Evaluation of Cognitive Impairment in Older Adults
Abstract
Cognitive complaints are common in older persons. With the shifting demographic pattern toward more aged individuals, more physicians will be needed who have competency in the differential diagnosis for cognitive impairment because there are not enough geriatric subspecialists. Psychiatrists who can evaluate patients for mood and different cognitive disorders will be key societal contributors in caring for the growing elderly population. We describe major differential diagnostic categories for cognitive impairment and characterize their examination, cognitive, laboratory, and neuroimaging findings. We discuss the 3 D’s (depression, delirium, dementia) as common conditions for which older persons are at risk, as well as key differential diagnostic features for the common causes of dementing disorders. Because these disorders may be comorbid, the practitioner needs to carefully evaluate and interpret a range of signs and symptoms indicative of their underlying conditions and manage those accordingly, including referring to subspecialists when necessary. Psychiatrists and neurologists are well positioned to diagnose older patients who have cognitive impairment or dementia. The coauthors leverage their geriatric psychiatry and cognitive-behavioral neurology expertise in addressing this topic for practitioners and cite guidelines and tools that have application in clinical practice.
Introduction
Because the U.S. population of persons 65 years and older is expected to grow, the annual number of cases of Alzheimer’s and other dementias is projected to double by 2050 (1). Current estimates of prevalence are that 10%−20% of persons over 65 have mild cognitive impairment (MCI), while 11% of those over 65 and 32% of those over 85 have dementia due to Alzheimer’s disease. Prevalence of all forms of dementia in the U.S. in those 71 or older is 13.9% (1). Among dementias, Alzheimer’s disease (AD) is by far the most common type, accounting for 50%−60% of cases (1, 2) with vascular, frontotemporal, and Lewy body dementias accounting for another 35% (3, 4). Although aging is the most consistent risk factor, those with fewer years of education are at higher risk for dementia related to having lower cognitive reserve (5).
The evaluation of cognitive disorders in older persons is complicated by the challenge of distinguishing them from depression and delirium, conditions that present with elements of cognitive and noncognitive neuropsychiatric symptoms that overlap with MCI and dementia. Further, they can be comorbid with dementia or its earlier phase, MCI. Another challenge is distinguishing normal aging from a dementing process. Education, cultural background, and primary language differences affect interpretation of symptoms and possible staging of a dementing disorder. The purpose of this article is to provide an overview of the approach to patients, as well as sufficient detail about practical clinical aspects of differential diagnosis of cognitive impairment in older persons, to benefit physicians who do not have additional training in geriatrics and wish to expand their competency, even if they eventually refer patients to a dementia specialist. We focus on the more prevalent conditions, whereas the subspecialist can diagnose atypical and less common disorders.
Depression and delirium are usually quite treatable disorders with high reversibility and therefore always need to be recognized and managed before considering a progressive condition as the cause of cognitive impairment. Delirium can be subsyndromal or even chronic and masquerade as a dementing condition. Delirium is often under-ecognized and misattributed to depression or dementia (6). Delirium is also often comorbid with dementia, ranging from approximately 15% (Alzheimer’s) to 35% (vascular dementia) (7). Major depression can cause memory deficits and be mistaken for MCI. Conversely, dementing illnesses can be accompanied by mood or apathy symptoms that get misattributed to depression. When mild, any of these may be confused as “normal aging.”
MCI, also called mild neurocognitive disorder (NCD) in DSM−5 (8), can have numerous etiologies, including depression, but may also herald the beginning stage of a dementing disorder. Some will revert to normal and many remain stable but approximately 10%−20% per year will convert to dementia with variations in rate depending on the length of follow-up (9, 10). Medical problems such as vitamin deficiencies, thyroid conditions, and adverse effects of drug therapies need to be carefully sought as sole causes or contributing factors to any of the 3 D’s (depression, delirium, dementia). Clinical interview, examination, and laboratory testing will be similar at the beginning of the evaluation irrespective of which of the 3 D’s is being considered. More advanced testing such as neuropsychological testing, neuroimaging, CSF analysis, or genomic testing will depend on the particular disorder(s) being differentially diagnosed for the individual patient; some testing might necessitate referral to a specialist.
The application of neuropathological, genomic, and newer neuroimaging techniques in research has revealed a significant rate of clinical misdiagnosis for types of dementias in both clinical and research settings, even when using strict diagnostic criteria (11, 12). Beach et al. (13) reported sensitivity ranging from 70.9% to 87.3% and specificity from 44.3% to 70.8% for possible or probable Alzheimer’s dementia as compared with autopsy diagnosis using longitudinal data from the National Alzheimer’s Coordination Center’s research database. As compared with neuropathological diagnosis at autopsy when using research criteria, Hogervorst et al. (14) found that identifying patients as a group who were possibly or probably demented had a higher (76%−100%) positive predictive value for Alzheimer’s disease as the cause, but a lower (18%−76%) negative predictive value. This highlights that while clinical evaluation can fairly reliably detect that MCI or dementia is present, advanced adjunctive testing targeting the underlying pathological process may be necessary to determine the actual diagnosis (2). Because prognosis, treatment, and temporal course vary with etiology, obtaining the right diagnosis is important. This article describes the typical clinical features of the major dementing disorders that, when complemented by objective diagnostic testing, may enable greater specificity than was possible prior to the advent of recent neuroimaging and laboratory biomarker tests (15).
The clinical rule of thumb is to first identify any component attributable to a major depression and/or delirium prior to considering a dementing disorder, acknowledging the complexity when these disorders are comorbid (see Figure 1). Some symptoms overlap across conditions, such as dysphoric mood, apathy, or forgetfulness, necessitating a careful characterization of the type of memory loss (secondary memory deficits due to inattention from depression or delirium; forgetfulness due to a retrieval memory disturbance related to white matter disease; amnestic memory pattern with the inability to learn and remember new information due to AD). Serial evaluations would include laboratory or imaging tests to identify reversible/treatable causes of cognitive impairment. In a meta-analysis (with 39 studies and 5620 dementia patients), potentially reversible causes were reported in 9% of dementia cases, with 0.6% cases actually resolved (0.29% partially, 0.31% fully) (16). These included metabolic (hypothyroidism, vitamin B12 deficiency), normal pressure hydrocephalus, brain tumor, depression, alcohol, infection, subdural hematoma, trauma, anoxia, and medications. This means that almost 1 patient in every 10 with dementia has a potentially reversible cause that should not be missed.
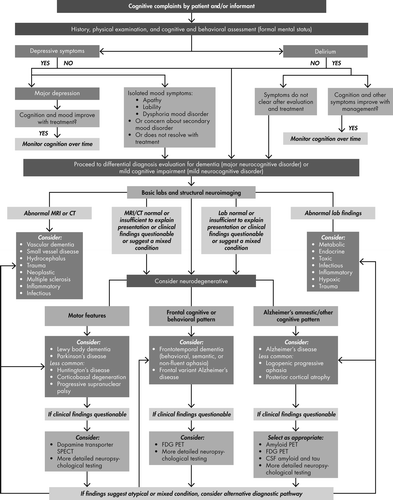
Assessment of the 3 D’s (depression, delirium, dementia) in older persons begins with ruling out depression and delirium, followed by consideration of examination findings in conjunction with neuroimaging or laboratory tests targeted to diagnose or rule out particular dementing disorders as noted in the schematic. Clinically available brain scans are listed related to relevant diagnostic considerations. Information in Tables 1–3 provides additional practical details for the clinician while working through the decision tree.
No matter the ultimate diagnosis determined, many patients and their caregivers/families are keenly aware of and fearful about the specter of having Alzheimer’s disease, which will often be top of mind and needs to be compassionately managed during the evaluation process (17, 18). Further, there is value in knowing the particular cause of MCI or dementia so appropriate medical management and psychosocial, financial, and occupational planning can occur more proactively (19–21). Caregivers note delays of about 3 years after symptom onset in obtaining a firm diagnosis of dementia type (usually by referral to a specialist) when general practitioners provide medical care (22–25). Delays and missed diagnoses—as many as 50% (26)—may be attributed to physicians’ discomfort with making a diagnosis or perceived harm (27).
Identifying cognitive impairment sooner may lead to earlier treatment of the cognitive condition, allow increased supervision of individuals to more adequately perform their activities of daily living (ADLs), improve treatment adherence rates for their other chronic conditions, reduce medication use errors, decrease ER and hospital visits, improve quality of life for the patient, and reduce financial and caregiver burden. For those patients with AD, we know that the current cognitive drugs work better the earlier they are started (28) and that when disease-modifying agents are discovered, earlier identification of cognitive impairment will be critical. Delaying the onset of or preventing AD would save billions of health care dollars and improve the quality of life for millions (1).
