Psychiatric Aspects of Organ Transplantation
Abstract
Psychiatrists working with transplant populations need to understand the organ transplant process, transplant-specific psychosocial issues, and the medical complexity of organ transplant candidates and recipients in order to provide appropriate psychiatric care. Strategies for psychiatric assessment and treatment have been developed with these transplant-related issues in mind. Patients with complex psychiatric histories present particular challenges to the transplant team and specific considerations in these cases are discussed. For living organ donors the psychiatrist’s role focuses on the protection of the psychological health and well-being of the donors and the pre-donation assessment must consider risks for poor post-donation psychosocial outcomes. Following transplantation, nonadherence to transplant directives is a significant problem in transplant recipients, and psychiatrists may be asked to assist in developing/providing strategies to improve patient adherence.
For over three decades, solid organ transplantation has been the standard of care for patients with advanced organ disease and certain types of cancers. By the end of 2012, nearly 120,000 patients were active on the U.S. transplant wait-list with the majority, >90,000, being kidney transplant candidates. Unfortunately, organ transplant candidates may wait years to receive an organ, and each year 10%−15% will die while waiting. The scarcity of donated organs, coupled with the enormous investment in transplant recipients from both the local transplant teams and the national medical system, requires the selection and preparation of those best able to survive the arduous surgery and postoperative recovery period and have good overall long-term outcomes. However, advances in transplant medicine complicate these mandates by providing transplant programs the ability to accept and transplant older and sicker candidates. In response to these trends, many transplant programs have removed prior age or illness restrictions, and over the past 10 years the numbers of transplant patients aged 65 and older has doubled. The pressures of an enlarging transplant waitlist have increasingly led to the consideration and use of living organ donors. Living organ donors offer to undergo complex surgery and put their own health and wellbeing at risk in order to improve the health and lifespan of another individual.
Postoperatively, innovations in surgical techniques (e.g., the use of laproscopic surgery for kidney transplant), immunological strategies (e.g., development of less toxic immunosuppressive medications), and improved medical management have resulted in reduced morbidity and gains in long-term survival for transplant recipients. Nevertheless, for kidney, liver, and heart transplant recipients of deceased donor organs, only 55%−60% will survive 10 years; for lung transplant recipients, only 30% will survive that period. Living kidney and liver recipients have somewhat better outcomes of 70%−80% survival at 10 years. Survival is dependent on the patient’s consistent adherence to their immunosuppressive medications and posttransplant regimens, which means adopting lifelong self-management techniques.
Psychiatrists are requested to assist with the evaluation of both patients and living donors as they are considered for transplant/donation and to address psychiatric and behavioral issues as they arise postoperatively. Transplant candidates and recipients present treatment challenges due to the complexities of their comorbid medical and surgical issues. Living donors require careful consideration of their psychological health, motives, and desire to donate. In this Clinical Synthesis article we will review these key areas of psychosomatic medicine as it applies to transplantation issues in adults.
As transplantation has distinct phases with critical transition points, it is helpful to consider the patient at their point along the continuum of the transplant process. Figure 1 shows these phases delineated by specific surgical and recovery events with potential medical and psychological stresses inherent in each phase listed.
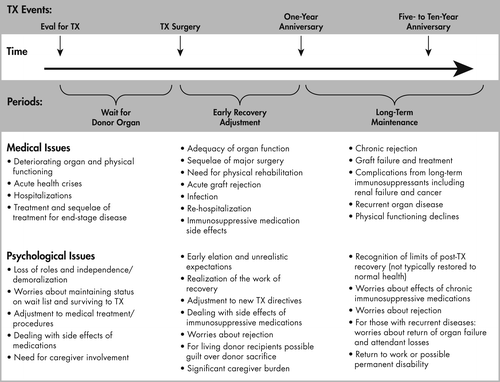
On average, distress levels are high pretransplant and tend to abate posttransplant. The rates of mood disorders following transplantation are higher than other medical/surgical populations, especially in the early posttransplant years. Depressive disorders occur in 60% or more of recipients, with anxiety disorders in 30% of recipients (1). Rates are lowest in kidney recipients. It has not been fully established that these disorders lead to worse medical outcomes. However, increasing evidence suggests that depressive disorders, particularly in the posttransplant phase, are associated with poorer patient and graft survival (2). There is less evidence that pretransplant disorders result in such outcomes. The first posttransplant year seems to be a critical time period during which repeated assessment for mood disorders may be essential to early identification and treatment with possible reduction in poor outcomes (3).
