 34.4 | |
| Ouimet et al. (2007) | Canada | ICU | ICU≥24 h | Comatose | 537 | 63 | 40 | ? | Score 1–3 on ICDSC | 6 | 3/day | 33.3a | ||
| Tan et al. (2008) | USA | SI | Elective cardiac surgery | Pre-operative delirium | 53 | 63 | 0 | ? | 1 or more CAM core symptoms | 7 | Daily | – | 34 | |
| Leonard et al. (2009) | Ireland | P | – | Glasgow Coma Scale ≤ 3 | 100 | 69 | 51 | ? | 1 or more CAM core symptoms | 7 | Weekly | 27.4 | 12.3 | |
| Ceriana et al. (2010) | Italy | SDU | – | Coma, delirium | 234 | 70 | 43 | ? | Score 1-3 on ICDSC | 29 | Daily | 4.6 | 2.9 | |
| Skrobik et al. (2010) | Canada | ICU | – | Coma | 1025 | 63 (est) | 59 (est) | ? | Score 1-3 on ICDSC | 5–6 | 3/day | 31.4a | ||
| Cole et al. (2011, 2012) | Canada | LTC | 65+ | – | 104 | 84 | 60 | 49 | (1) 1 or more CAM core symptoms | 180 | Weekly | – | 2.5 | |
| 138 | 85 | 65 | 70 | (2) 2 or more CAM core symptoms | 180 | Weekly | – | 0.9 |
Diagnostic criteria
In the 12 studies, SSD was diagnosed using three different sets of criteria (Table 1). Three studies (Levkoff et al., 1996, Cole et al., 2003, Liptzin et al., 2005) defined SSD as the presence of one or two (or more) symptoms of delirium, not meeting the criteria for delirium; the symptoms included inattention, altered level of consciousness, disorientation and perceptual disturbances. Six studies (Marcantonio et al., 2002, Bourdel-Marchasson et al., 2004, Tan et al., 2008, Leonard et al., 2009, Cole et al., 2011, Cole et al., 2012) defined SSD as the presence of one or two (or more) Confusion Assessment Method core symptoms of delirium, not meeting the criteria for delirium; the core symptoms were acute onset and fluctuation, inattention, disorganized thinking and altered level of consciousness. Finally, three studies (Ouimet et al., 2007, Ceriana et al., 2010, Skrobik et al., 2010) defined SSD as the presence of 1–3 symptoms of delirium on the Intensive Care Delirium Screening Checklist; the symptoms included altered level of consciousness, inattention, disorientation, hallucinations or delusions, agitation or retardation, inappropriate speech or mood, sleep/wake cycle disturbance and symptom fluctuation.
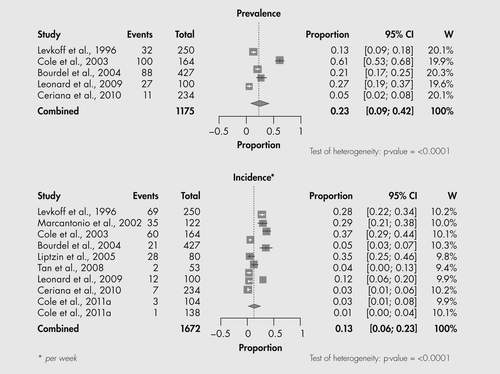
Prevalence and incidence
Eleven studies yielded information about the prevalence or incidence of SSD (Table 1). The validity of these studies was assessed according to four criteria for studies of prevalence and incidence derived from (Barker et al. (1998). These criteria included the following: (1) appropriate study population; (2) systematic study sample; (3) response rate of more than 75%; and (4) use of a reliable and valid diagnostic instrument. Each study was scored with respect to meeting (+) or not meeting (−) each of the above criteria. Most studies met most of the four criteria; however, eight reported a response rate of less than 75%.
In the qualitative analysis, the prevalence of SSD varied from 12.6% to 60.9%; the incidence (per week) varied from 0.9% to 36.5%. Variation in reported rates could not be explained by study site, population, response rate of more than 75%, study design, diagnostic criteria, age, gender, presence of dementia or period of observation. In one study, a requirement for two or more as opposed to one Confusion Assessment Method core symptom(s) resulted in a lower incidence rate (Cole et al., 2011).
In the quantitative analysis (Figure 1), the combined estimate of prevalence was 23% (95% CI, 9–42%); the combined estimate of incidence/week was 13% (95% CI, 6–23%). Of note, there was significant heterogeneity in reported prevalence and incidence rates. In meta-regression analysis, there were no predictors of higher prevalence, but higher incidence was predicted by higher frequency of observation (more often than weekly versus weekly).
