Diagnosis, Pathophysiology, and Management of Mood Disorders in Pregnant and Postpartum Women
Abstract
Mood disorders disproportionately affect women across the lifespan. Mood disorders in pregnancy and the postpartum period are common and have profound implications for women and their children. These include obstetric and neonatal complications, impaired mother–infant interactions, and, at the extreme, maternal suicide and infanticide. Because obstetrician–gynecologists are often the first (and sometimes the only) point of contact for young women in the health care system, familiarity with the presentation and treatment of depressive illness in the perinatal period is imperative. The goal of this review is to synthesize essential information on depressive illness in the perinatal period with a focus on its most common and severe presentations, major depressive disorder and bipolar disorder. Accurate diagnosis of unipolar major depressive disorder from bipolar disorder can facilitate the selection of the best possible treatment alternatives. Counseling may be sufficient for perinatal women who have mild to moderate depression, but women who are severely depressed are likely to require antidepressant treatment. Women with bipolar disorder are at high risk for relapse if mood stabilizer medication is discontinued, and they are vulnerable to relapse near the time of delivery. Comanagement of their care with psychiatrists will increase their chances of avoiding a recurrence of illness.
(Reprinted with permission from Obstet Gynecol 2011; 117:961–977)
Approximately 20% of the adult female population in the United States will experience an episode of major depressive disorder at some point in their lives (1, 2). Studies consistently show that the likelihood of major depressive disorder developing is nearly double in women compared with men, and that the highest risk period for women occurs during their fertile years (3). Approximately 1% of women experience comorbid manic symptoms and thus experience bipolar disorder, an illness that is among the most severe mood disorders that occur in the perinatal period. Mood disorders affect a woman's quality of life and her ability to fulfill her usual roles as worker, mother, and spouse. There is also good evidence to show that mood disorders, when concurrent with general medical problems such as diabetes, heart disease, and asthma, worsen medical outcomes. In the United States, obstetricians and gynecologists are often the central medical caregivers for younger women and, as such, are likely to be the first or only medical providers to care for women who have a mood disorder. This review on mood disorders in perinatal women provides an overview of diagnostic considerations, the public health significance, incidence, and etiopathology, as well as the management of major depressive disorder and bipolar disorder in pregnant and postpartum women.
General diagnostic considerations for mood disorders in women
Although the general public commonly refers to “depression,” the term connotes a range of disorders that are collectively termed “mood disorders.” Classification of mood disorder subtype depends on the course of the mood disturbance, comorbidity with manic symptoms, and severity. Each subtype has treatment implications, and thus it is helpful for clinicians to be familiar with the differences between conditions. According to the American Psychiatric Association Diagnostic and Statistical Manual, fourth edition (4), an episode of major depressive disorder (Box 1) can occur in a woman with no history of a mood disorder (unipolar major depressive disorder, single episode) or one who has had many episodes in her life, ie, “recurrent unipolar major depressive disorder.” A major depressive episode can be classified as mild, moderate, or severe, depending on the number and severity of symptoms. In nonpregnant women, antidepressant treatment is a recommended first-line treatment for major depression of any severity and psychotherapy is recommended as first-line therapy for mild to moderate depressive episodes (5). The greater the number of previous episodes of major depressive disorder, the higher the likelihood that another episode will occur. In general, approximately one-half of women who have one episode of major depressive disorder will have another event, whereas a history of two episodes increases the risk to approximately 80% (6). The treatment implications are that once a woman has had at least three episodes of major depressive disorder in her life, her risk of a recurrence is sufficiently high that clinicians should consider maintenance, lifetime, antidepressant therapy (5). Of the various types of mood disorders, “unipolar” major depressive disorder, that is depression without lifetime mania or hypomania, is the most common. Other types of unipolar mood disorders can be chronic or acute and include dysthymic disorder and minor depressive disorder. Dysthymic disorder is a mood disturbance that endures for 2 years or more and, although it is characterized by fewer and less severe symptoms of depression, the chronicity of the condition confers morbidity. Minor depressive disorder is acute and the candidate symptoms are the same as those found for major depressive disorder (Box 1), but only two to four symptoms are required for the majority of 2 weeks, rather than the five symptoms required for major depressive disorder. Both of these disorders are associated with either clinically significant distress or functional impairment.
 |
Box 1. Diagnosis of a Depressive Episode
Women with unipolar major depressive disorder who present either in pregnancy or after delivery commonly have difficulties with sleep, energy, and appetite. To differentiate these symptoms from typical experiences of pregnancy or being postpartum, obstetricians are advised to rely on assessment of cognitive symptoms that occur in depressed individuals. Mothers with depression are often anxious and worried about the health of their infants, but they may be despondent and strikingly uninterested in either their pregnancy or the activities of their infants. It is typical for women to express guilt and feelings of worthlessness that can spill over into concerns about their capacity to be an adequate mother. Whereas reassurance can be helpful to women who have mild depressive symptoms, a patient with major depressive disorder typically does not respond to support and encouragement and will require a therapeutic regimen such as medication, psychotherapy, or both of these.