Treatment Strategies and Evidence
Behavioral and Psychotherapeutic Interventions
A range of psychotherapeutic and behavioral interventions have been developed specifically for solid organ transplant candidates and recipients and tested empirically. Most psychotherapeutic interventions target patients and their families during the waiting period because it is typically one of the most psychologically and physically stressful periods of the entire transplant experience (4). Interventions that focus on managing the emotional demands of waiting for the transplant while also dealing with the symptoms of advanced organ disease are most effective. Quality of Life Therapy (QOLT) is a cognitive-behavioral intervention that targets the areas of life (e.g., relationships, goals, self-esteem) the patient identifies as essential for his/her happiness and overall life satisfaction but are currently compromised (5). QOLT produced improvements in quality of life, psychological distress, and social intimacy in lung (6) and kidney (7) candidates who received weekly sessions over 8–15 weeks. Notably, caregivers of candidates receiving QOLT experienced vicarious improvements in quality of life, mood and social intimacy (8); this is an important benefit of QOLT given that candidates’ perceptions of social support have been found to decrease after waiting times greater than 3 months (9). Coping skills training and similar cognitive-behavioral therapies tailored to transplant specific needs also offer benefits for quality of life, anxiety and depressive symptoms, and perceived social support (10, 11).
Although many transplant centers offer supportive services to patients and their families during the waiting period and immediately posttransplant, psychiatrists on the transplant team have an opportunity to further optimize patients’ psychological outcomes by using these evidence-based psychotherapies. Remote intervention delivery is a viable option, and evidence supports the feasibility and efficacy of telephone-based (6, 10, 11) and web-based (12) programs. This may be a solution to the logistical challenges of delivering regular psychotherapy when many transplant patients live hundreds of miles from the transplant center and are unable to travel due to their deteriorating health.
Complementary and alternative therapies have also shown promise as strategies to improve sleep quality and duration. Sleep is a common problem for transplant patients, due to both their underlying disease and chronic immunosuppression (13) and the effects of poor sleep can carry over into mental health and well-being. Transplant recipients who participated in an 8-week mindfulness-based stress reduction program experienced improvements in quality of life and quality of sleep and decreases in anxiety and depression symptoms that were sustained up to 1 year (14, 15).
Lastly, exercise interventions have received attention because of their direct benefits on physical functioning and their indirect benefits on quality of life (16). Empirically tested exercise programs have taken forms ranging from a 3–4 week intensive inpatient rehabilitation program for long-term transplant survivors (17) to a thrice-weekly supervised cross training program for the first 3 months after discharge from the transplant hospitalization (18). The physical functioning gains demonstrated by these programs give reason to be optimistic about the potential for transplant survivors, particularly those who have survived the first year, to reach the functioning levels of the general population and, in so doing, improve their overall quality of life (19). Psychiatrists should thus encourage their transplant survivors to seek out formal exercise programs or, at the very least, establish a regular aerobic activity routine.
Following transplantation, problems psychologically incorporating the organ can lead to emotional difficulties. In part, transplant programs’ policies to preserve deceased donor confidentiality may actually facilitate better psychological incorporation of the organ, to the extent that they preclude undue focus and rumination by the recipient on donor characteristics such as race/ethnicity, specific age, or other personal characteristics. For recipients who appear distressed about their new organ, directly interviewing them may help to uncover facets that seem particularly disturbing to them or steps that they might take to become more at peace with the transplant. Recipients may also provide revealing and clinically useful information via other techniques, including a questionnaire focused on organ incorporation (20), and artwork and projective test drawings (21) have been used to bring forth emotionally laden issues (even guilt, debt, or culpability) that may not be easy for a recipient to verbalize. Through these techniques recipients of living donation may express guilt over putting someone else at risk (21). Psychological rejection of the organ may lead to poorer survival (20) and interventions based on psychological rejection should aim to change the recipient’s attitude toward the transplanted organ and thus facilitate incorporation.
Pharmacological Issues
Psychotropic Therapies
There are no absolute contraindications to the use of specific psychotropic medications for transplant patients, but there is a need for greater caution with choice of agent and dosing. Prior to transplant, physiologic changes due to advanced organ disease cause alterations in pharmacokinetics affecting drug absorption, distribution, metabolism, and elimination. Patients awaiting transplantation are often seriously ill and less able to tolerate medication side effects such as nausea or sedation or physiological effects such as blood dyscrasias. Prescribing psychotropic medications before transplantation generally requires the use of lower initial doses and careful monitoring of tolerability and clinical response. Doses should be adjusted gradually. The final dose will often be lower than that used in a healthy individual. Closer monitoring, both symptomatically and physiologically, is recommended, and combining follow-up with regular pretransplant medical appointments may facilitate this. Some drugs may be less optimal to use. For example, maintaining stable nontoxic lithium levels within a narrow therapeutic window is complicated in advanced organ disease. Dehydration, congestive heart failure, cirrhosis, renal dysfunction, or cystic fibrosis reduce lithium clearance, increasing levels, while fluid overload (e.g., edema, ascites) reduces lithium levels. As another example, the use of antipsychotic medications requires additional care during the pretransplant period, as patients often have increased risk for QT prolongation and possible torsades de pointes due to electrolyte imbalances, presence of cardiac, renal, or hepatic failure, left ventricular hypertrophy, or other QT-prolonging medications (for detailed review of these issues, see Ferrando et al. [22]).