Risk factors
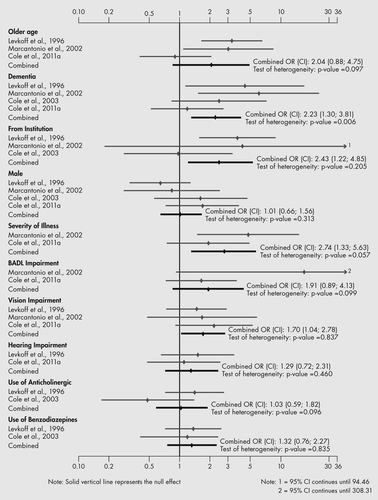
Six studies yielded information about risk factors for SSD (Levkoff et al., 1996, Marcantonio et al., 2002, Cole et al., 2003, Ouimet et al., 2007, Ceriana et al., 2010, Cole et al., 2011) (Table 2). The validity of these studies was assessed according to the four primary criteria for risk factor studies described by the Evidence-Based Medicine Working Group (Levine et al., 1994): (1) clearly identified comparison groups that were similar with respect to important determinants of the outcome, other than the one of interest (or differences in important determinants were controlled for in the analysis); (2) exposures and outcomes were measured in the same way in comparison groups; (3) follow-up was sufficiently long; and (4) follow-up was sufficiently complete (i.e., 80% of inception cohort). Most studies met most of the four criteria, but three did not have comparison groups that were similar with respect to important determinants of the outcome or did not adjust for differences between groups in the analysis.
| Study characteristics | Risk factors | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| Author (year) | Population | N | Type of case | Definition of SSD | Risk factors for prevalent SSD | Risk factors for incident SSD | Outcome(s) determined at | Outcomes (compared to subjects with no SSD) |
| Levkoff et al. (1996) | MI | 250 | I | 1 or more of clouding of consciousness, inattention, disorientation, perceptual disturbances | – | 80+, dementia, from LTC, more BADL decline and illness severity, fracture, infection, dehydration, fever | Discharge | More institutionalization |
| Marcantonio et al. (2002) | SI | 122 | I | 1 or more CAM core symptoms | – | 80+, dementia, more comorbidity | 1 and 6 months | More institutionalization, death, BADL and ambulation decline |
| Cole et al. (2003) | MI | 164 | I or P | Prevalent SSD: 2 or more of; clouding of consciousness, inattention, disorientation, perceptual disturbances; incident SSD: 1 or more of clouding of consciousness, inattention, disorientation, perceptual disturbances | Dementia, higher APS, CCI, illness severity scores and lower BADL score | Similar to risk factors for prevalent SSD | 12 months | Longer length of hospital stay, more death, and cognitive and functional decline |
| Bourdel et al. (2004) | GU | 427 | Combined I and P | 1 or more CAM core symptoms | – | – | Discharge | More institutionalization |
| Ouimet et al. (2007) | ICU | 537 | Combined I and P | Scores 1–3 on ICDSC | Older age, higher APS scorea | Discharge | Longer ICU and hospital length of stay, more institutionalization | |
| Ceriana et al. (2010) | SDU | 234 | P | Scores 1–3 on ICDSC | Older age, delirium in ICU, mechanical ventilation | – | – | – |
| Cole et al. (2011) | LTC | 104 | I | (1) 1 or more CAM core symptoms | – | male, dementia, more cognitive and ADL | 6 months | No significant differences |
| 138 | I | (2) 2 or more CAM core symptoms | – | impairment and depressive symptoms | 6 months | More cognitive decline | ||
In the qualitative analysis, the three studies that did not have similar comparison groups and did not adjust for differences in the analysis reported a greater numbers of risk factors, but the risk factors were similar to those reported in the remaining studies. Risk factors for prevalent or incident SSD identified most often included older age, dementia, more cognitive and basic activities of daily living impairment, more severe physical illness and more comorbidity (Table 2). Additional risk factors identified in at least one study each included admission from LTC, received mechanical ventilation, male gender and depressive symptoms. Identified risk factors did not appear to be related to study site, population, study design, diagnostic criteria, age, gender, dementia, or period and frequency of observation.
In the quantitative analysis (Figure 2), 10 risk factors for incident SSD had usable data from two or more studies. Four statistically significant risk factors were dementia, admitted from an institution, increasing severity of medical illness and vision impairment. Of note, there was significant unexplained heterogeneity in the results for dementia and severity of illness. No risk factors for prevalent SSD had usable data from two or more studies.