Key Features of Nondementing Disorders
Depression Characteristics
Major depression can be recurrent or new onset in older persons. Sadness, dysphoric mood, guilt-ridden thoughts, apathy, hopelessness, anhedonia, psychomotor slowing/agitation, suicidality, and negative thoughts are often accompanied by appetite and sleep disturbances, painful somatic symptoms, and mild forgetfulness. Apathy (loss of interest in usual activities with awareness) needs to be distinguished from abulia (loss of motivation without concern) where the latter is evidence of a primary neurological disorder. Further, apathy is often mistakenly described by patients and care partners as “depression” when in fact symptoms are better attributed to delirium, frontotemporal dementia, or early AD (29), especially in the absence of usual symptoms of depression such as sadness, sleep-wake cycle and appetite disturbances, hopelessness, etc. Neuropsychiatric symptoms, including apathy, depressed mood, irritability, agitation, and anxiety, are common in MCI (30), and we find the occurrence of at least one symptom in 80% of dementia patients, with depressed mood and apathy being the most common (31). Depressive disorders are reported as more common in vascular (about 44%) and unspecified dementia (about 32%) than AD (about 19%) (32). Characteristics that definitively distinguish primary depression from that associated with AD dementia are not well-defined and much symptomatic overlap exists (33–35). Additionally, depression is associated with an increased risk for AD dementia (36, 37).
Depression secondary to an underlying neuropathological process such as stroke, brain tumor, remote effects of cancer, or thyroid disease also need to be considered (36). A neurological examination for localizing signs may offer clues about a comorbid/causative condition. Assessment for depressive symptoms with atypical presentations or insufficient symptoms to meet criteria for major depression may herald a mood disorder associated with a neurological or medical condition including dementia (see Figure 1, secondary mood symptoms). Cognitive deficits in major depression can be varied and include inattention, impaired verbal learning, executive dysfunction, impaired working memory, and poor concentration that can secondarily affect memory in an inconsistent fashion (38). Measurement of cognition using a structured office-based multidomain test is important to detect the degree and type of deficits and then to document their improvement after the depression has been treated.
When a major depression is present, treatment using antidepressants in sufficient doses for adequate periods of time is warranted and would be expected to improve the depression and associated cognitive deficits. Once a primary depressive disorder has been ruled out or treated, persistent cognitive deficits need to be further evaluated because they may indicate a mild or major neurocognitive disorder (per DSM−5, dementia).
Delirium Characteristics
Delirium is an acute alteration of consciousness affecting attention and all higher cortical functions. It exists on a continuum between normal alertness and stupor or coma and can range between subtle (i.e., subsyndromal delirium) to more severe presentations. Delirium has three core domains of symptoms (cognitive, circadian, and higher-level thinking). Its cardinal symptom is inattention and is accompanied by deficits of other cognitive domains (short- and long-term memory, visuospatial ability, and orientation to time, place and person), circadian disturbances of motor activity and sleep-wake cycle, and impairments of higher-level thinking (comprehension, thought process, and executive function) (39). Circadian disturbances are common and are manifested by sleep and wake times being out of sync with normal diurnal patterns. Increased motor activity often occurs in the middle of the night when sleep is expected and hypoactivity during the day is often mistaken for depression. Impairment of higher-level thinking is reflected through a variety of deficits such as of executive and social cognition, situational awareness, comprehension and coherent communication, information processing, and making sound judgments and decisions (39). Delirium at times is also accompanied by affective lability, delusions, or misperceptions, which are less frequent than core symptoms but more likely to gain notice by observers than cognitive deficits (40).
Misperceptions are most often visual or auditory but can be tactile, olfactory, or gustatory and may present as illusions or hallucinations. Abnormalities of thought content are typically paranoid or grandiose although can be somatic. Delirium is usually not associated with a particular mood but rather with a rapidly changing, labile affect that is under poor control and incongruent with context. Some of these symptoms overlap with dementing conditions depending on the stage, especially in AD, although a temporal history of acute onset (hours to days) and fluctuating course of symptoms helps distinguish these disorders. Delusions in delirium tend not to be systemized, whereas in AD they are persistent and may be delusional misidentifications. Presence of extrapyramidal signs can help distinguish Lewy body dementia, which also involves visual hallucinations and fluctuating severity, from delirium.
Delirium is usually short-lived (days to a week) and reversible after detection and active management (6). It is more abrupt in its onset of cognitive impairment than are most dementias. Prolonged delirium can occur in the severely medically ill such as in hospice, long ICU stays, or institutionalization after severe traumatic brain injury or stroke.
Delirium is a very serious condition and a medical emergency requiring immediate evaluation of its causes and management to alter those etiologies. It is presumed reversible especially if caught early and if the underlying causes are not continuing. A careful medical and neuropsychiatric history including a review of all prescribed and over-the-counter medications may indicate situations with an increased risk for delirium. Anticholinergics, benzodiazepines, and opiates are associated with such risk and should be discontinued in a safe tapering method (41). Other risk factors include advanced age, underlying dementia, subtle cognitive impairment, low serum albumin, and dehydration. Administration of an office-based cognitive screening test is important to detect delirium because subsyndromal delirium has milder impairment than full delirium (42). Administration of a validated delirium scale to measure both noncognitive and cognitive symptoms will help distinguish delirium. Whenever delirium is suspected, an antidepressant medication should not be initiated until delirium has resolved. When diagnosis is difficult an electroencephalogram (EEG) may be obtained, where generalized slowing is the characteristic pattern for delirium but unlikely in depression or early stages of a dementia. Even if a dementia is suspected or preexisting, any acute decline in mental status should be considered delirium until proven otherwise and immediately lead to initiation of a cascade of laboratory and other testing to search for causes. Common causes include infection, autoimmune disorders, endocrine/metabolic disturbance, hyper/hypoglycemia, stroke, hypoperfusion, organ insufficiency, hypoxia, trauma, neoplasm, hematoma, etc.
Delirium is associated with increased morbidity and mortality. In older persons, an episode of delirium is also associated with decreased independent living during the year following and increased risk for dementia (43–45). In the elderly, the co-occurrence of delirium and depression carries a worse prognosis than either alone (46). Because the risk of delirium occurrence is increased by the presence of any preexisting (whether previously detected or not) cognitive impairment or dementing process (47–49), older patients need more monitoring following an episode of resolved delirium. Subsyndromal delirium has milder symptoms of inattention and other core symptoms (40) but is associated with intermediate morbidity between no and full delirium (50). Chronic subsyndromal delirium can masquerade as early dementia and medication adverse effects are an underappreciated cause.
Mild Cognitive Impairment and Dementia Characteristics
Overview
Mild cognitive impairment (mild NCD) can progress to dementia (major NCD), may revert to normal, or occasionally persist without progression. About 12% of patients with MCI (51) convert to dementia per year. The differential diagnosis for MCI is the same as for dementias, although the percentage due to a neurodegenerative or vascular etiology is lower than at a dementia stage. National Institute on Aging/Alzheimer’s Association (NIA/AA) guidelines (52) for MCI diagnosis require a reported decline in cognition, objective evidence on testing of one or more cognitive domains being abnormal for expected level of education and age, and no or mild functional impairment of instrumental ADLs defined as requiring only minimal assistance. Many physicians will differentiate between MCI patients that have primarily memory impairments on testing (either retrieval or amnestic memory deficits) called amnestic MCI and those with primarily nonmemory cognitive impairments (e.g., language, visuospatial, executive deficits) called nonamnestic MCI or, if applicable, multidomain MCI (53).
Some patients with complaints of cognitive decline do not have objective impairment on testing and are categorized as subjective memory complaints or subjective cognitive impairment (54). There is yet no standardized definition for subjective cognitive impairment, although emerging research using sensitive neuropsychological testing and neuroimaging suggests subtle abnormalities can be detected (55). These individuals are considered to possibly have a pre-MCI condition.
While evaluation and biomarkers may assist in determining the cause of MCI, in as many as half the cases MCI is not due to a progressive or neurodegenerative condition. The course of illness is therefore unstable in many cases. Causes include reversible conditions such as depression, use of anticholinergic, opioid, and benzodiazepine drugs (56), thiamine deficiency, dehydration, malnutrition, seizures, and hypothyroidism as well as dementing degenerative conditions.
Dementia is characterized by progressive impairments involving multiple cognitive domains of such severity as to result in a decline of basic and/or instrumental ADLs. If only memory loss is present without significant impact on ADLs, then amnestic disorder is diagnosed instead. When there is only language impairment, it is termed aphasia and not dementia. Typically dementia involves impairment of memory plus other cognitive domains such as executive, language, or visuospatial functions. Neuropsychiatric features commonly occur including depression, disinhibition, anxiety, psychosis, apathy, restless behaviors, impulsivity, obsessive-compulsive traits, sleep and appetite disturbances, and agitation.