In the weeks following transplantation, some recipients will have transient physiological abnormalities that could also affect pharmacokinetics (e.g., liver congestion and/or renal hypoperfusion in heart recipients, fluid overload in renal recipients, and liver hypoperfusion and fluid overload in liver recipients). Even with normalized organ functioning, immunosuppressants and other medications increase the risk for drug-drug interactions. Most immunosuppressants have significant toxicities and narrow therapeutic indices. Adequate levels must be maintained to prevent organ rejection. The most commonly used immunosuppressants (glucocorticoids, calcineurin inhibitors [tacrolimus and cyclosporine], sirolimus, and corticosteroids) are all CYP3A4 substrates. Inhibitors and inducers of CYP3A4 may cause clinically significant drug level changes, resulting in toxicity or inadequate immunosuppression. Thus the selection of psychotropics should include consideration of agents with less potential to inhibit or induce CYP450 enzymes (see Table 1).
| Medication | Pharmacokinetic Effect | Effect on Immunosuppressant Drug and Management |
|---|---|---|
| CarbamazepinePhenytoinOxcarbazepineArmodafinilModafinilb | Induces CYP 3A4 | Increased metabolism and reduced exposure and therapeutic effect of CYP 3A4 substrates including: corticosteroids, cyclosporine, tacrolimus, sirolimus. |
| St. John’s wortc | Induces CYP 3A4 and P-glycoprotein | Reduced bioavailability and increased metabolism leading to reduced exposure and therapeutic effect of CYP 3A4/P- glycoprotein substrates including: corticosteroids, cyclosporine, tacrolimus, sirolimus. |
| FluoxetineFluvoxamineNefazodoned | Inhibits CYP 3A4 | Reduced metabolism and increased exposure and toxicities of CYP 3A4 substrates including: corticosteroids, cyclosporine, tacrolimus, sirolimus. |
Immunosuppressant Medications
While there can be many possible etiologies for neuropsychiatric symptoms or changes in mental status, especially in the early posttransplant period, the possibility that symptoms reflect immunosuppressive medication side effects should always be entertained. Symptoms often diminish with reduction in immunosuppressive medications.
Questions and Controversies
Complex Patients
Most transplant programs engage social workers and additionally mental health clinicians to assist in determining the suitability of candidates for transplantation. While guidelines exist in the literature, there are no national listing criteria for either medical/surgical or psychosocial/psychiatric issues—each transplant team must decide for themselves what factors are considered relevant and their willingness to take risks on less optimal candidates. Psychiatrists are most often asked to render opinions on patients with mental health histories, especially complex patients and those with substance use disorders. Assistance with preparing potential candidates for transplant is a typical strategy, although for some the severity of their medical condition or the need for urgent transplantation simply does not allow them to accomplish necessary goals (e.g., addiction rehabilitation for an active substance user). Below we review some of the more complex scenarios and patients who could present a psychiatric challenge for the transplant team. It is important to note that with expert psychiatric management, good social supports, and a longitudinal relationship with the transplant team, even complicated patients such as those with severe psychiatric disorders can have successful posttransplant outcomes.
Substance Use Disorders
Perhaps due to careful psychosocial screening, relapse rates following transplantation are low relative to the general population. However, rates of any alcohol use after transplantation for alcoholic liver disease approaches 6% per year with cumulative rates of heavy use approaching 10%−15% by 5 years (23). Substance use can impair adherence to posttransplant care. Posttransplant screening for substance use is required and relapse can often be effectively elicited by direct nonjudgmental questioning during routine clinic visits.
Tobacco use is now recognized as problematic for all types of solid organ transplantation, not heart and lung alone. A significantly increased risk of malignancies, vasculopathies, infections, graft loss, and other complications are observed in posttransplant smokers. Many liver and kidney transplant programs are now also requiring patients to be tobacco-free prior to transplant listing. Despite similar emphasis on maintenance of lifelong smoking cessation in lung and heart transplantation, following lung transplantation the rates of posttransplant smoking are 4.8% per year (higher than for any other organ) and 3.2% per year for heart transplant recipients (24). Patients who are stable in methadone or suboxone maintenance programs should not be required to stop these therapies as a condition for transplant eligibility, as this increases the risk for relapse, especially during the stressful pretransplant period.