Course
There were two studies of the course of SSD. The first described the course over 7 days in 53 post-cardiac surgery patients (Tan et al., 2008). Fifteen patients (28.3%) had one episode, and three (5.7%) had two or more episodes. Most episodes lasted 1–3 days and ended in recovery. The second described the course over 180 days in 68 LTC residents (Cole et al., 2012). Thirty-two residents had one episode, and 36 had two or more episodes. Episodes lasted 7–133 days and most ended in recovery. Use of a more restrictive definition of SSD resulted in a more protracted course.
Outcomes
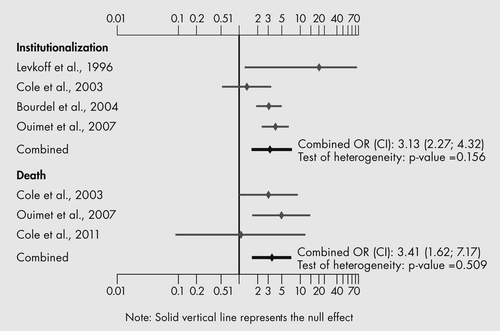
Six studies yielded information about the outcomes of SSD (Levkoff et al., 1996, Marcantonio et al., 2002, Cole et al., 2003, Bourdel-Marchasson et al., 2004, Ouimet et al., 2007, Cole et al., 2011) (Table 2). The validity of these studies was assessed according to the five criteria for studies of prognosis described by the Evidence-Based Medicine Working Group (Laupacis et al., 1994): (1) formation of an inception cohort of incident cases only; (2) adequate length of follow-up to determine outcome; (3) complete follow-up (determination of outcomes for at least 80% of the inception cohort); (4) objective outcome criteria; and (5) adjustment for extraneous prognostic factors (e.g., age and severity of physical illness). Most studies met most of the five criteria.
In the qualitative analysis, SSD was associated with many poor outcomes including cognitive and functional decline, increased length of hospital stay and increased rates of admission to LTC institutions. Poor outcomes did not appear to be related to study site, population or design, diagnostic criteria, age, gender, dementia, or period and frequency of observation.
In the quantitative analysis (Figure 3), only two outcomes had usable data from two or more studies. Both outcomes, rates of institutionalization and death, were significantly worse for groups with SSD.
Discussion
Subsyndromal delirium was defined as the presence of one or more symptoms of delirium, not meeting criteria for delirium and not progressing to delirium. We located 12 studies that yielded information about the frequency, risk factors, course and outcomes of SSD in older people. Of note, there was significant unexplained heterogeneity in the results of studies of prevalence, incidence and some risk factors; consequently, the results of this review must be interpreted cautiously.
Subsyndromal delirium appears to be frequent in older populations. The combined prevalence was 23% (95% CI, 9–42%); the combined incidence was 13% (95% CI, 6–23%). Fewer numbers of symptoms required for diagnosis and higher frequency of observation (i.e., more often than weekly) may be related to higher incidence.
Risk factors for SSD appear to include older age, dementia, more cognitive and basic activities of daily living impairment, admitted from an institution, increasing severity of medical illness, vision impairment and more comorbidity. These risk factors are similar to the risk factors that predict the onset of DSM-defined delirium (Elie et al., 1998); moreover, the frequencies of these risk factors were often intermediate between those of risk factors in populations with and without delirium (Levkoff et al., 1996, Cole et al., 2003).
Episodes of SSD appeared to lasted up to 133 days and most ended in recovery. Use of a more restrictive definition of SSD resulted in a more protracted course (Cole et al., 2012). There are, however, only two studies of course, and these studies are not comparable because of substantial differences in study populations and methodology.
Finally, the outcomes of SSD (i.e., cognitive decline, functional decline, increased length of hospital stay, and increased rates of admission to LTC institutions and death) were poor. These outcomes were often intermediate between the outcomes of older people with and without delirium (Levkoff et al., 1996, Cole et al., 2003).
Of note, five studies that did not re-screen enrolled subjects to exclude those progressing to full delirium were excluded from this review (Kiely et al., 2003, Marcantonio et al., 2005, Dosa et al., 2007, Voyer et al., 2009, von Gunten and Mosimann, 2010). These five studies reported prevalence rates of subsyndromal symptoms ranging from 11.9% to 51%; the median rate was 39.5%, much higher than the combined prevalence rate of SSD (i.e., 23%) reported in this paper. Two of these studies (Kiely et al., 2003, Marcantonio et al., 2005) reported risk factors similar to those in this review, and one (Marcantonio et al., 2005) reported outcomes similar to those in this review. Thus, the inclusion of subjects with subsyndromal symptoms that may progress to full delirium appears to increase prevalence substantially but may not change risk factors or outcomes.