If a woman with major depressive disorder also has a history of mania or has experienced manic symptoms with depression (Box 2), then her illness is bipolar disorder rather than unipolar major depressive disorder. Manic symptoms may have occurred in temporal proximity to major depressive disorder or may have occurred years earlier. A first-degree family member with a history of mania should alert clinicians to the possibility of bipolarity in the patient with major depressive disorder. The familiality of bipolar disorder is strong in that the rate of a bipolar spectrum disorder is increased 14-fold for offspring who have a parent with bipolar disorder. In actual percentages, approximately 5% of children will have bipolar disorder develop and 10% will have a related spectrum disorder if a parent has bipolar disorder (7). Mood stabilizers (ie, lithium, various anticonvulsant medications, and atypical antipsychotic medications) are the optimal therapeutic choice for individuals with bipolar disorder because antidepressant treatment alone can trigger or worsen manic symptoms. There is increased likelihood of underlying bipolar disorder among women who have a severe and sudden onset of major depressive disorder soon after delivery (8).
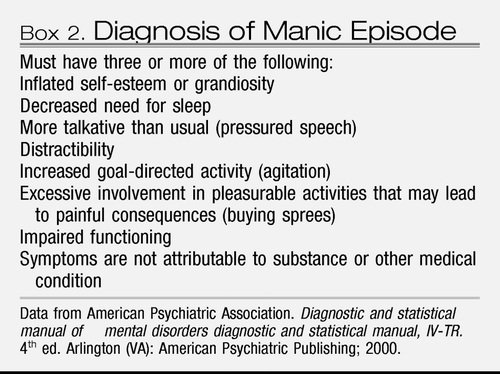 |
Box 2. Diagnosis of Manic Episode
Postpartum psychosis is a term that is used to describe postpartum women with a variety of psychiatric illnesses that do not map onto a single Diagnostic and Statistical Manual, fourth edition, diagnosis. Women who become psychotic during or immediately after pregnancy may experience any of the following: a single psychotic episode, schizophrenia, schizoaffective disorder, unipolar major depressive disorder, or bipolar disorder. Studies that have conducted long-term follow-up find that mood disorders predominate in women who become psychotic near the time of delivery (9–12); among women with mood disorders and psychosis after delivery, the majority express a clinical course consistent with bipolar disorder over the ensuing years (9–12). Given the preponderance of mood disorders among those with postpartum psychosis, we do not specifically discuss postpartum psychosis but instead unipolar and bipolar disorder with psychosis.
Epidemiology of unipolar major depressive disorder during pregnancy and the postpartum period
A quantitative review commissioned by the Agency for Healthcare Research and Quality (13, 14) provides the most comprehensive investigation to date of the incidence and prevalence for both minor and major depressive disorder during the perinatal period. These are found in Table 1. Estimates vary according to time frame, point of pregnancy, and postdelivery interval. The best estimate for period prevalence of any depressive disorder during pregnancy is 18.4% (95% confidence interval [CI] 14.3%–23.3%), whereas the corresponding estimate for the 3 months postpartum is 19.2% (95% CI 10.7%–31.9%).
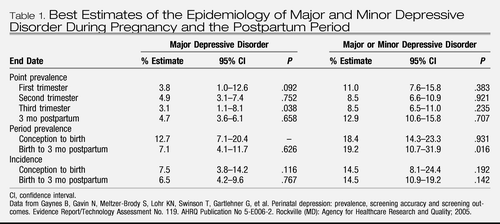 |
Table 1. Best Estimates of the Epidemiology of Major and Minor Depressive Disorder During Pregnancy and the Postpartum Period
The estimated rate of new cases of major depressive disorder was 7.5% in pregnancy and 6.5% in the 3 months after delivery. Only one investigation of major depressive disorder during pregnancy (15) and three studies of depression during the postnatal period (16–18) included a nonperinatal comparison group and none reported a significant difference in point or period prevalence of major depressive disorder between perinatal and nonperinatal women. However, the lone study of incidence of major depressive disorder found significantly increased rates during the first 5 weeks postpartum. This difference disappeared by 6 months postpartum (17).
As an update to the aforementioned meta-analytic review, we identified eight additional studies published since 2004 that estimated the prevalence of unipolar major depressive disorder during pregnancy. Point prevalence and period prevalence estimates for a diagnosis any time during pregnancy (6.9% and 7.3%) were within the CIs of the rates estimated by the Gaynes et al 2005 review (13, 14) and rates estimated for adult women in general (19).
In contrast, four studies included a nonperinatal comparison group but reported lower risk for major depressive disorder or psychiatric admission or both during pregnancy relative to risk during for nonperinatal women (20–23). However, three of these investigations (20, 21, 23) were from two historical cohort studies, which may be less reliable because of recall bias, and one was a registry study that reported treatment rates rather than illness prevalence or incidence rates (22). Others have found that treatment increases in the postpartum period, even if the actual rates of illness are not higher (24).
Our search also identified additional studies reporting on the prevalence of unipolar major depression during the first postpartum year. Generally, prevalence rates were consistent with those found by Gaynes, although one (25) of the two studies (25, 26) in developing nations had substantially higher estimates than those found in developed countries (18.6%) (25).