Personality Disorder
Patients referred for transplant evaluation are seen at a time they are already facing declining health, limited prognosis, disability, financial stress, role loss, and family strain. Under these circumstances, mature coping mechanisms are often compromised even in psychologically healthy individuals. Among patients with comorbid personality traits/disorders, regression to immature and inflexible defenses can compromise their ability to cope with the demands of the transplant evaluation and the long-term requirements for self-management. For example, borderline and narcissistic patients can be excessively demanding, overly critical, and may disrupt the cohesive functioning of transplant team members and other healthcare providers through splitting. While the attention to details and need for control and order may make obsessive-compulsive traits seem valuable to transplant self-management, a patient with such traits can become overly controlling about their medical care, argumentative about treatment recommendations, and intolerant of the inevitable delays or setbacks. Concerns arise with these patients regarding their ability to cope with transplantation and work collaboratively with the transplant team while adhering to medications and the necessary follow-up posttransplantation. Psychiatrists can assist transplant teams by helping them to recognize the presence of personality pathology and to develop care plans that can help to reduce miscommunication, anger, frustration, and acting out. For patients with more severe psychopathology, a behavioral contract may be needed between patient and transplant team outlining clear expectations about treatment adherence, appropriate communication with healthcare providers, involvement of their support system, and mandatory psychiatric treatment. While this is not a legal contract, it can help to define expectations between patient and transplant team, reducing miscommunication and splitting. Eligibility for transplantation can be made contingent on fulfillment of contract requirements.
Psychotic Disorders
Transplant teams are often hesitant to consider candidates with pre-existing psychotic disorders (e.g., schizophrenia, bipolar disorder). Their hesitance often arises from concerns about the patient’s ability to adhere to their posttransplant care and their risk of developing “steroid psychosis.” However, evidence does not suggest that these patients are at greater risk for steroid-induced neuropsychiatric complications. Limited information exists on transplant outcomes for patients with psychotic disorders. Case reports and a survey reporting on 35 patients across several transplant centers indicate successful transplantation can occur with careful psychiatric assessment and planning (25–30). According to survey findings, risk factors for posttransplant complications (e.g., suicide attempts, nonadherence) included presence of comorbid antisocial or borderline personality features, history of assault, living alone/homeless, recent positive psychotic symptoms, and family history of schizophrenia. In the survey, rates of posttransplant nonadherence with medications and laboratory testing, reduced graft function or graft loss due to nonadherence, were similar to rates observed in general transplant populations. Transplant candidates should be evaluated on an individual basis with added attention to response to psychiatric treatment, stability of psychiatric symptoms, adherence to medical and psychiatric care, and availability of support before and after transplantation. Developing a coordinated care plan is needed to ensure that there are no lapses in psychiatric treatment and follow-up, as this can easily occur both before and after transplantation given the greater focus on physical health and medical care.
Fulminant Organ Failure / Critically Ill Patients
In some cases, patients present emergently for transplant consideration due to fulminant organ failure and are unable to participate in a pretransplant psychosocial assessment. These patients often arrive in a dramatic fashion such as cardiogenic shock following a massive myocardial infarction or fulminant hepatic failure following an acetaminophen overdose. Patients are unable to be interviewed face-to-face and evaluation requires urgent but thorough collection of collateral information from other healthcare providers, family, friends, and other close associates. Since informed consent from the patient is not possible, discussions with family members and significant others are crucial to determine the patient’s possible interest in transplantation and ability to adjust to posttransplant life (e.g., chronic immunosuppressant therapy, side effects, lifestyle restrictions, self-monitoring, medical follow-up). The availability of support for the patient after transplant should be determined. For patients presenting with fulminant hepatic failure due to intentional acetaminophen overdose, additional information needs to be considered for transplant candidacy including circumstances surrounding the suicide attempt, presence and severity of active psychiatric symptoms, history of prior suicide attempts or psychiatric hospitalizations, comorbid substance abuse, adherence to mental healthcare, and coping skills. At times, families and friends may be hesitant to provide information that they fear may hinder the patient’s eligibility for transplant, but openness should be encouraged. For all cases of emergent transplantation, families and others will need added support to cope with the acute nature of the patient’s decline and often higher chance of poor outcome, even with transplantation. Patients who attempt suicide are at heightened risk for another attempt and posttransplant management should not neglect the need for long-term psychiatric care and risk monitoring. While many patients experience a honeymoon period in the early stages after transplant—being elated to be alive—the realities of living as an organ recipient appear quickly and such stresses can be disabling, especially for an already psychologically fragile patient.
Transplant Nonadherence
A patient’s ability to learn, incorporate, and maintain consistent health behaviors should be thoroughly assessed prior to transplantation during the psychosocial evaluation. Identified issues should be remediated through education or behavioral or psychological therapies addressed above. While many of these interventions are employed posttransplant when a problem is discovered, proactive identification pretransplant can both assist the patient in meeting transplant requirements and aid in the identification of patients unable to consistently comply with directives.