The above findings may support the notion of a continuum of acute neurocognitive disorder in older people. Within this continuum, the available evidence suggests that increasing number and severity of risk factors for delirium and increasing number and duration of symptoms may predict increasingly adverse outcomes. SSD may represent a point on this continuum, intermediate between no symptoms and full delirium. As such, SSD may be a marker of underlying medical conditions (e.g., infection and drug toxicity) not severe enough to cause full delirium. This hypothesis may be testable by comparing repeated measures of medical and physiological variables at the beginning and end of episodes of SSD and full delirium, respectively.
The above findings may have implications for clinical practice. Because the outcomes of SSD appear to be poor, the presence of even one or two symptoms of delirium may identify older people who warrant clinical attention. Efforts to prevent or detect and treat SSD may be justified. As to prevention, programs that have proved effective in preventing delirium (Inouye et al., 1999) may be adapted to prevent SSD. As to detection and treatment, interventions may lead to recovery from SSD and improved outcomes. Indeed, one study reports that hospital inpatients who recovered from SSD by 8 weeks had better outcomes than those who did not recover (Cole et al., 2008).
The above findings may have implications for clinical research. The prevalence and incidence of SSD should be determined using different diagnostic thresholds that include both the number and severity of symptoms. The putative causes (precipitating factors) of SSD should be determined to inform efforts to develop interventions to prevent, or detect and treat SSD. Management of SSD should be recorded in detail and related to rates of recovery and outcomes. Those with SSD should be followed and re-assessed frequently to determine the evolution of this condition. Rates of recovery from SSD should be determined. It will be important to examine if SSD is associated with behavior problems, increased burden on nursing staff and increased costs of care. Because the diagnosis of SSD may result from a relatively small number of observations of fluctuating symptoms of full delirium (Blazer and van Nieuwenhuizen, 2012), more frequent observations (e.g., every second day) or use of additional sources of information such as daily nurse-observed symptoms of delirium (McCusker et al., 2010)) may result in detection of more symptoms and a diagnosis of full delirium; furthermore, future studies must try to account for the fact that many subjects may be receiving medical interventions that probably prevent the emergence of full delirium. Finally, even though SSD is probably a delirium spectrum disorder rather than a distinct entity, further research is necessary to clarify the relationship between SSD and full delirium.
This systematic review has six strengths. Only studies that re-screened cases to exclude those progressing to delirium were included. The validity of included studies was systematically assessed. There was a qualitative and quantitative synthesis of results. When possible, meta-regression analysis was used to examine study variables to account for variability in study results. Nine of the studies enrolled subjects with incident SSD and provide particularly strong evidence of risk factors and outcomes. The results of studies of prevalent SSD were presented separately and, for the most part, supported the findings of studies of incident SSD.
This systematic review has four potential limitations. The literature search was conducted by one author only and limited to articles published in English and French because there were no resources to translate articles written in other languages. The data were abstracted by one author only. Finally, there was significant unexplained heterogeneity in the results of studies of prevalence, incidence and some risk factors; it is arguable that such heterogeneity should have precluded the combining of the results of the different studies.
Conclusion
Subsyndromal delirium may be a frequent and clinically important condition that falls on a continuum between no symptoms and DSM-defined delirium. Because of significant unexplained heterogeneity in the results of studies of SSD, however, the results of this review must be interpreted cautiously. Further research is necessary.