In summary, estimates for the period prevalence of major depressive disorder in pregnant and postpartum women vary widely among different studies and populations. Available data do not show substantial differences between point prevalence or incidence estimates in pregnancy compared with after delivery, but large variations among studies make it difficult to accurately determine differences. Despite significant gaps in the evidence base, the available data suggest that unipolar major depression may be marginally more prevalent during the postpartum period than among women in the general population.
Etiopathology of unipolar major depressive disorder in pregnant and postpartum women
It is likely that biological, psychological, and social factors interact to trigger an episode of unipolar major depressive disorder in pregnant and postpartum women. Known risk factors for major depressive disorder in postpartum women include a previous episode of major depressive disorder, life stress, and lack of social support in the postpartum period (27–29). Risk factors for major depressive disorder in pregnancy have been less well-studied but appear to be similar (30). However, not every woman who experiences these risk factors will have major depressive disorder develop, and there continues to be exploration of the biological mechanisms that will help practitioners identify who will have major depressive disorder develop and how it can best be treated.
The structure and function of various cortical regions such as the prefrontal cortex, anterior cingulate gyrus, and other areas of the limbic system are implicated generally in the etiology of major depressive disorder (31). Researchers also have focused on aberrations in selected neurotransmitter systems, including serotonin, norepinephrine, and dopamine, as well as the possible role of stress and the glucocorticoid system (for a comprehensive review on the etiopathology of major depressive disorder, see Belmaker and Agam 2008 [32]). However, the unique timing of major depressive disorder and biology of the perinatal period (particularly the postpartum period) has sparked investigation into whether major depressive disorder that occurs in pregnancy or the postpartum period has unique biological underpinnings relative to nonperinatal major depressive disorder. A synopsis of available literature on the biological factors related to major depressive disorder in perinatal women is reviewed here. The majority of studies relate to investigations of postpartum women, but those that are relevant to pregnant women are included when available.
Sex hormones
The dramatic changes in hormone levels that occur in pregnancy and after delivery have made this a popular area of exploration. Most of this work has assessed biological changes in women with postpartum depression. An interesting study of postnatal onset of unipolar major depressive disorder entailed administration of estrogen and progesterone in doses that would mimic the hormonal milieu of pregnancy (33). Women with a history of postpartum depression compared with those without such a history were more likely to experience depressive symptoms after hormonal withdrawal, which supports biological vulnerability to the disorder given the appropriate hormonal changes.
Perhaps one of the stronger pieces of evidence is that postpartum estrogen supplementation, which slows the postpartum decline in estrogen levels, leads to resolution of depressive symptoms (34, 35). However, estrogen has a myriad of effects on neurotransmitter systems that have been implicated in major depressive disorder and, thus, its influence may be indirect.
Neurotransmitter systems
Because of the known interplay between hormones and neurotransmitter systems, neurotransmitters implicated in major depressive disorder such as monoamine oxidases (MAOs), ie, MAO-A and MAO-B, serotonin, and norepinephrine have been specifically studied in perinatal populations. Through the use of positron emission tomography, researchers found that MAO-A levels in the early postpartum period correlate with a decrease in estrogen levels (36); this suggests a model in which an acute monoamine-lowering process contributes to the mood change indicative of postpartum blues and postpartum major depressive disorder. There is partial support for this among research that has assessed peripheral markers of neurotransmitter activity. For example, platelet serotonin is lower in postpartum women with major depressive disorder than in those without major depressive disorder (37). Serotonin transporter activity is greater in postpartum women with, as compared with those without, depressive symptoms and serotonin levels in platelets correlate with the severity of blues at 5 days postpartum (38). These results imply that lower synaptic serotonin may lead to depressive symptoms in postpartum women. However, one of these studies found that a metabolite of norepinephrine was elevated after delivery in women with, compared with women without, depressive symptoms (38). The research group hypothesizes that the later finding is a reaction to stress.
Hypothalamic-pituitary-adrenal axis abnormalities
Major depressive disorder has long been associated with hypothalamic-pituitary-adrenal axis dysfunction, and events of pregnancy and parturition may perturb this system. Nonpregnant individuals have an elegant feedback mechanism whereby cortisol, corticorticotropin-releasing hormone (CRH), and adrenocorticotropin (ACTH) autoreceptors decrease hormone production in the hypothalamus, anterior pituitary, and adrenal cortex. In pregnancy, the placenta independently produces a number of hormones (39) (eg, CRH, ACTH, and cortisol) that are regulated in a feed-forward manner, which leads to down-regulation of autoreceptors in the hypothalamus and anterior pituitary. This process of receptor down-regulation and the transition to a nonpregnant hormonal state has been hypothesized by some to constitute a period of vulnerability for mood disorders (40). Although depressed and nondepressed postpartum women do not suppress cortisol in response to dexamethasone, suppression of ACTH to bovine CRH is more profound among depressed postpartum women with than those without depressive symptoms (40). Further evidence suggests that the cortisol response is uncoupled from ACTH secretion in depressed as compared with nondepressed individuals (41). Overall elevations in hypothalamic-pituitary-adrenal axis are consistent with work that finds elevated CRH levels at 25 weeks of gestational age (42) and augmented cortisol response to stress (43) in pregnant women who go on to express postpartum depressive symptoms.