Rates and Predictors of Nonadherence
Following transplantation, recipients must consistently take their immunosuppressive medications, have routine blood work and biopsies (if indicated), and come to all requested clinic appointments and procedures. Additional self-management skills that need to be adopted include being able to monitor their symptoms and know when to report concerning symptoms to the transplant team. Nonadherence to these components of the transplant medical regimen is a persistent problem (31) and has been consistently shown to predict transplant related morbidity and mortality (32).
Immunosuppression is the most important element of the transplant regimen and requires daily adherence. Across all organ types the average rate for immunosuppressant nonadherence is 23% annually, with kidney recipients having the highest medication nonadherence at 36% (24). Rates of nonadherence are slightly better for attending clinic appointments (6%) and getting blood work and tests (12%), but less so for self-monitoring behaviors such as monitoring vital signs (21%) (24). Nonwhite ethnicity, poorer social supports and poorer perceived health are associated with greater immunosuppressive medication nonadherence, suggesting these patients may benefit from closer monitoring by the transplant team. The magnitude of these rates is discouraging given the shortage of organs and the psychosocial screening of candidates that occurs prior to transplantation, which includes pretransplant education emphasizing the necessity of lifelong adherence to transplant directives.
Clinical Issues and Monitoring Adherence
While patients are informed of their postoperative self-care requirements prior to transplant, the real education begins shortly after surgery in the hospital and includes both patient and caregiver. Written materials are provided as are strategies and tools for managing the complex medication regimens. Patients and their caregivers are expected to learn and adopt these complex regimens and to be capable of monitoring symptoms and appropriately report them to the transplant team. These directives are reviewed most intensively during the early postoperative period when recipients come to clinic and are interviewed by the transplant team. As patients stabilize and have fewer, less frequent appointments with the transplant team, there is less opportunity to provide these face-to-face reinforcement sessions; at some point patients are expected to have integrated these skills without significant involvement by the transplant team. Patients will have a transplant coordinator assigned to them who will know them best and follows their course. The transplant coordinator responds to calls and questions from the patient and often knows essential information on the patient’s medication taking, pharmacy refills, appointment attendance, getting labs and biopsies as directed, and returning phone calls.
Self-report assessments by clinical interview are the standard in identifying nonadherence, and strategies that maximize the accuracy of self-reporting (e.g., establishing rapport; wording questions so that they assume that nonadherence can and will occur) are important when relying on self-report information (see Table 2). Other methods, such as electronic medicine cap monitoring, may not be as revealing (e.g., patients tire of using special pill bottles) and are expensive and labor intensive to use clinically.
| • Conduct a clinical interview |
|---|
| Ask directly using a nonjudgmental, nonconfrontational approach |
| Frame questions as if it is assumed nonadherence has occurred/will occur |
| Be specific about medication dosages, timing, frequency, and consistency |
| Ask about late as well as missed dosages |
| Ask about late pharmacy refills |
| Elicit reasons for missed meds (e.g., forgot, other activities interfered, side effects) |
| • Examine medication drug levels |
| • Ask pharmacy about refills – timeliness, meds not being refilled |
| • Ask transplant coordinator about whether the patient completes appointments, labs, and procedures; returns phone calls; appropriately seeks assistance for problems |
Immunosuppressive medication levels are routinely checked, since maintaining adequate blood levels of these medications is critical to preventing organ rejection. In the clinical setting this is one of the primary methods of monitoring adherence. In addition to blood levels at a single time point, investigators have determined that wide fluctuations in immunosuppressive medication levels (i.e., standard deviations of ≥2) can indicate patients who have erratic or inconsistent medication taking and subsequently are at risk for rejection (26).
Strategies and Interventions to Address Nonadherence
Strategies to improve adherence include re-education about transplant-specific directives, although additional therapies are often necessary to address psychological and psychosocial issues related to nonadherence. Patients for whom the transplant was abrupt and there was little time to adjust to the need for transplantation (such as in acute or fulminant failure) or those who have been emotionally traumatized by the experience (and may even have developed transplant-related PTSD) may be unwilling to or have difficulty with incorporating their transplant identity and struggle with following directives. Some patients find the side effects of immunosuppressive medications intolerable. Others might not perceive a need to continue taking them as they do not immediately feel any symptoms of organ rejection so they do not perceive the dangerous effects of having stopped their medications. Depression and substance use can also lead to nonadherence, so identifying and addressing psychiatric disorders may improve adherence. For some the expenses of medications, loss of insurance, transportation, or other logistical issues need to be addressed by the social worker on the transplant team.