There are many etiologies for dementia. Neurodegenerative conditions (AD, Parkinson’s and Lewy body dementias, frontotemporal dementia) are the most common causes of dementia along with vascular dementia. Less common causes include normal-pressure hydrocephalus, immunological/inflammatory (systemic lupus erythematosus, vasculitis, paraneoplastic, multiple sclerosis), infectious (prion, syphilis, HIV, herpes), anoxia, obstructive sleep apnea, posttraumatic, neoplastic (CNS and metastatic), toxic/metabolic (hypothyroidism, drug induced, uremia, hepatic encephalopathy), vitamin deficiencies (B12, folate, thiamine, niacin), Huntington’s disease, alcohol-induced, and the dementia of depression (57).
Characteristics of memory deficits relate to the brain region affected: working memory (frontal), amnesia/episodic memory (temporal/hippocampal), or retrieval memory (subcortical/white matter). Executive dysfunction affects self-organization, planning, decision-making, abstraction, sequencing, multitasking, insight, and judgment. Language impairment may cause difficulties with naming, verbal fluency, comprehension, repetition, reading, and writing. Visuospatial deficits often result in impaired construction abilities (copying figures). In late MCI and early stages of dementia there are difficulties with independent performance of instrumental ADLs such as paying bills, working, participating in community affairs, shopping, and cooking, that subtly but noticeably affect the individual’s ability to function. As the dementia worsens, performing basic ADLs like dressing, feeding oneself, bathing, and toileting require assistance. Table 1 describes key clinical features that characterize the most common dementing disorders.
| Disorder | Temporal Course | Presenting Symptoms | Physical Exam | Neuropathology | Laboratory | Neuroimaging |
|---|---|---|---|---|---|---|
| MOST COMMON | ||||||
| Alzheimer’s Disease | Insidious onset, progressive course, typically in those over 70 but can start in 40s | Memory loss (amnestic) and difficulty with new learning, word-finding difficulty, anomia, visuospatial deficits, impaired insight, dysphoria, anxiety, apathy | Normal exam early; apraxia common later | Cortical atrophy, neuritic plaques (amyloid), neurofibrillary tangles (tau), amyloid angiopathy | Blood: normal or not contributory CSF: decreased amyloid- β1–42 and increased tau and phospho-tau Genetic: polygenic common; APOE-ε4 alleles confer greater risk for sporadic AD; rare autosomal dominant (familial) mutations in genes for APP, PS1 or PS2 | CT or MRI: generalized cortical atrophy, ventricles larger than expected for age MRI: hippocampal and cortical atrophy FDG PET: temporal/parietal hypometabolism Florbetapir PET: positive for brain amyloid |
| Frontotemporal Dementias | Insidious onset, progressive course, varies with subtype, typically in those 45–70 | Personality changes (disinhibition, self-care neglect, obsessive-compulsive) or language disturbance, executive impairment | Normal exam early; apraxia common later | Tau (sometimes as Pick bodies) or ubiquitin and TDP–43 proteins | Blood: normal or not contributory Genetic: familial forms with mutations on chromosome 17 (tau or progranulin gene) or chromosome 9 | CT or MRI: frontal and/or anterior temporal atrophy FDG PET: frontal and/or temporal hypometabolism Florbetapir PET: negative for brain amyloid |
| Vascular Dementia | Abrupt onset and stepwise deterioration for large vessel disease; slowly progressive for small vessel disease | Any cognitive or behavioral pattern; depressed mood common; inattention, retrieval memory complaints common | Focal neurological deficits; gait disturbances; frontal release signs | Ischemic, hemorrhagic or hypoxic lesions; subcortical lacunes, small vessel ischemia, and/or large vessel infarcts; vasculitis; amyloid angiopathy | Blood: normal or evidence of hyperlipidemia or diabetes | CT or MRI: multiple strokes, ischemia, or hemorrhage MRI: subcortical lacunes, periventricular white matter changes, large vessel strokes, or hemorrhage |
| Dementia with Lewy Bodies | Progressive course but onset can appear to be subacute at times given the fluctuations and hallucinations that mimics delirium | Prominent visuospatial deficits, hallucinations, gait disturbance and falls | Extrapyramidal motor signs such as hypokinesia, rigidity and impaired balance | Lewy bodies (alpha-synuclein) | Blood: normal or noncontributory | Dopamine transporter scan: decreased dopamine integrity in basal ganglia Florbetapir PET scan: amyloid present in a substantial subgroup |
| Parkinson’s Disease Dementia | Insidious onset, progressive course | Visuospatial, executive, and retrieval memory deficits begin after long history of Parkinson’s disease | Extrapyramidal motor signs such as hypokinesia, festinating gait, resting tremor, and cogwheeling, and impaired balance | Lewy bodies (alpha-synuclein) | Blood: normal or noncontributory | Dopamine transporter scan: decreased dopamine integrity in basal ganglia Florbetapir PET scan: amyloid present in a small subgroup |
| LESS COMMON | ||||||
| HIV Dementia / Neurocognitive Disorder | Subacute onset, static or progressive course over weeks to months | Mental slowness, impaired concentration, forgetfulness, and apathy | Slowed motor skills, ataxia, weakness, leading to paraplegia | Reactive gliosis, microglial nodules in subcortical white matter and loss of cortical neurons | Blood: Positive for HIV CSF: abnormal but nonspecific | CT or MRI: generalized atrophy, periventricular white matter abnormalities |
| Prion / Creutzfeldt-Jakob Disease | Rapid dementia with death after several months to one year | Fatigue, mood issues, insomnia, memory, executive, visuospatial and language deficits | Startle, myoclonus, tremors, incoordination, gait disturbance | Neuronal loss, vacuolization of gray matter (spongiform appearance), gliosis | CSF: elevated 14–3–3 protein, neuron-specific enloase, total tau, phospho-tau EEG: bilateral periodic discharges | MRI FLAIR: multifocal cortical>subcortical hyperintensities FDG PET: multiple diffuse regions of cortical and subcortical hypometabolism |
| Huntington’s Disease | Gradual decline | Personality changes, executive function abnormalities | Chorea and athetoid movements | Neuronal loss in striatum and at times in cortical regions | Genetic: >26 CAG trinucleotide repeats on short arm of chromosome 4 | CT or MRI: caudate atrophy, boxcar ventricles |
Dementia due to Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common dementia condition (58, 59). Cognitive impairment starts insidiously and has a gradually progressive course without remissions. Onset is rarely before age 50 for sporadic AD, and incidence increases with every decade of life. Familial AD is genetic and has onset in early adulthood. Earliest cognitive deficits involve episodic memory loss (related to hippocampal impairments), impaired insight, anomia, visuospatial impairments, and disorientation. The pattern of memory loss in AD patients is characterized by impaired delayed recall of word lists even after clues are provided (amnestic memory pattern). Noncognitive neuropsychiatric disturbances are common and typically involve apathy, irritability, agitation, restless behaviors, anxiety, and depression, which may occur at any stage of the disease, while psychosis and aggression are more common later in the disease course. Progressive functional decline and apraxia precede death about 8 to 12 years after diagnosis.
Factors other than age and genetics that increase the risk of AD include head trauma, female sex, race/ethnicity, hypertension, obesity, diabetes, depression, smoking, use of progestin/estrogen medication, and cerebrovascular disease (60). Potential nongenetic protective factors against developing AD include mental and physical exercise, and higher education or cognitive reserve (60). Individuals who are able to improve any of these modifiable risk factors may reduce their risk of developing AD (61).
Pathology reveals accumulation of beta-amyloid proteins deposited in neuritic plaques outside of neurons and intraneuronal accumulation of hyperphosphorylated tau proteins twisted into neurofibrillary tangles (62). The proteinopathy of AD accumulates in specific neuroanatomical regions. Beta-amyloid plaques are initially found in the neocortex before accumulation in the hippocampus, while tau proteins (forming neurofibrillary tangles) initially accumulate in the entorhinal cortex and hippocampal region, posterior cingulate, and precuneus, and progress in characteristic fashion along known neural pathways to involve the frontal, temporal, and parietal cortical regions. The neuropathology of AD begins many years prior to symptoms appearing and it may be nearly 30 years before dementia occurs (63). Polygenetic inheritance in sporadic AD is common, whereas in dominantly inherited familial AD, specific genes have been identified. Apolipoprotein-E (APOE) genotype with ε4 allele(s) confers increased risk of AD and at an earlier age while the ε2 allele(s) decrease the risk for Alzheimer’s disease (64). While APOE genotype is often used in research trials, it is seldom used in clinical practice, as it is not useful to rule in or out the disease.