Immune system
Delivery of a newborn stimulates a pro-inflammatory state presumably attributable to pain, physical exertion, and tissue injury involved in delivery (44). Pro-inflammatory cytokines are also linked to hypothalamic-pituitary-adrenal axis activity and have been associated with mood disorders in nonpregnant individuals (45, 46). Interleukin-1β, a potent pro-inflammatory cytokine released from white blood cells, is elevated in the first month postpartum (47). In a cross-sectional study, serum interferon-γ or interleukin-10 and cortisol levels were measured in postpartum women and were lower in those who had depressive symptoms, suggesting a hypoactive hypothalamic-pituitary-adrenal and mixed pro-inflammatory immune response (48).
Thyroid function
A relationship between abnormalities in thyroid function and mood disorders is well-established. The thyroid axis is also affected by pregnancy via the effects of chorionic gonadotropin on the thyroid gland and may constitute an area of biological vulnerability for depression in pregnancy or after delivery. Associations between various thyroid indices and mood changes have been found for both pregnant (49, 50) and postpartum (49–53) women. These indices have included elevations of thyroid-stimulating hormone that would indicate hypothyroidism (50, 52, 53) and elevations of free T4 that would be consistent with subclinical hyperthyroidism (49). Some work also shows that elevations in thyroid autoantibodies are a risk factor for depressive symptoms in pregnancy and after delivery (50, 51, 54–56), although this finding is not uniform (49). It is reasonable to assess thyroid status in pregnant and postpartum women with depressive disorders because this may indicate thyroid dysfunction and may assist in the management of depression. However, it is not clear that abnormalities in thyroid parameters explain all cases of perinatal depressive disorders.
Melatonin
Parry et al (57) found that pregnant women with major depressive disorder had lower melatonin levels and postpartum women with major depressive disorder had higher melatonin levels as compared with their nondepressed counterparts. These findings are consistent with earlier work from this group that showed a beneficial effect of critically timed sleep deprivation on mood symptoms in postpartum women with major depressive disorder (58). Such results need to be replicated before they can be strongly implicated in the pathophysiology of major depressive disorder in pregnancy or after delivery.
Familial aggregation
An important finding is that major depressive disorder in pregnancy or after delivery tends to cluster within families. For example, using 838 female twin pairs from an Australian national twin registry, Treloar et al (1999) found that genetic factors accounted for 25% of the variance in interview-assessed postpartum major depressive disorder and 38% of the variance of postpartum depressive symptoms (59). Although the retrospective nature of the study is a limitation, two additional historical cohorts that included women with diagnosed major depressive disorder found evidence for familiality. In the multicenter Depression Network Study, investigators diagnosed postpartum major depressive disorder in 42% of 31 women with a sister who had experienced major depressive disorder after delivery compared with only 15% of 59 women whose sisters had not experienced postpartum major depressive disorder (60). A report from the Genetics of Recurrent Early-Onset major depressive disorder study revealed that having a sibling with major depressive disorder during pregnancy or after delivery increased the odds of a similar episode in the other sibling by 2.28 (95% CI 1.13–4.58); a sibling with postpartum major depressive disorder increased the odds of having postpartum major depressive disorder by 4.96 (95% CI 1.51–10.42) (61).
Genetic studies
Findings from familial aggregation studies have spurred the search for underlying genetic mechanisms. Polymorphisms in the serotonin transporter gene (5HTTLPR) lead to either low or high expression of the serotonin transporter. High expression of the transporter gene is linked with depressive symptoms, particularly when the serotonin precursor, tryptophan, is relatively depleted. The postpartum period is a time when tryptophan is relatively depleted and the high expression of polymorphisms is associated with elevated scores on the Edinburgh Postnatal Depression scale at 8 weeks postpartum, although not at 32 weeks postpartum (62). In a genome-wide linkage and association analysis of women with a postpartum mood disorder (bipolar or unipolar), two potential candidate genes were identified on chromosome 1, hemicentin 1 and methyltransferesae-like 13, although they did not retain statistical significance after correction for multiple testing (63). Hemicentin 1, which is highly expressed in the hippocampus, contains four estrogen binding sites and is altered by postpartum decreases in estrogen levels in rats. Methyltransferase-like 13 is likely to be involved in DNA methyltransferase activity, which plays a role in estrogen-receptor-induced gene transcription.
An additional investigation found evidence for possible genetic risk among pregnant and postpartum women. In a prospective cohort study of perinatal women, the low functioning polymorphism of the serotonin transporter was associated with depressive symptoms at 6 weeks postpartum (64). Additionally, the low functioning polymorphisms of two enzymes that degrade neurotransmitters, monoamine oxidase-A and catechol-O-methyl-transferase, were associated with depressive symptoms during the third trimester and 6-week time point after delivery. The contrasting results of this study with this genetic investigation and other biological work illustrate that this literature is not yet mature, but it suggests that ultimately we may understand genetic risk factors that increase the likelihood of depressive symptoms in pregnancy and after delivery.