Clinicians commonly target nonadherence by providing reading materials about immunosuppression, training patients to self-administer medications, and providing printed medication instructions and teaching patients to link medication-taking with their daily routines (33). In the literature, only five of 12 empirically-tested adherence interventions included in a 2009 review yielded adherence benefits (34). Of these, those that combined educational, behavioral, and psychological dimensions were most effective, which is consistent with the multimodal, collaborative approach to chronic disease management. More recently, mobile health technologies have been used to track adherence to all elements of the medical regimen, including medications and self-monitoring tasks such as spirometry for lung recipients. These technologies have enhanced traditional adherence interventions by facilitating communication with the transplant care team and have been well accepted even by patients who are older and less familiar with using computers (35, 36). Automated text messaging reminders delivered via mobile telephones for taking medications has been demonstrated to improve adherence to medication schedules and reduce rejection episodes (37). Other promising strategies include pharmacist-led longitudinal interventions that begin before hospital discharge and continue with quarterly meetings to discuss adherence throughout the first posttransplant year (38, 39), as well as interventions conducted before hospital discharge from the transplant surgery to educate recipients about the importance of adherence and preemptively address expected barriers and facilitators to adhering (40). Given that the most effective strategies are multimodal, there is a key role for psychiatrists on the transplant team to coordinate these efforts to promote adherence.
Living Organ Donors
Although there are no national standards for the exact content or process of conducting the psychosocial evaluation, the domains listed above cover the elements currently required by Organ Procurement and Transplantation Network (OPTN) policy (48). The OPTN/UNOS Living Kidney Donor Psychosocial Evaluation Checklist provides a useful tool to ensure that essential elements are covered in the evaluation (49). This checklist could also be used as a model for the evaluation of other types of living donors (e.g., liver donors or the rare living lung donor).
Because transplant teams care for and evaluate both donors and transplant candidates, to prevent a conflict of interest all programs in the United States are required to have an independent living donor advocate (ILDA) to work with donors. The ILDA provides assistance to the donor unencumbered by the needs of the intended recipient or transplant team. The ILDA also evaluates the ability of the prospective donor to give informed consent, and may either provide additional psychosocial assessment or observe portions of the psychosocial evaluation. The ILDA is often a mental health professional, and he/she provides additional critical input regarding whether the prospective donor should be approved for donation.
Elements of the process and timing of the psychosocial evaluation are important to consider. Given the overall goal of preserving donor safety and well-being, it is important to ensure that the evaluation process is not rushed and that there is time to build enough trust with the prospective donor that he/she will feel able to openly discuss all of the issues raised in the evaluation. This can sometimes be difficult due to the urgency of the transplant candidate’s medical condition. It is also important to allow some time for the prospective donor to reconsider his/her decision based on all of the information provided by the donor team (i.e., a “cooling off” period), as well as time to have additional consultation with the ILDA as a follow-up to the completion of the medical and psychosocial evaluation.
After donation, although U.S. centers are required to collect follow-up information on living donors through 2 years postdonation, this information pertains primarily to laboratory data and physical health indices. Psychiatrists rarely see living donors post-donation unless major mental health issues are identified through routine (and increasingly infrequent) clinic visits. The majority of living donors do well in terms of psychosocial outcomes post-donation (50), although a significant minority may develop sustained somatic complaints (e.g., pain, fatigue), psychological distress (e.g., depression or anxiety), or other psychosocial problems related to the donation (e.g., family strains, financial stress due to unexpected medical costs) (51). Ensuring that prospective donors understand the donation process and both the medical and psychosocial risks of donation is therefore critically important so that individuals can be fully prepared and make an informed decision about donation. A recent study found that offering donors additional intervention to assist them to resolve any remaining ambivalent feelings about donation was helpful for reducing the likelihood of somatic and psychological problems postdonation (52).
Palliative Care
Despite extensive overlap of patient populations served, palliative care and transplant are not often formally connected. However the nature of the day-to-day clinical problems (e.g., critical illness, life threatening condition) and the intensity of commitment to the patient and families are very similar between these two medical disciplines. For transplant teams, with their intensive focus on aggressive treatment often to rescue patients at the very end of life, consideration of palliative care can present seemingly contradictory goals. For patients and their families, the request for palliative care involvement might inadvertently signal that the transplant team has given up on the patient. However, palliative care can be employed in tandem with the intent to move forward with transplantation if the patient is on the wait list and for all transplant patients who are critically ill. Such care can improve satisfaction and reduce symptom burden regardless of prognosis or ultimate treatment goals. Psychiatrists may be in a unique position to listen to the concerns and hopes of the patient and their families and assist in suggesting such additional services. Several recent intervention studies using palliative care services in transplant cohorts show improved continuity of care and treatment planning and increased discussions of goals of care. However, while mortality rate was unchanged between those in the intervention and those who were not, Do Not Resuscitate (DNR) status and withdrawal of life support increased (53–55).