Accurate diagnosis requires having the typical clinical features and ruling out other medical conditions with lab tests and structural neuroimaging (CT or MRI). If the clinical features are unclear, atypical, or mixed, then adjunctive evaluation of CSF for amyloid and tau levels, amyloid-positron emission tomography (PET) neuroimaging for amyloid plaque density, or fluorodeoxyglucose (FDG) PET neuroimaging for hypometabolism in the parietal and temporal regions can be useful for diagnosis.
Rarer conditions that are in the AD spectrum are posterior cortical atrophy, which progressively affects visual processing skills with predominant degeneration of posterior regions (65), and logopenic progressive aphasia, with prominent word-finding difficulty and other language features (66). Current treatments are all symptomatic and include cholinesterase inhibitors (donepezil, rivastigmine, galantamine) and NMDA antagonists (memantine). Symptomatic treatments improve symptoms but do not alter the accumulation of amyloid and tau proteins, which are toxic to neurons. Current research is heavily focused on disease modification aimed at reducing the burden of tau and amyloid proteins affecting brain function. While there are no FDA-approved therapies for dementia-related behaviors, caregiver education, environmental manipulation, and psychopharmacological treatment are often needed.
Vascular Dementia
The prevalence of vascular dementia (VD) is highly dependent on the comorbid frequencies of hypertension, diabetes, and hyperlipidemia that exist in the population being evaluated (67). Onset can be abrupt with focal neurological deficits and a stepwise course of deterioration if large vessel strokes/hemorrhages are prominent or slowly progressive with gait and cognitive loss when small vessel disease predominates. With small vessel disease, attentional and executive deficits are common, and the pattern of memory loss is characterized by impaired delayed recall of word lists that improves significantly when clues are provided (so-called retrieval memory pattern). Clinical presentation depends on the extent, location (cortical-subcortical), and persistence of vascular lesions (ischemic, hemorrhagic) (68). Impaired judgment or poor planning ability may predominate over memory deficits, reflecting frontal or subcortical influence on executive function. Depression is also very common. MRI neuroimaging helps to best define the etiology and location of the lesion(s): multi-infarct dementia (multiple cortical large vessel and small vessel vascular infarcts); subcortical ischemic vascular dementia (small vessel vascular ischemia or infarcts); hemorrhagic dementia; or strategic-infarct dementia (dominant hemisphere parietal/temporal infarct). Hypoperfusion dementia and dementia caused by specific arteriopathies, including cerebral amyloid angiopathy, may not be obvious on a brain MRI scan.
Progression of the cognitive impairment can be lessened by interventions aimed toward stroke prevention, such as elimination of stroke risk factors (hypertension, smoking, diabetes, hyperlipidemia), elimination of sources of emboli, and treatment with antiplatelet agents or, if indicated, anticoagulants. Vascular dementia is a common comorbidity with AD (mixed dementia) (69). Mixed dementia presents with mixed clinical symptoms, where either diagnostic component may be missed depending on which diagnosis is first considered, and both structural and amyloid neuroimaging may be needed to make the diagnosis.
Dementia With Lewy Bodies
Lewy body pathology dementias comprise the second most common neurodegenerative dementia type and include two presentations: dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD) (70). DLB may account for 10% to 15% of dementia cases based on autopsy studies (71, 72). Patients must meet criteria for a dementia condition that began prior to or concurrently within a year of extrapyramidal signs. Core diagnostic features of DLB include fluctuation in cognitive abilities with marked variations in attention and alertness, recurrent visual hallucinations (seen in about 40%), and spontaneous features of parkinsonism (bradykinesia and rigidity, with tremors less common) (73). Suggestive features include REM sleep behavior disorder, severe neuroleptic sensitivity (even resembling neuroleptic malignant syndrome) and low dopamine transport uptake in the basal ganglia on single photon emission computed tomography (SPECT) or PET. If two of the three core features or one core feature and at least one suggestive feature are present, probable DLB can be diagnosed.
DLB and PDD display some similar symptoms including syncope, repeated falls, systematized delusions, excessive sleepiness, and obstructive sleep apnea which occur more frequently than in AD patients. DLB cases may have some features of AD but with prominent extrapyramidal signs, especially rigidity (73). Cognitive deficits are most prominent on tests of attention, retrieval memory, executive function, and visuospatial ability (71, 72) and differ from AD where memory impairment is episodic (hippocampal) and inattention occurs late in the disease. Olfactory dysfunction, constipation, and increased salivation occur early as compared with AD (74). The fluctuations seen in DLB along with visual hallucinations and sleep disturbances can be mistaken for delirium, and careful observation for the other core features of each condition is required for accurate diagnosis. DLB progresses more rapidly than AD, often leading to death in 6 to 8 years.
Neuropathology reveals cortical and subcortical Lewy bodies, although in a substantial number of patients amyloid pathology is also seen (75–77). Low dopamine transporter uptake in the basal ganglia on single photon emission computed tomography (SPECT) or PET is supportive of the diagnosis (and of Parkinson’s). Alhough there are no FDA-approved treatments for the cognitive, behavioral, or motor features of DLB, cholinesterase inhibitors and levodopa may be beneficial, whereas neuroleptics are generally avoided unless psychosis is otherwise not responsive to cholinesterase inhibitors (72, 78). Clinicians using antipsychotic medications must start at very low doses to avoid the potential morbidity and even mortality related to the severe neuroleptic sensitivity seen in some DLB patients.
Parkinson’s Disease Dementia
Parkinson’s disease (PD) must be diagnosed a year or more (mean approximately 10 years) prior to the onset of the dementia (70, 72, 79) in order to diagnose PDD. Clinical motor features in PDD typically include extrapyramidal signs (bradykinesia, rigidity, resting tremors) and postural instability. Cognitive testing characteristically shows impaired attention, executive dysfunction, visuospatial disturbances, and retrieval memory impairment. Apathy, depression, anxiety, visual hallucinations, delusions, and excessive daytime somnolence are common neuropsychiatric symptoms, which can reflect subcortical and cortical pathology or medication adverse effects (e.g., dopaminergic).
Alpha-synuclein proteins accumulate in the nuclei of neurons and form into Lewy bodies located in subcortical regions on neuropathology. Dopamine deficiency underlies PDD and can be detected using the dopamine transporter SPECT scan in early and unclear cases (80). Although less common than mixed AD and DLB, patients with PDD can have coexisting AD neuropathology (81), which complicates diagnosis and may produce a mixed dementia syndrome clinically.
Less commonly seen extrapyramidal disorders in the differential diagnosis include progressive supranuclear palsy, which has a faster course of decline, frontal dementia syndrome, vertical gaze palsy, postural instability, and tau pathology (82). Corticobasal degeneration is very rare, with severe rigidity, asymmetric limb apraxia, postural instability, progressive dementia, and poor response to levodopa treatment.
Frontotemporal Dementia
Frontotemporal dementia (FTD), also known as frontotemporal lobar degeneration (see Table 1) is the third most common neurodegenerative dementia with onset between 45 and 70 years of age, typically younger than AD. FTD is characterized by progressive early behavioral changes in personality and executive function or by progressive language difficulties (83, 84). When abulia and apathy occur in FTD they can be misattributed to depression. However, lack of guilt, dysphoria, and sadness point toward FTD. FTD is not a single disease but rather the moniker for a heterogeneous group of disorders including Pick’s disease. It has been associated, as well, in those with motor neuron disease (e.g., amyotrophic lateral sclerosis). There are three main recognized forms of FTD:
| 1. | Behavioral variant (bvFTD) is characterized by disinhibition, social dysdecorum, self-absorption, mental inflexibility, obsessive-compulsive traits, neglect of self-care, and executive impairment (poor planning, organization, problem solving and judgment). Right or bilateral frontal involvement is common. | ||||
| 2. | In progressive nonfluent aphasia (PNFA), patients speak in shorter and shorter phrase lengths, develop stereotyped output and eventually become mute. Memory and executive impairments are common. Left frontal involvement occurs before the right. | ||||
| 3. | Semantic dementia (SD) patients progressively develop loss of word meaning with the inability to recognize the correct name for objects and have stereotyped verbal output. Involvement of left anterior temporal cortex precedes the right. | ||||
For all the FTD conditions, retrieval memory deficits are typical, while calculations and visuospatial skills are relatively spared early in the course in contrast to AD. Personality changes occur early in FTD in contrast to AD. Tau (found in Pick’s disease, bvFTD, and most PNFA cases) and ubiquitin with TDP−43 proteins (found in bvFTD and most SD cases) are the most common proteinopathies noted (84). Familial FTD has been associated mostly with mutations on chromosome 17 (tau or progranulin genes) and chromosome 9 (hexonucleotide repeat). Accurate diagnosis requires having the typical clinical features and ruling out other conditions with lab tests and standard neuroimaging (CT or MRI). If the clinical features are unclear, atypical, or mixed, evaluating for brain hypometabolism (often asymmetrical) in the frontal and/or anterior temporal regions using FDG PET imaging can help confirm the diagnosis. Sometimes the frontal variant of AD presents as FTD and an amyloid PET scan can identify this pathology. While there are no FDA-approved therapies for FTD, supportive management and treatment of behavioral issues are critical. Cholinesterase inhibitors and memantine are not efficacious in FTD (85–87), although serotonergic antidepressants may have some benefit.