In summary, there is evidence from correlational, familial, and genetic studies that there may be a subgroup of women who are uniquely at risk for unipolar major depressive disorder in pregnancy or in the postpartum period. The mechanisms for this increased biological risk continue to be poorly characterized but may be related to abrupt changes in sex steroids and monoamine levels; postnatal failure to achieve optimal regulation of thyroid hormone, immune responses, and the hypothalamic-pituitary-adrenal axis are additional contributors to unipolar mood disorders. There is limited evidence that some of these factors (eg, CRH in the second trimester of pregnancy) may be of promise as biological markers to help identify women at risk for perinatal depression.
Screening or case finding and diagnostic approach for women with a unipolar depressive disorder
Whether there are benefits to systematic screening for major depressive disorder in primary care settings has been the focus of numerous reports and is summarized by a recent meta-analysis (65) and recommendations from the United States Task Force on Preventive Services (66). In general, screening has increased case-finding, but whether this translates into superior patient outcomes is not clear. The United States Task Force on Preventive Services recommends screening when coupled with system changes that increase treatment availability or provide integrated treatment for major depressive disorder. Although this pertains to general primary care settings, there has been little research with pregnant and postpartum women. Preliminary work from a group in Olmsted County, Minnesota, found that screening increased case finding and utilization of mental health services (67), but discrepant findings also exist (68). The lack of data in support of screening led the American College of Obstetricians and Gynecologists to state that there are insufficient data to recommend the practice (69). However, future information may support the benefit of screening. Systematic follow-up and assessment of high-risk groups, such as women with a history of major depressive disorder or women who acknowledge substantial stress, may benefit from case-finding strategies.
Whether targeted or systematic screening procedures are implemented, variation among the subtypes of mood disorders can complicate the diagnostic work-up for an individual with a suspected mood disorder. A reasonable approach is to first determine if a woman is having an episode of major depressive disorder as indicated by expressing at least five of the symptoms listed in Box 1 for most of the day and for at least 2 weeks. Personal history of manic symptoms (Box 2) or a report that depressive symptoms only occurred in the setting of another medical condition (eg, hypothyroidism, hyperemesis gravidarum) will allow the clinician to determine whether this is unipolar major depressive disorder, part of a bipolar syndrome, or symptoms of another medical condition that is presenting as major depressive disorder. Women who do not have five symptoms but have two, three, or four symptoms most of the day for at least 2 weeks may also experience distress and impairment and may require treatment. Tools that can be helpful in case-finding for major depressive disorder are instruments such as the Patient Health Questionnaire-9 (70), which includes all of the items outlined in the Diagnostic and Statistical Manual, fourth edition, for major depressive disorder. A score of 5–9 indicates mild depression, a score of 10–14 indicates moderate depression, a score of 15–19 indicates moderate to severe depression, and a score higher than 19 indicates severe depression. Also useful is the Edinburgh Postnatal Depression Scale, which is designed for use in perinatal women (71). A score of greater than 12 in pregnancy or greater than 10 in the postpartum period indicates that the woman is likely experiencing major or minor depressive disorder. These measures are only screening questionnaires and scores will be elevated in women who have other psychiatric or drug use disorders. Thus, positive screens should be followed by a clinical interview.
Treatment of women with unipolar depression
Pregnant and postpartum women may worry about the stigma associated with mental illness (72). In the postpartum period, this is particularly heightened because women may fear child custody implications of their illness (72, 73). Thus, stigma and lack of knowledge about depression may contribute to, and hinder the treatment of, postpartum depression. This reinforces the importance of making treatment decisions shared between the patient, family members, and health care providers. A discussion of the risks and benefits of all interventions should be carefully documented in the patient's chart.
Preventive interventions
This work has focused on women at risk for postpartum unipolar depression. A review of 15 trials of various psychosocial and psychological interventions concluded that intensive postpartum support provided by a health professional such as a public health nurse or midwife was the most effective intervention for mitigating the onset of a postpartum mood disorder (74). These results may be bolstered by the benefit related to continuity of care. More recent evidence suggests that peer support via the telephone also may be effective in the prevention of postpartum depression (75).
Approach to treatment
We present an algorithm for the treatment of pregnant and postpartum women with mood disorders (Fig. 1). Four critical questions need to be addressed initially. First, does the woman have current or past bipolar disorder? Second, does the woman have symptoms of psychosis? Third, does the woman have suicidal or homicidal (eg, toward baby) thoughts? Fourth, does the woman have major or minor depressive disorder without a history of bipolar disorder? The treatment recommendations that follow should guide clinicians through the management of women who meet various scenarios. In this section, we discuss women for whom the answers to the first two questions are “no.” Treatment recommendations for women with bipolar disorder can be found in that section.
Behavioral treatments
Psychosocial and psychological treatments for major depressive disorder can be applied alone or in combination with medication therapy. In general, psychosocial and psychological treatments without medication therapy are recommended as first-line treatments for women with mild or mild-moderate depressive illness (5). Behavioral studies have examined the effectiveness of various psychosocial and psychological interventions for minor depressive disorder and major depressive disorder during pregnancy and the postpartum period. Tested interventions include antenatal and postnatal classes (76, 77), group psycho-education (78–81), postpartum debriefing before leaving the hospital or clinic (82–84), nondirective counseling (85, 86), interpersonal psychotherapy (87–91), and cognitive behavioral therapy (92–97). Despite methodologic insufficiencies in some studies (122), these data indicate that individual psychotherapy is helpful for both pregnant and postpartum women with mild-moderate depression. A limitation is that evidence is strongest for structured psychotherapies, particularly interpersonal psychotherapy and cognitive behavioral therapy (97, 98). These treatments may not be feasible or readily available for some patients. In areas where such resources are not accessible, other psychotherapeutic interventions also may prove useful.