Key Points
| •. | Subsyndromal delirium appears to be frequent in older populations. | ||||
| •. | Risk factors for subsyndromal delirium appear to be similar to those for delirium. | ||||
| •. | Outcomes of subsyndromal delirium are poor. | ||||
| •. | Because of significant unexplained heterogeneity in the results of studies of SSD, the results of this review must be interpreted cautiously. | ||||
. 1998. Epidemiology in Medical Practice. Elsevier Health Sciences: London, UK.Google Scholar
. 2012. Evidence for the diagnostic criteria of delirium: an update. Curr Opin Psychiatry 25: 239–43.Crossref, Google Scholar
. 2004. Delirium symptoms and low dietary intake in older inpatients are independent predictors of institutionalization: a 1-year prospective population-based study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 59A: 350–4.Crossref, Google Scholar
. 2010. Delirium in patients admitted to a step-down unit: analysis of incidence and risk factors. J Crit Care 25: 136–43.Crossref, Google Scholar
. 2003. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc 51: 754–60.Crossref, Google Scholar
. 2012. The course of subsyndromal delirium in older long-term care residents. Am J Geriatr Psychiatry doi: 10.1097/JGP.0b013e3182447f91.Google Scholar
. 2008. The 6 and 12 month outcomes of older medical inpatients who recover from subsyndromal delirium. J Am Geriatr Soc 56: 2093–9.Crossref, Google Scholar
. 2011. Subsyndromal delirium in older long-term care residents: incidence, risk factors, and outcomes. J Am Geriatr Soc 59: 1829–36.Crossref, Google Scholar
. 1971. Medical Dissertations of Psychiatric Interest, Printed Before 1750. S. Karger: Basel, New York,.Google Scholar
. 2007. Preliminary derivation of a nursing home confusion assessment method based on data from the minimum data set. J Am Geriatr Soc 55: 1099–105.Crossref, Google Scholar
. 2001. Systematic Reviews in Health Care: Meta-analysis in Context. BMJ Publishing Group: London, UK.Crossref, Google Scholar
. 1998. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med 13: 204–12.Crossref, Google Scholar
. 1999. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 340: 669–720.Crossref, Google Scholar
. 2010. DSM-V Neurocognitive Criteria, Draft 1/7/10. A Proposal from the DSM-V Neurocognitive Disorders Work Group: Dilip Jeste (Chair), Deborah Blacker, Dan Blazer, Mary Ganguli, Igor Grant, Jane Paulsen, Ronald Petersen, and Perminder Sachdev. Arlington, Va: American Psychiatric AssociationGoogle Scholar
. 2003. Delirium among newly admitted postacute facility patients: prevalence, symptoms, and severity. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 58: M441–M5.Crossref, Google Scholar
. 1994. Users’ guides to the medical literature: V. How to use an article about prognosis. JAMA 272: 234–7.Crossref, Google Scholar
. 2009. Symptoms of depression and delirium assessed serially in palliative-care inpatients. Psychosomatics 50: 506.Crossref, Google Scholar
. 1994. Users’ guides to the medical literature. IV. How to use an article about harm. JAMA 271: 1615–9.Crossref, Google Scholar
. 1996. Subsyndromal delirium. Am J Geriatr Psychiatry 4: 320–9.Crossref, Google Scholar
. 1990. Delirium: Acute Confusional States. Oxford Universiy Press: NY, USA.Google Scholar
. 2005. Donepezil in the prevention and treatment of post-surgical delirium. Am J Geriatr Psychiatry 13: 1100–6.Crossref, Google Scholar
. 2002. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc 50: 850–7.Crossref, Google Scholar
. 2005. Outcomes of older people admitted to postacute facilities with delirium. J Am Geriatr Soc 53: 963–9.Crossref, Google Scholar
. 2010. Use of nurse-observed symptoms of delirium in long-term care: effects on prevalence and outcomes of delirium. Int Psychogeriatr 23: 602–8.Crossref, Google Scholar
. 2011. Prevalence and incidence of delirium in long-term care. Int J Geriatr Psychiatry 26: 1152–61.Google Scholar
. 2007. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med 33: 1007–13.Crossref, Google Scholar
. 1994. Users’ guides to the medical literature: VI. How to use an overview. JAMA 272: 1367–71.Crossref, Google Scholar
. 1993. The occurrence and duration of symptoms in elderly patients with delirium. J Gerontol 48: M162–M6.Crossref, Google Scholar
. 2006. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 35: 350–64.Crossref, Google Scholar
. 2010. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg 111: 451–63.Crossref, Google Scholar
. 2008. Incidence and predictors of post-cardiotomy delirium. Am J Geriatr Psychiatry 16: 575.Crossref, Google Scholar
. 2010. Delirium upon admission to Swiss nursing homes: a cross-sectional study. Swiss Med Wkly 140: 376–81.Google Scholar
. 2009. Detecting delirium and subsyndromal delirium using different diagnostic criteria among demented long-term care residents. J Am Med Dir Assoc 10: 181–8.Crossref, Google Scholar
. 2010. Delirium in elderly patients and the risk of post discharge mortality, institutionalization, and dementia: a meta-analysis. JAMA 304: 443.Crossref, Google Scholar