Conclusions
Evaluating and treating transplant patients requires awareness and consideration of transplant specific scenarios and medical/surgical issues. Psychotherapeutic strategies to deal with transplant-related psychosocial and psychiatric issues have been developed. The logistics of treating complex transplant patients requires coordination of service with the transplant team and understanding of the sometimes fragile nature of the patient’s physiology. Following transplantation, mood disorders are common; prompt recognition and treatment may reduce the potential for poor long-term outcomes. Early identification and treatment of the resumption of substance and tobacco may also prevent poor outcomes. Maintenance of lifelong compliance to transplant directives is essential for graft and patient survival, and psychiatrists can assist in the implementation of strategies to address nonadherence.
We did not address issues of pediatric transplantation, which are different in significant ways from adult transplantation, so some excellent overviews of assessment and management of psychosocial issues in pediatric transplantation are recommended (56–60). While our clinical work has provided substantial information to address psychiatric issues related to transplantation, further clinical and research initiatives are needed to address long term outcomes and adapt newer psychotherapeutic technologies to the complex needs of transplant patients.
1 : Organ transplantation, in Kaplan and Sadock’s Comprehensive textbook of psychiatry, 9th ed, vol. 2. Edited by Sadock BJSadock VARuiz P. Philadelphia, Lippincott Williams & Wilkins, 2009, pp 2441–2456Google Scholar
2 : Psychiatric disorders as risk factors for adverse medical outcomes after solid organ transplantation. Curr Opin Organ Transplant 2012; 17:188–192Crossref, Google Scholar
3 : Early treatment of depressive symptoms and long-term survival after liver transplantation. Am J Transplant 2013; 13:928–935Crossref, Google Scholar
4 : Psychosocial issues facing lung transplant candidates, recipients and family caregivers. Thorac Surg Clin 2012; 22:517–529Crossref, Google Scholar
5 : Quality of life therapy and assessment in health care. Clin Psychol 1998; 5:19–40Google Scholar
6 : A randomized evaluation of quality-of-life therapy with patients awaiting lung transplantation. Am J Transplant 2005; 5:2425–2432Crossref, Google Scholar
7 : A psychological intervention to improve quality of life and reduce psychological distress in adults awaiting kidney transplantation. Nephrol Dial Transplant 2011; 26:709–715Crossref, Google Scholar
8 : Caregivers of lung transplant candidates: do they benefit when the patient is receiving psychological services? Prog Transplant 2006; 16:336–342Crossref, Google Scholar
9 : Hope and social support as coping resources for adults waiting for cardiac transplantation. Can J Nurs Res 1994; 26:31–48Google Scholar
10 ;
11 : Telephone-based coping skills training for patients awaiting lung transplantation. J Consult Clin Psychol 2006; 74:535–544Crossref, Google Scholar
12 : An internet-based intervention to improve psychosocial outcomes in heart transplant recipients and family caregivers: development and evaluation. J Heart Lung Transplant 2004; 23:745–758Crossref, Google Scholar
13 : Symptom experience after solid organ transplantation. J Psychosom Res 2009; 66:101–110Crossref, Google Scholar
14 : Mindfulness-based stress reduction for solid organ transplant recipients: a randomized controlled trial. Altern Ther Health Med 2010; 16:30–38Google Scholar
15 : Longitudinal impact of mindfulness meditation on illness burden in solid-organ transplant recipients. Prog Transplant 2005; 15:166–172Crossref, Google Scholar
16 : Kidney transplantation: a systematic review of interventional and observational studies of physical activity on intermediate outcomes. Adv Chronic Kidney Dis 2009; 16:482–500Crossref, Google Scholar
17 ;
18 : Exercise training after lung transplantation improves participation in daily activity: a randomized controlled trial. Am J Transplant 2012; 12:1584–1592Crossref, Google Scholar
19 ;
20 : The Transplanted Organ Questionnaire: a validation study. J Psychosom Res 2012; 73:319–324Crossref, Google Scholar
21 : Association between pretransplant psychological assessments and posttransplant psychiatric disorders in living-related transplantation. Psychosomatics 2002; 43:49–54Crossref, Google Scholar
22 Ferrando SJ, Levenson JL, Owen JA (eds): Clinical Manual of Psychopharmacology in the Medically Ill. Washington, DC, American Psychiatric Press, 2010.Google Scholar
23 : Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transpl 2008; 14:159–172Crossref, Google Scholar
24 : Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation 2007; 83:858–873Crossref, Google Scholar
25 : Rational guidelines for transplantation in patients with psychotic disorders. Curr Opin Organ Transplant 2007; 7:385–388Crossref, Google Scholar
26 : Heart transplant in a young man with schizophrenia. Am J Psychiatry 2005; 162:453–457Crossref, Google Scholar