Office Evaluation of Depression, Delirium and Dementing Disorders
Depression and delirium need to be identified and managed prior to embarking on a workup for a dementing process (Figure 1). After treatment occurs for depression and/or delirium, if symptoms remain, the patient should be evaluated for a possible dementing process. A substantial stroke can result in an initial delirium that clears to reveal a significant depression and a more permanent cognitive problem. Isolated depressive symptoms that are subthreshold to a major depression may be secondary to a delirium, a dementing process or another medical/neurological condition.
Subtle cognitive deficits that are stable over a period of months and temporally related to mood changes suggest possible depression. Acute onset of cognitive impairment suggests delirium or a major neurological insult such as stroke. Fluctuating cognitive impairment suggests delirium or DLB. Chronic mild cognitive deficits suggest MCI or mild NCD or subsyndromal delirium. A rapid trajectory of decline but without all the features of delirium suggests a prion neurodegenerative process. Gradual persistent cognitive decline with increasing functional impairment suggests a neurodegenerative dementing process.
In addition to a careful history with an informant, the next step is formal mental status testing and a physical examination with an emphasis on measuring mood, behaviors, cognition, function (ADLs), and neurological signs. Administration of standardized scales for depression or delirium and cognitive tests objectively measure severity and patterns of symptoms. Tests can be repeated over time to monitor the patient’s trajectory. Findings on physical, laboratory, and neuroimaging examinations can help identify underlying medical conditions that may be causative.
Once depression and delirium are ruled out or managed, the diagnosis of dementia commences and involves a multistep approach. Additional medical history and physical examination may need to be performed, including assessment of risk factors and early warning signs (2, 57). Results from office-based cognitive and standard laboratory tests may need to be supplemented by formal neuropsychological testing, neuroimaging, and cerebrospinal fluid (CSF) examination to enable a more specific diagnosis. Some degenerative disorders are not well detected with current methods (e.g., tauopathies). The presentation of dementia symptoms may differ based on the nature of affected brain regions and stage of progression of the underlying neuropathology both inter- and intraindividually. Nonetheless, characteristic phenotypes (see disease state sections) can provide some direction to the order of tests performed in an evaluation. It is important to be aware that more than one etiology for a dementing process may be present so that mixed dementias need to be considered. AD mixed with either vascular or DLB etiologies is not uncommon and produces atypical clinical presentations.
Office-Based Cognitive Assessment
Objective assessment of cognitive abilities is an initial step in differential diagnosis. The cognitive loss in those developing MCI and degenerative dementia usually starts insidiously. These patients may have impaired insight and typically do not complain of significant memory loss, and casual conversation with their physician does not uncover deficits. Consequently, these patients present to a physician an average of 3 to 4 years after cognitive symptoms began (88). Screening for cognitive impairment in physician offices may help with detection in older at-risk patients (89). Each person has different natural talents and will have different baseline scores on their cognitive testing. Therefore, it would be ideal for every senior to have screening cognitive assessments prior to any decline in their cognitive abilities. This may practically be performed in the U.S. during the Medicare Annual Wellness Visit (90).
It is important to distinguish among cognitive screening (not in response to a cognitive complaint), office-based multidomain cognitive assessments to aid diagnosis, and formal neuropsychological testing also to aid diagnosis (performed by computer, neuropsychologist or practitioner). Patients with subtle or atypical patterns of cognitive impairment may require neuropsychological testing, whereas for those with mild to moderate cognitive symptoms, an office-based multidomain test may suffice.
When evaluating cognitive decline, the first step is objective assessment appropriate to the suspected condition. For example, delirium may be tested using simple tests of attention such as having the individual repeat a series of numbers or recite the months of the year backward, whereas depression has subtler deficits requiring more challenging tests. In MCI or dementia, patterns of deficits can be helpful in the total patient evaluation. For example, deficits in executive function suggest prefrontal dysfunction, and deficits on word list learning tests suggests hippocampal dysfunction. However, cognitive tests alone cannot determine the etiology of a delirium or dementia process.
A multidomain brief cognitive test (see Table 2) such as the well-known Mini-Mental State Exam (MMSE) (91), the Montreal Cognitive Assessment (MoCA) (92), Saint Louis University Mental Status (SLUMS) (93), or the Self-Administered Gerocognitive Examination (SAGE) (94) is an easy way to obtain a standardized score across a range of cognitive domains and takes about 10 to 15 minutes to complete. While they alone cannot distinguish among the 3 D’s, they offer a quick measure of severity of overall cognitive impairment. Delirium and dementia patients would be expected to score lower than those with MCI or depression.
| Features | MMSE | MoCA | AD8 | SLUMS | SAGE |
|---|---|---|---|---|---|
| Scoring range | 0–30 (higher score better) | 0–30 (higher score better) | 0–8 (score >2 indicates impairment) | 0–30 (higher score better) | 0–22 (higher score better) |
| Domains tested | Orientation, attention, comprehension, calculations, memory, language, constructions | Orientation, memory, clock, constructions, fluency, language, abstraction, calculations, executive, attention | Informant rating of patient’s judgment, interests, memory, ADLs, orientation | Orientation, memory, animal fluency, attention, clock drawing | Orientation, language, calculations, memory, abstraction, executive, constructional ability |
| Administration | Clinician with patient | Clinician with patient | Clinician with informant and patient | Clinician with patient | Patient (self-administered) |
| Advantages | Well-known scale; used also in delirium but cannot distinguish delirium and dementia; often used as proxy to stage dementia severity | Less ceiling effect than MMSE due to greater difficulty and more executive function tests; more sensitive to mild impairments than MMSE; used in delirium also | More aimed at dementia than mild stages; not for use in delirium | Less ceiling effect than MMSE due to greater difficulty; emphasizes memory tasks; different cutoffs for Mild NCD and dementia | Done without clinician; good correlation to clinician-administered tests; less ceiling effect than MMSE due to greater difficulty and more executive function tests; able to distinguish between MCI and dementia |
| Pitfalls | Ceiling effect especially in more educated patients; executive testing limited, does not distinguish between MCI and dementia | Cannot distinguish between MCI and dementia | Does not measure cognition directly | Less evaluation of language, constructions, and executive abilities than MoCA or SAGE | Memory testing limited |
| Time to administer | 7–10 minutes | 10–13 minutes | 3 minutes | 10 minutes | 10–15 minutes |
| Cost | $1.23 to PAR | free | free | free | free |
| Specificity/ sensitivity to detect dementia | 84%/78% with cutoff 26 or less (122) | 87%/100% with cutoff of 25 or less (92) | 80%/84% with cutoff 2 or more (123) | Comparable to MMSE but better at detecting mild NCD (93) | 95%/79% with a cutoff of 16 or less to detect cognitive impairment and 95%/95% with a cutoff of 16 or less to detect dementia (94) |
| Obtaining test | Psychological Assessment Resources (PAR) | Mocatest.org | http://alzheimer.wustl.edu/about_us/pdfs/ad8form2005.pdf | medschool.slu.edu/aging successfully/pdfsurveys/slumsexam_05.pdf | sagetest.osu.edu |
Depressed patients will have subtle cognitive deficits where their complaints may be out of proportion to the actual deficits on objective testing. An office-based screening test may be suitable for initial testing. Follow-up for unresolved deficits after the depression has remitted is recommended. Delirium is defined by attentional deficits that, if severe, make it difficult to test other cognitive domains.
Office-based cognitive tests for MCI need to be sensitive enough to detect early deficits. For example, the MoCA can measure executive function and the impairments in language and memory often found in early stages of AD better than the MMSE (95). The SLUMS has some similarities to the MoCA but has more items related to memory and fewer to language, executive, and visuospatial tasks.