Antidepressant medication in pregnancy
Decisions about how to treat women with major depressive disorder during pregnancy involve consideration of the risks of untreated depression to mother and fetus, as well as the potential risks of treatment itself. Untreated depression during pregnancy and at delivery is associated with a number of complications that include poor prenatal care, substance use, preterm birth, and low birth weight (99–103). Behavioral treatments such as those outlined avoid fetal exposure to medication and are efficacious, but they may require several months before improvements are observed and may be more effective for mild to moderate mood episodes. Antidepressants can be required for many depressed women who are pregnant or who recently delivered, although some work suggests that they do not come without risk.
A recent summary of the risks of antidepressant use in pregnancy was published by a committee appointed by the American Psychiatric Association and American College of Obstetricians and Gynecologists (103). The report reviewed studies that found connections between antidepressant use in pregnancy and miscarriage, preterm birth, cardiac defects (first trimester exposure), persistent pulmonary hypertension of the newborn (second and third trimester exposure), and neonatal adaptation syndrome (a self-limited withdrawal syndrome seen after late third trimester exposure). Results suggest that risk differs across outcome but for all of these potential adverse events, the magnitude of risk was small. The report also notes the inconsistencies in the literature and the possibility that patient characteristics, underlying illness, and poor health habits contributed to the various effects.
When patients do not have comorbid conditions that are severe or psychotic, obstetricians may elect to start an antidepressant medication. For women with a history of treatment, a reasonable guide is to reinstate a medication that has been well-tolerated and efficacious for the given patient. If the patient has not been using medication previously, then the newer antidepressants (selective serotonin reuptake inhibitors and buporprion) are better-tolerated and of equivalent efficacy to older medications (5). If a patient or clinician prefers the use of a tricyclic medication, then blood levels can be monitored through pregnancy. These agents will likely require dosage increases as pregnancy progresses, although clinical assessment is a better marker of response than are blood levels (104). With the possible exception of citalopram, dosage adjustments because of the changing pharmacokinetic parameters of pregnancy are not required for most newer medications (105). Women who smoke may want to consider bupropion because this can assist with smoking cessation. Some clinicians have concerns about the use of paroxetine given its association with heart malformations in some studies, although most experts do not consider this agent teratogenic (106, 107). Moreover, other medications in this class have been implicated in a variety of malformations, although the literature is inconsistent (108). Persistent pulmonary hypertension has been linked to a six-fold increased risk with usage of serotonin reuptake inhibitors after 20 weeks of gestation (109, 110). It is not clear if this finding is confounded by cumulative exposure to antidepressants in pregnancy, psychiatric illness, or other factors. If the association between serotonin reuptake inhibitors and persistent pulmonary hypertension is causal, then the absolute risk is six to 12 afflicted offspring per 1,000 (109).
As shown in Figure 1, concurrent psychotic symptoms or suicidal ideation should trigger an immediate referral to a psychiatrist. At the same time, causes of these symptoms such as illicit drug use or concurrent medical problems (eg, thyroid disorder) should be ruled out as a central or contributory cause.
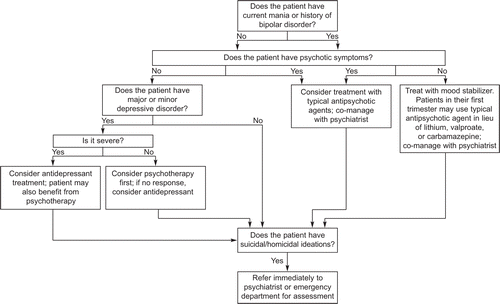
Yonkers. Mood Disorders in Pregnant and Postpartum Women. Obstet Gynecol 2011.
Alternative somatic treatment options
Electroconvulsive therapy has been used to treat major depressive disorder and bipolar disorder in pregnant women and has been shown to be highly efficacious. It requires a general anesthetic and is associated with side effects for patients such as memory loss. A review of the literature of electroconvulsive therapy use in pregnancy shows that it has been associated with adverse cardiac effects, as well as some adverse neonatal outcomes (111). As such, its use in pregnancy is usually limited to severe treatment-resistant cases of depression, acute suicidal tendencies, depression with psychotic features, or severe dehydration or malnutrition that can occur as part of a depressive syndrome. Although it is unlikely that an obstetrician will initiate electroconvulsive-therapy treatment, he or she likely will be involved in the decision to use it and in the comanagement of care for patients who require it.
Techniques such as repetitive transcranial magnetic stimulation are being investigated widely for the treatment of severe or treatment-resistant major depressive disorder. Because magnetic fields have been associated with increased risk of miscarriage, there is a theoretical risk to administering transcranial magnetic stimulation in pregnant women (112). Although it is not yet recommended for pregnant women, it is an experimental treatment that is undergoing study in pregnant populations.