27 : Heart transplantation and schizophrenia. Psychosomatics 2003; 44:264–265Crossref, Google Scholar
28 : Organ transplantation and paranoid schizophrenia. Psychosomatics 1994; 35:159–161Crossref, Google Scholar
29 : Liver transplantation in an undiagnosed schizophrenic. J R Soc Med 1997; 90:563Crossref, Google Scholar
30 : Lung transplantation in a Japanese patient with schizophrenia from brain-dead donor. Gen Hosp Psychiatry 2013; 35:e11–e13Crossref, Google Scholar
31 : Adherence to the therapeutic regimen in heart, lung, and heart-lung transplant recipients. J Cardiovasc Nurs 2005; 20(Suppl):S88–S98Crossref, Google Scholar
32 : Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant 2010; 14:940–943Crossref, Google Scholar
33 : Interventions used by health care professionals to enhance medication adherence in transplant patients: a survey of current clinical practice. Prog Transplant 2011; 21:322–331Crossref, Google Scholar
34 : Interventions to improve medication-adherence after transplantation: a systematic review. Transpl Int 2009; 22:780–797Crossref, Google Scholar
35 : Evaluation of a hand-held, computer-based intervention to promote early self-care behaviors after lung transplant. Clin Transplant 2009; 23:537–545Crossref, Google Scholar
36 : Use of telehealth technology for home spirometry after lung transplantation: a randomized controlled trial. Prog Transplant 2010; 20:310–317Crossref, Google Scholar
37 : Improved adherence and outcomes for pediatric liver transplant recipients by using text messaging. Pediatrics 2009; 124:e844–e850Crossref, Google Scholar
38 : Impact of a pharmaceutical care program on liver transplant patients’ compliance with immunosuppressive medication: a prospective, randomized, controlled trial using electronic monitoring. Transplantation 2009; 87:839–847Crossref, Google Scholar
39 : Improving Outcomes of Renal Transplant Recipients With Behavioral Adherence Contracts: A Randomized Controlled Trial. Am J Transplant (Epub ahead of print: Jul 2, 2013)Google Scholar
40 : A brief psychological intervention to improve adherence following transplantation. Ann Transplant 2005; 10:52–57Google Scholar
41 ;
42 : Organ transplantation, in The American Psychiatric Publishing textbook of psychosomatic medicine: Psychiatric care of the medically ill, 2nd ed. Edited by Levenson JL. Washington, DC, American Psychiatric Publishing, Inc., 2010, pp 725–758Google Scholar
43 : Psychosocial factors in living organ donation: clinical and ethical challenges. Transplant Rev (Orlando) 2008; 22:192–195Crossref, Google Scholar
44 : Psychosocial assessment of living organ donors: clinical and ethical considerations. Prog Transplant 2001; 11:40–49Crossref, Google Scholar
45 : Psychosocial evaluation interview protocol for living related and living unrelated kidney donors. Soc Work Health Care 2003; 38:39–61Crossref, Google Scholar
46 : Guidelines for conducting a psychiatric evaluation of the unrelated kidney donor. Psychosomatics 2003; 44:452–460Crossref, Google Scholar
47 : Consideration of psychosocial factors in the evaluation of living donors. Prog Transplant 2008; 18:41–48, quiz 49Crossref, Google Scholar
48 Organ Procurement and Transplantation Network, Policy 12, Living donation. http://Optn.transplant.hrsa.gov/policiesandbylaws2/policies/pdfs/policy_172.pdf. Last accessed April 21, 2013.Google Scholar
49 Organ Procurement and Transplantation Network. Living kidney donor psychosocial evaluation checklist. http://optn.transplant.hrsa.gov/news/newsDetail.asp?id=1590. Last accessed April 21, 2013.Google Scholar
50 ;
51 : Prevention of poor psychosocial outcomes in living organ donors: from description to theory-driven intervention development and initial feasibility testing. Prog Transplant 2012; 22:280–292, quiz 293Crossref, Google Scholar
52 : Preventive intervention for living donor psychosocial outcomes: Feasibility and efficacy in a randomized controlled trial. Am J Transplant (in press)Google Scholar
53 : Changing end-of-life care practice for liver transplant service patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage 2012; 44:508–519Crossref, Google Scholar
54 : Barriers to optimal palliative care of lung transplant candidates. Chest 2013; 143:736–743Crossref, Google Scholar
55 : Pilot study of palliative care consultation in patients with advanced heart failure referred for cardiac transplantation. J Palliat Med 2012; 15:12–15Crossref, Google Scholar
56 : Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14:1229–1236Crossref, Google Scholar
57 : Psychiatric issues in pediatric organ transplantation. Child Adolesc Psychiatr Clin N Am 2010; 19:285–300, viii–ixCrossref, Google Scholar
58 : The psychosocial challenges of solid organ transplant recipients during childhood. Pediatr Transplant 2012; 16:803–811Crossref, Google Scholar
59 : Solid-organ transplantation in childhood: transitioning to adult health care. Pediatrics 2011; 127:742–753Crossref, Google Scholar
60 : Outcomes in pediatric solid-organ transplantation. Pediatr Transplant 2011; 15:128–141Crossref, Google Scholar