The SAGE can identify both MCI and early dementia (94). The SAGE is unique in that it is self-administered in pen and paper format and does not require staff time to administer. It has four equivalent interchangeable forms. It can be used for cognitive screening or as an office-based multidomain test.
The AD-8 is an 8-item informant-based dementia screening questionnaire inquiring about the presence or absence of cognitive and functional symptoms but does not directly measure cognition (96). Scores of 2 points or more suggest cognitive impairment. Using amyloid biomarkers, the AD-8 was found superior in detecting dementia of the Alzheimer’s type than the MMSE (97). Simple word list learning tests like the Memory Impairment Screen (MIS) and animal fluency have been advocated in primary care settings as a cognitive screen followed by more sensitive tests (98).
Laboratory and Neuroimaging Testing
Table 3 lists types of laboratory and neuroimaging tests that are performed during differential diagnosis for depression, delirium, and dementing disorders. Blood and urine laboratory tests are similar when evaluating among the 3D’s and serve to help determine underlying medical problems causing a delirium or reversible causes of cognitive impairment such as vitamin deficiency, urinary tract infections, and hypothyroidism. These are performed to identify and manage possibly reversible conditions prior to obtaining CSF examination and neuroimaging, the latter being reserved for when there is suspicion of a CNS abnormality that is causative for the symptoms. It is not uncommon to obtain structural neuroimaging (CT or MRI) in delirium and dementia but less common for depression. Depending on the dementia etiology suspected, an MRI may often be the wiser choice because it detects more subtle abnormalities such as white matter hyperintensities that are associated with slow information processing speed. MRI is very good at detecting abnormalities such as strokes, tumors, hematomas, infectious cysts, inflammation, hemorrhages, and subtle lesions in the brainstem. Although MRI may detect cortical or hippocampal atrophy patterns and ventricular dilation associated with some dementias (e.g., AD, FTD), it is unable to reliably determine the cause for degenerative dementias such as AD, DLB, PDD, and FTD which require specific molecular detection methods (e.g., of beta amyloid, tau, dopamine, and so on). Because a structural brain scan (e.g., MRI) can provide information about a wide variety of lesions that might be attributed to the cognitive impairment, it is always ordered prior to PET scans or CSF examination. However, because conditions can be comorbid—for example, AD and small strokes—both MRI and PET may be ordered to more fully evaluate the underlying etiologies to differentially diagnose the individual patient.
| Test | Major Depression | Delirium | Mild and Major Neurocognitive Disorders due to Neurodegeneration |
|---|---|---|---|
| Urinalysis | X | X | X |
| Blood | |||
| Hematology | X | X | X |
| Electrolytes, calcium, magnesium, phosphorus | X | X | |
| BUN and creatinine | X | X | |
| Fasting blood sugar | X | X | |
| Liver function testing | X | X | |
| Lipid profile | X | ||
| B12 and folate | X | X | X |
| TSH | X | X | X |
| Sedimentation rate | X | X | |
| Syphilis testing | X | X | |
| APOE | Typically for AD research | ||
| CSF | |||
| WBCs | As needed | ||
| VDRL | As needed | ||
| Amyloid-betab | If questioning AD | ||
| Tau and p-taub | If questioning AD | ||
| MRI | |||
| Structural | X | X | |
| Hippocampal volume | If questioning AD | ||
| Periventricular white matter changes | X | ||
| Florbetapir PET | If questioning AD | ||
| FDG PET | For differentiating AD and FTD | ||
| Dopamine transporter SPECT | If questioning DLB or Parkinson’s | ||
| Electroencephalogram | If clinical picture is unclear |
The question often arises as to whether, when, and in what order to obtain CSF and other neuroimaging tests on an individual patient (Figure 1). Unclear, mixed and atypical findings may precipitate consideration of these additional evaluations. According to Jack et al. (99), amyloid is the earliest biomarker that can be detected as abnormal in those with AD. There is a progression of biomarkers that appear later that can be detected after amyloid in those with AD pathology: markers of neurodegeneration using MRI looking for cortical and hippocampal atrophy and metabolic defects that may reflect reduced synaptic activity by using FDG PET.
PET neuroimaging available clinically includes amyloid plaque detection, an important marker of AD neuropathology, and FDG PET to measure brain metabolism. Florbetapir-F18 PET is currently the only FDA-approved amyloid PET radiotracer. Florbetapir specifically labels and estimates the burden of beta amyloid plaques (positive scan indicates moderate to frequent plaques and negative scan indicates none to sparse plaques) that are found in patients with AD and some other amyloidogenic conditions (100). (See also Appropriate Use Criteria for amyloid PET [101]). Pittsburgh Imaging Compound-C11 (PIB) also labels amyloid plaques but is used in research settings. Amyloid PET should be considered only in those with documented cognitive deficits suspected of being due to AD and interpreted in the context of an individual patient’s other clinical information. A percentage of normal older persons can have positive amyloid PET scans where the prognostic value is unclear at this time, although those with amyloid have poorer neuropsychological performance than those without it (102, 103). Emerging research also finds faster trajectories of decline in those who have amyloid pathology than those who do not (104–107).
Those with atypical presentations often have AD pathology that can be detected using amyloid PET while their metabolic patterns on FDG PET reflect the clinical phenotype (110). Mixed VD is most likely to be comorbid with AD than with other neurodegenerative diseases (69), where both MRI and amyloid PET can be applied. FDG PET detects frontal hypometabolism that underlies FTD but also in frontal variant AD that presents with prominent executive dysfunction and can be mistaken for FTD (111), in which case amyloid PET may also be needed.
Figure 2 displays results of MRI, FDG PET, and florbetapir PET scans from two patients diagnosed as either FTD or AD to show how they contribute different types of information about the brain. In AD the MRI shows more generalized cortical atrophy often more prominent in hippocampal regions, a temporoparietal pattern of metabolic defect on FDG PET, and abnormal levels of amyloid plaque on florbetapir PET. In FTD the MRI shows frontotemporal cortical atrophy, FDG PET shows metabolic defects in anterior regions, and florbetapir PET does not show abnormal levels of amyloid plaque.
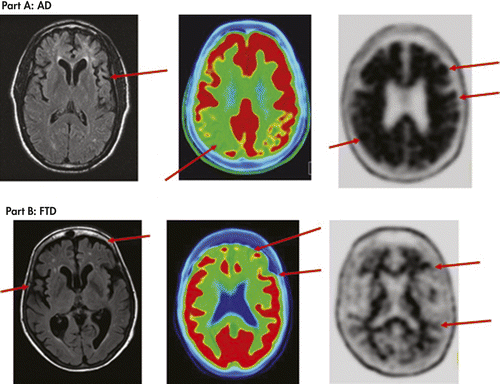
Findings shown by three types of neuroimaging (MRI, amyloid PET and FDG PET scans) in two case examples. Arrows denote key findings. Part A is a patient with dementia due to Alzheimer’s disease. MRI FLAIR transverse image shows diffuse cortical atrophy consistent with gray matter volume loss due to neurodegeneration. FDG PET fused with a CT scan image, where red areas represent the highest metabolism, shows reduced red in the posterior temporal-parietal cortical region (greater on the right), a hypometabolism pattern consistent with Alzheimer’s disease. Florbetapir F18 PET image shows black in the cortical gray matter and a loss of the gray/white matter border in more than two cortical regions, consistent with moderate to frequent density of cortical amyloid plaques and a pathological diagnosis of Alzheimer’s disease. Part B is a patient with frontotemporal dementia. MRI FLAIR transverse image shows significant bilateral atrophy of frontal and temporal cortices. FDG PET fused with CT scan image shows bilateral frontal and anterior temporal cortical areas with decreased red as compared with more posterior regions, a pattern of hypometabolism consistent with clinical diagnosis of frontotemporal dementia. Florbetapir F18 PET scan is negative for amyloid plaques (having none to sparse density), and shows white matter pathways (in black) and clear borders between white matter pathways and cortical gray matter (in gray), which is inconsistent with a pathological diagnosis of Alzheimer’s disease.
When extrapyramidal motor symptoms are present and PDD or DLB are in the differential diagnosis, a SPECT scan that labels the dopamine transporter can detect reduced dopamine integrity in the striatum. Because DLB and PD can have coexisting Alzheimer’s pathology, an amyloid PET scan may also be useful for amyloid detection (76). Rates of AD are much higher in DLB than PD patients (75) and amyloid presence may impact disability (77).
CSF assays of biomarkers for AD (Amyloid-beta 42 and tau levels) as well as more routinely performed CSF assays for blood cells and immunoglobulins, proteins, bacteria, and viruses can be adjunctive in the diagnostic process depending on the suspected etiologies. Low levels of amyloid-beta 42 (Aβ42) and high levels of tau and phosphorylated tau in the CSF are consistent with AD. CSF assays for AD biomarkers still need more standardization and have not been approved by the FDA (112). CSF Aβ42 has high correlations with amyloid PET (107). Referral to a dementia clinic for CSF examination when appropriate is recommended.