Other alternative treatments
There is limited support for some nonpharmacologic alternatives to psychotherapy, including dietary calcium (113), exercise (114), massage therapy (115, 116), and bright light therapy (117, 118). If there is benefit, then it is likely for women with mild depressive illness and should not be recommended as the standard of care for women with moderate to severe major depressive disorder. There was early enthusiasm for omega-3 fatty acids, although most randomized clinical trials to date have failed to show that the active treatment differs from placebo (119–121). Similarly, despite literature support for estrogen treatment in postpartum women (34, 35), there are insufficient data to support estrogen as a standard treatment for major depressive disorder. Moreover, there is a concern about estrogen promotion of thromboembolic events, which can complicate its use in postpartum women.
Lactation
It appears that the amount of drug that passes into breast milk is small but extremely discrepant between women and within the same woman at different times. Variations result from genetic influences on maternal and child enzyme activity, a point at which the child feeds relative to the mother's ingestion of medication (122, 123), and properties of the breast milk. In the existing literature, antidepressant exposure through breast milk is not associated with material risks to full-term and healthy infants, although minimal data are available for preterm infants. However, as outlined in a recent review (124), certain considerations should be addressed before initiation of antidepressants while breastfeeding: 1) consider behavioral treatments as first-line interventions, particularly for mild to moderate depression; 2) obtain thorough informed consent and provide a risk-benefit analysis to the mother and her family; 3) moderate to severe major depressive disorder are appropriately treated by antidepressants; and 4) clinical factors, such as previous history of treatment response, should drive the selection of the antidepressant.
Epidemiology of bipolar disorder during pregnancy and the postpartum period
Information about the rates of illness for bipolar disorder in pregnant and postpartum women are complicated by several factors: 1) the overall prevalence rate for bipolar disorder is only approximately 0.5%–1% (125), mandating large sample sizes to provide reliable incidence and prevalence estimates; 2) bipolar disorder in the puerperium may present as postpartum psychosis, yet not all women who become psychotic in the few months after delivery have bipolar disorder; 3) women who experience bipolar disorder may have their symptoms misdiagnosed as a unipolar depressive disorder after delivery (126); and 4) risk of relapse for bipolar disorder is tightly linked with medication discontinuation (127) and, because a number of agents that are used to treat bipolar disorder are teratogens (128), many women stop medications during pregnancy (129).
Despite these complexities, a large linked Swedish registry study found that the incidence of hospitalization for a bipolar (manic or depressive) episode in the 3 months after delivery was .03%, which constitutes approximately half of the women who were hospitalized for postpartum psychosis (130). However, this is likely an underestimate of the exacerbation of bipolar disorder either in pregnancy or after delivery because this indicates hospitalizations rather than illness episodes. Between 25%–30% of women with bipolar disorder who become pregnant and deliver will experience an episode of depression or mania either in pregnancy or after delivery (131–133). The latter statistic illustrates the high risk that women with bipolar disorder have for illness relapse in pregnancy or after delivery.
There is no evidence that the period prevalence of bipolar disorder differs between pregnant and nonpregnant women. However, the linkage between medication discontinuation and relapse that we note is profound and suggests little protective effect of pregnancy on risk of bipolar disorder relapse (127, 134, 135). Studies find that between 80% and 100% of women who stop mood stabilizer medication during pregnancy will experience relapse soon after medication discontinuation; these rates are two-fold to three-fold higher than rates found for pregnant women with bipolar illness who continue medication in pregnancy (134, 135). Women who experience relapse appear to be more likely to have relapse into an episode of depression or mixed-mood episode that is characterized by both manic and depressive symptoms.
Although medication discontinuation largely explains the relapse one sees among pregnant women with bipolar disorder, the biological processes that occur in late pregnancy and postpartum appear to contribute to relapse in the postpartum period (136). Registry studies indicate that hospitalization and outpatient treatment for bipolar disorder increase after delivery as compared with pregnancy (130, 137, 138). To illustrate, a comparison of pregnant and nonpregnant women who discontinued lithium treatment showed that the rate of relapse was the same for pregnant women and nonpregnant women. However, the risk was nearly three-times higher in postpartum (70%) than in nonpregnant (24%) women (127). It is critical to note that in women with bipolar disorder, relapse or illness onset for a first episode occurs rapidly and often within a few days after delivery and may differentiate unipolar major depressive disorder from bipolar depression or mania (127, 137).
Etiopathology of bipolar disorder onset or relapse in pregnant and postpartum women
Psychosocial stress, interpersonal difficulties, and environmental events are mentioned as risk factors for unipolar major depressive disorder. In contrast, these factors do not play a great role in the perinatal risk of mania or depression among women with bipolar disorder (see also Sit et al for review [136]) (12, 131, 139). Women with bipolar disorder who become ill after delivery are more likely to have biological risk factors of younger age (12, 131) and first pregnancy (12, 131, 140).
Neurotransmitter systems
It is also possible that some of the neuroendocrine systems that confer risk for unipolar illness also increase the liability to experience a manic or mixed episode in pregnancy or after delivery. This has received only scarce attention in the literature, and what little information is available has focused on dopamine systems.