Further, genomic testing is useful for certain conditions such as Huntington’s disease. Apolipoprotein E epsilon 4 allele has been associated with greater risk and earlier onset of sporadic AD but itself is insufficient for a diagnosis of AD. Autosomal dominant genes implicated in familial AD include mutations of APP, PS1 and PS2 genes. Certain genetic biomarkers are being established for FTD (see Table 1).
Other Neuropsychiatric Office Tests
When delirium is suspected, in addition to cognitive testing, a more complete assessment of the range of delirium symptoms can be accomplished using validated tools like the 16-item Delirium Rating Scale-Revised−98 (DRS-R98) (113) for diagnosis and severity ratings or the Memorial Delirium Assessment Scale (MDAS) (114) for symptom severity rating, both designed for use by psychiatrists. Patients who are not rousable for interview may instead be stuporous or sedated by medications, requiring the evaluation be delayed until sedation is reduced or level of arousal improved to allow consciousness to be evaluated for presence of delirium symptoms.
Routine use of a symptom checklist or depression rating scale such as the Geriatric Depression Scale (GDS) (115) and maintaining a high index of suspicion for atypical depression is important, rather than simply accepting the patient or care partner’s report of “depression.”
The Clinical Dementia Rating (CDR) scale is a global measure where the clinician rates the patient’s orientation, executive skills, memory, and functional abilities after a careful evaluation (116). The score ranges from 0 to 3 with 0.5 suggesting mild cognitive impairment or very early dementia and 1 or more suggesting a dementing condition.
The Neuropsychiatric Inventory-Community version (NPI-Q) (117) can be completed by an observer or caregiver who knows the patient. It rates severity of 12 items on a 3-point Likert scale: dysphoria/depression, irritability/lability, anxiety, euphoria/elation, apathy/indifference, aberrant motor behavior, agitation/aggression, disinhibition, delusions, hallucinations, nighttime disturbances, and appetite/eating disturbances. These noncognitive neuropsychiatric symptoms are commonly associated with dementing conditions, where symptom patterns may vary depending on the brain regions affected by the disease.
The Functional Activities Questionnaire (FAQ) (118) is a validated tool that is easy to use in office settings to rate 10 Independent Activities of Daily Living and some basic ADL items on a severity scale from 0 to 3 that can be completed by the patient or the informant. Scores range from 0 to 30 and those greater than 6 may indicate functional impairment in Independent Activities of Daily Living (119). The FAQ can be used across the range of cognitive impairment from milder to full dementia stages.
The Mayo Fluctuations Scale can assist with the quantification of fluctuations in symptom severity when DLB is in the differential diagnosis, where fluctuation is a core defining feature (120).
The modified Hachinski index (121) is a short scale capturing the essence of stepwise deterioration of vascular disease that can lead to cognitive impairment. Scores ≤4 are consistent with AD and scores ≥7 points are consistent with a likely ischemic origin of symptoms and can be adjunctive in evaluation of possible vascular dementia.
Conclusions
The information provided in this article discusses common disorders impairing cognition that may present in patients coming to the general psychiatrist’s office and practical ways to identify and diagnose these conditions in an office-based practice. The demographic shift toward a growing elder population means many more physicians beyond dementia specialists will be needed to perform thorough assessments to identify and diagnose cognitive impairments, especially at their earlier stages when management and interventions targeted at the disease, the patient’s welfare and functioning, and the family’s needs can make an enormous difference in the lives of patients. Many patients and care partners want to know the specific diagnosis of their cognitive impairment, even if it is a dementia diagnosis, and psychiatric training is well-suited to conduct both the neuropsychiatric evaluations and medical discussions that are sensitive.
1 ;
2 ;
3 ;
4 : Islington study of dementia subtypes in the community. Br J Psychiatry 2002; 180:270–276Crossref, Google Scholar
5 : Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012; 11:1006–1012Crossref, Google Scholar
6 : Delirium, in American Psychiatric Publishing Textbook of Psychosomatic Medicine, 2nd ed. Edited by Levenson J. Washington, DC, American Psychiatric Publishing, 2011, pp 71–114Google Scholar
7 : Prevalence of delirium among outpatients with dementia. International Psychogeriatrics, available at CJO2013. DOI: 10.1017/S1041610213001191Google Scholar
8
9 : Caring for older adults with mild cognitive impairment: an update for nurses. J Gerontol Nurs 2012; 38:22–35, quiz 36–37Crossref, Google Scholar
10 ;
11 : Diagnostic evaluation of dementia in the secondary health care sector. Dement Geriatr Cogn Disord 2009; 27:534–542Crossref, Google Scholar
12 : Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology 2001; 57:226–234Crossref, Google Scholar
13 : Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol 2012; 71:266–273Crossref, Google Scholar
14 : Diagnosing dementia: interrater reliability assessment and accuracy of the NINCDS/ADRDA criteria versus CERAD histopathological criteria for Alzheimer’s disease. Dement Geriatr Cogn Disord 2000; 11:107–113Crossref, Google Scholar
15 : Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement 2013; 9:414–421Crossref, Google Scholar
16 : The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med 2003; 163:2219–2229Crossref, Google Scholar
17 : Would you like to know what is wrong with you? On telling the truth to patients with dementia. J Med Ethics 2000; 26:108–113Crossref, Google Scholar
18 : Expectations, experiences, and tensions in the memory clinic: the process of diagnosis disclosure of dementia within a triad. Int Psychogeriatr 2012; 24:1756–1770Crossref, Google Scholar
19 : Early identification and treatment of Alzheimer’s disease: social and fiscal outcomes. Alzheimers Dement 2009; 5:215–226Crossref, Google Scholar
20 : Caregivers’ perspectives on the pre-diagnostic period in early onset dementia: a long and winding road. Int Psychogeriatr 2011; 23:1393–1404Crossref, Google Scholar
21 : Do older adults presenting with memory complaints wish to be told if later diagnosed with Alzheimer’s disease? Int J Geriatr Psychiatry 2006; 21:419–425Crossref, Google Scholar
22 : Delays in the diagnosis of dementia: perspectives of family caregivers. Am J Alzheimers Dis Other Demen 1999; 14:20–26Crossref, Google Scholar
23 : The pathway to dementia diagnosis. Med J Aust 2008; 189:487–489Crossref, Google Scholar
24 ;
25 ;
26 : Diagnosing cognitive impairment and dementia in primary health care — a more active approach is needed. Age Ageing 2003; 32:606–612Crossref, Google Scholar
27 : Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord 2009; 23:306–314Crossref, Google Scholar
28 ;
29 : Apathy: prevalence, associated factors, and prognostic value among frail, older inpatients. J Am Med Dir Assoc 2012; 13:541–545Crossref, Google Scholar
30 : Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 2008; 65:1193–1198Crossref, Google Scholar
31 : Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002; 288:1475–1483Crossref, Google Scholar
32 : Subtypes of depression among patients with Alzheimer’s disease and other dementias. Alzheimers Dement 2010; 6:63–69Crossref, Google Scholar
33 : Blowing hot and cold over depression and cognitive impairment. Neurology 2010; 75:12–14Crossref, Google Scholar
34 : Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry 2002; 10:125–128Crossref, Google Scholar
35 : A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatry 2003; 160:857–866Crossref, Google Scholar
36 : Depression and incident Alzheimer disease: the impact of disease severity. Am J Geriatr Psychiatry (Epub ahead of print; doi: 10.1016/j.jagp.2013.02.011)Google Scholar
37 : The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry 2013; 21:685–695. Available at doi: 10.1016/j.jagp.2013.01.006Crossref, Google Scholar
38 : Cognitive impairment in major depression. Eur J Pharmacol 2010; 626(1):83–86Google Scholar
39 : Phenomenology of delirium: assessment of 100 adult cases using standardised measures. Br J Psychiatry 2007; 190:135–141Crossref, Google Scholar
40 : Three core domains of delirium validated using exploratory and confirmatory factor analyses. Psychosomatics 2013; 54:227–238Crossref, Google Scholar
41 : Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med 2009; 37:177–183Crossref, Google Scholar
42 : Phenotype of subsyndromal delirium using pooled multicultural Delirium Rating Scale—Revised-98 data. J Psychosom Res 2012; 73:10–17Crossref, Google Scholar
43 : Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc 2010; 58:643–649Crossref, Google Scholar
44 : Impact of delirium on distress, health-related quality of life, and cognition 6 months and 1 year after hematopoietic cell transplant. Biol Blood Marrow Transplant 2010; 16:824–831Crossref, Google Scholar
45 : Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med 2010; 56:244–252, e1Crossref, Google Scholar
46 : The overlap syndrome of depression and delirium in older hospitalized patients. J Am Geriatr Soc 2009; 57:1347–1353Crossref, Google Scholar
47 : Subtle attentional deficits in the absence of dementia are associated with an increased risk of post-operative delirium. Dement Geriatr Cogn Disord 2007; 23:390–394Crossref, Google Scholar
48 : Impaired executive function is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc 2006; 54:937–941Crossref, Google Scholar
49 : Validating the diagnosis of delirium and evaluating its association with deterioration over a one-year period. Am J Geriatr Psychiatry 2001; 9:148–159Crossref, Google Scholar
50 : Subsyndromal delirium in older people: a systematic review of frequency, risk factors, course and outcomes. Int J Geriatr Psychiatry 2013; 28:771–780Crossref, Google Scholar
51 : Amyloid imaging as a biomarker for cerebral β-amyloidosis and risk prediction for Alzheimer dementia. Neurobiol Aging 2011; 32(Suppl 1):S20–S36Crossref, Google Scholar
52 : The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279Crossref, Google Scholar
53 : Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256:183–194Crossref, Google Scholar
54 : Cognitive interventions targeting subjective cognitive complaints. Am J Alzheimers Dis Other Demen 2013; 28:560–567Crossref, Google Scholar
55 : Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006; 67:834–842Crossref, Google Scholar
56 : A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging 2012; 29:639–658Google Scholar
57 ;
58 : Alzheimer’s disease: a clinical practice-oriented review. Front Neurol (Epub ahead of print April 20, 2012; doi: 10.3389/fneur.2012.00063Google Scholar
59 : Changing concepts of Alzheimer disease. JAMA 2011; 305:2458–2459Crossref, Google Scholar
60 : Scope of dementia: epidemiology and public health impact, in Long-Term Management of Dementia. Edited by Scharre DW. New York, Informa Healthcare, 2010, pp 1–24Google Scholar
61 : The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011; 10:819–828Crossref, Google Scholar
62 : The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:263–269Crossref, Google Scholar
63 ;
64 :.Genetics of Alzheimer’s disease. Biomed Res Int 2013; 2013:254954Google Scholar
65 : Shining a light on posterior cortical atrophy. Alzheimers Dement 2013; 9:463–465Crossref, Google Scholar
66 : FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer’s type. PLoS ONE 2013; 8:e62471 www.plosone.orgCrossref, Google Scholar
67 : Vascular dementia: distinguishing characteristics, treatment, and prevention. J Am Geriatr Soc 2003; 51(Suppl Dementia):S296–S304Crossref, Google Scholar
68 : Vascular cognitive impairment. Lancet Neurol 2003; 2:89–98 [Review]Crossref, Google Scholar
69 : Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013; ePub ahead of print. DOI: 10.1093/brain/awt188Google Scholar
70 ;
71 : Advances in the diagnosis and treatment of dementia with Lewy bodies. Introduction. Dement Geriatr Cogn Disord 2004; 17(Suppl 1):1–2Crossref, Google Scholar
72 ;
73 : More severe functional impairment in dementia with lewy bodies than Alzheimer disease is related to extrapyramidal motor dysfunction. Am J Geriatr Psychiatry 2006; 14:582–588Crossref, Google Scholar
74 : Retrospective survey of prodromal symptoms in dementia with Lewy bodies: comparison with Alzheimer’s disease. Dement Geriatr Cogn Disord 2012; 33:273–281. Available at doi: 10.1159/000339363Crossref, Google Scholar
75 : Imaging amyloid deposition in Lewy body diseases. Neurology 2008; 71:903–910Crossref, Google Scholar
76 : Assessing mild cognitive impairment with amyloid and dopamine terminal molecular imaging. J Nucl Med 2013; 54:887–893Crossref, Google Scholar
77 : Amyloid PET imaging in Lewy body disorders. Am J Geriatr Psychiatry 2013: pii: S1064-7481(13)00168-1. [Epub ahead of print] DOI: 10.1016/j.jagp.2013.03.001Google Scholar
78 : Dementia with Lewy bodies: diagnosis and management for primary care providers. Prim Care Companion CNS Disord 2011; 13(5): pii: PCC.11r01190. DOI: 10.4088/PCC.11r01190Google Scholar
79 : Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007; 22:1689–1707, quiz 1837Crossref, Google Scholar
80 : The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain 2011; 134:3146–3166Crossref, Google Scholar
81 : Principal component analysis of PiB distribution in Parkinson and Alzheimer diseases. Neurology 2013; 81:520–527Crossref, Google Scholar
82 ;
83 ;
84 : Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry 2011; 82:476–486Crossref, Google Scholar
85 : Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry 2007; 15:84–87Crossref, Google Scholar
86 : Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord 2008; 25:178–185Crossref, Google Scholar
87 : Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2013; 12:149–156Crossref, Google Scholar
88 : Pathways to diagnosis: exploring the experiences of problem recognition and obtaining a dementia diagnosis among Anglo-Canadians. Health Soc Care Community 2011; 19:372–381Crossref, Google Scholar
89 : Improving dementia care: the role of screening and detection of cognitive impairment. Alzheimers Dement 2013; 9:151–159Crossref, Google Scholar
90 ;
91 : “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Google Scholar
92 : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699Crossref, Google Scholar
93 : Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry 2006; 14:900–910Crossref, Google Scholar
94 : Self-administered Gerocognitive Examination (SAGE): a brief cognitive assessment Instrument for mild cognitive impairment (MCI) and early dementia. Alzheimer Dis Assoc Disord 2010; 24:64–71Crossref, Google Scholar
95 : Is the montreal cognitive assessment superior to the mini-mental state examination in detecting subtle cognitive impairment among middle-aged outpatient U.S. Military veterans? Arch Clin Neuropsychol 2012; 27:742–748Crossref, Google Scholar
96 : The AD8: a brief informant interview to detect dementia. Neurology 2005; 65:559–564Crossref, Google Scholar
97 : Relationship of dementia screening tests with biomarkers of Alzheimer’s disease. Brain 2010; 133:3290–3300Crossref, Google Scholar
98 : Primary care screen for early dementia. J Am Geriatr Soc 2008; 56:206–213Crossref, Google Scholar
99 : Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12:207–216Crossref, Google Scholar
100 ; AV45-A07 Study Group: Use of florbetapir-PET for imaging-amyloid pathology. JAMA 2011; 305:275–283 (correction: JAMA 2011; Mar 16; 305(11):1096Crossref, Google Scholar
101 ;
102 : Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 2010; 67:353–364Google Scholar
103 : β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology 2012; 78:387–395Crossref, Google Scholar
104 ;
105 : Cognitive trajectories associated with β-amyloid deposition in the oldest-old without dementia. Neurology 2013; 80:1378–1384Crossref, Google Scholar
106 : Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol 2009; 65:557–568Crossref, Google Scholar
107 : Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol 2013 Accepted Article, DOI: 10.1002/ana.23908Google Scholar
108 : FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 2007; 130:2616–2635Crossref, Google Scholar
109 : Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology 2011; 77:2034–2042Crossref, Google Scholar
110 : Amyloid imaging in dementias with atypical presentation. Alzheimers Dement 2012; 8:389–398Crossref, Google Scholar
111 ;
112 : Global standardization measurement of cerebral spinal fluid for Alzheimer’s disease: an update from the Alzheimer’s Association Global Biomarkers Consortium. Alzheimers Dement 2013; 9:137–140Crossref, Google Scholar
113 : Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci 2001; 13:229–242Crossref, Google Scholar
114 : The Memorial Delirium Assessment Scale. J Pain Symptom Manage 1997; 13:128–137Crossref, Google Scholar
115 : Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986; 5:165–173Crossref, Google Scholar
116 : Clinical Dementia Rating (CDR). Psychopharmacol Bull 1988; 24:637–639Google Scholar
117 : Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000; 12:233–239Link, Google Scholar
118 : Measurement of functional activities in older adults in the community. J Gerontol 1982; 37:323–329Crossref, Google Scholar
119 : Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord 2004; 18:241–246Google Scholar
120 : DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 2004; 62:181–187Crossref, Google Scholar
121 : Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637Crossref, Google Scholar
122 : Establishing the limits of the Mini-Mental State. Examination of ‘subtests’. Arch Neurol 1992; 49:87–92Crossref, Google Scholar
123 : Validity and reliability of the AD8 informant interview in dementia. Neurology 2006; 67:1942–1948Crossref, Google Scholar