Older antipsychotic agents block activity at dopamine receptors, a property that leads to the “dopamine hypothesis” for psychosis. There remains substantial evidence for dysregulation of dopamine and particularly dopamine receptor supersensitivity in psychotic and mood disorders. With reference to postpartum onset of mania or (psychotic) depression, estrogen acutely antagonizes dopamine activity by decreasing dopamine production and blocking activity at dopamine receptors. As estrogen is withdrawn after delivery, the dopamine system is supersensitized (141). Dopamine supersensitivity was assessed in one study (142) through administration of apomorphine to postpartum women with a history of bipolar or schizoaffective disorder. Women who experienced relapse had a more robust growth hormone response to apomorphine than did asymptomatic and nonpsychotic women, supporting the hypothesis of dopamine supersensitivity. However, an attempt by another group to replicate these findings, particularly in women with bipolar disorder, was unsuccessful (143).
Sleep and biological rhythm dysregulation
As with unipolar major depressive disorder, alterations in sleep and biological rhythms may elicit postpartum relapse of mania or depression among pregnant women with bipolar disorder (144, 145). Although there is some empirical support for this hypothesis (146), more work is needed.
Familial aggregation
The possibility that delivery is a genetically programmed trigger for relapse among women with bipolar disorder has been investigated by severalgroups. Puerperal relapse occurred in 20 of 27 (74%) women with bipolar disorder who had a sister with puerperal psychosis, whereas puerperal relapse only occurred in 38 of 125 (30%) women with bipolar disorder who did not have a family history of puerperal psychosis (132). Differential risk was found by a second group who estimated a 2.3-fold elevation (95% CI 1.21–4.40) in the odds of postpartum relapse of bipolar disorder (147). Linkage for puerperal psychosis has been found for a site on chromosome 16, although this has not been replicated (148).
In sum, there is less study of the biological risk factors associated with bipolar relapse in pregnancy or after delivery, although the literature suggests a strong signal for genetic factors. The low prevalence rate of bipolar disorder, treatment as a protective factor, and the potent effect of discontinuing treatment either in pregnancy or after delivery have made it difficult to study the biology of perinatal relapse in women with bipolar disorder (127, 135).
Screening or case finding and diagnostic approach for women with bipolar disorder
We are unaware of case-finding studies for bipolar disorder in perinatal women. Patients who seem pressured, “revved-up,” or overly euphoric should be questioned carefully about other symptoms consistent with mania (eg, racing thoughts, psychosis, and others; Box 2) as well as any psychiatric history. Similar precautions should be followed for women who have major depressive disorder because they may be experiencing the depressed pole of bipolar disorder. Obstetricians should also query the patient about use of drugs that could mimic a manic syndrome (eg, cocaine or stimulants) and should rule out mania triggered by antidepressant medication use. The latter may “unmask” a bipolar syndrome for a woman previously thought to have unipolar depression. A useful screening questionnaire for bipolar disorder is the Mood Disorder Questionnaire (150).
Treatment of women with bipolar disorder
Standard treatment for bipolar disorder includes typical mood stabilizers such as lithium, carbamazepine, valproic acid, and lamotrigine, as well as antipsychotic medication. Whereas older antipsychotics are not Food and Drug Administration-approved treatments, clinical practice supports their efficacy (151). Many of the newer atypical antipsychotics are Food and Drug Administration-approved for the management of bipolar disorder. Psychotherapy alone is not a recommended treatment, although it may be a useful adjunct to treatment (152).
Treatment of bipolar disorder in pregnancy is complex and requires the consideration of the risks and benefits of using mood stabilizers, some of which are associated with teratogenic properties (valproate, carbamazepine) (153, 154). Insufficient data are available to determine the safety of most novel antipsychotics, even though many clinicians are using these medications as alternatives to mood stabilizers (for a review of antipsychotic medication use in pregnancy, see Einarson and Boscovic 2009 [155]). Early reports warned of congenital heart disease in children exposed to lithium carbonate in utero, but subsequent analyses have shown these risks to be only slightly greater than those in the general population (156, 157).
Treatment of a pregnant or postpartum woman with bipolar disorder must consider the well-being of the mother and the fetus or infant. In pregnancy, it is not uncommon for a woman with bipolar disorder to present for treatment when she is past 28 days and thus beyond the period when neural tube closure occurs. Termination of treatment at this point puts the mother at risk while providing minimal benefit to the fetus. Even when the mother presents before pregnancy or early in pregnancy, the high relapse rates for women with bipolar disorder after medication discontinuation may argue for medication continuation. Mood stabilizers administered in pregnancy decrease the likelihood of recurrence into a manic or depressive episode (158, 159). However, if a woman is stable, then there does not appear to be prophylactic benefit of the mood stabilizer valproate against a postpartum recurrence of mania or depression (160). Clinical history can be helpful in this scenario. For some women who are asymptomatic, it is prudent to monitor them closely rather than reflexively re-initiating treatment. However, women who discontinued medication secondary to concerns about teratogenicity but who have a history of relapse after medication discontinuation should consider medication reinstatement.
Physicians and patients who have concerns about the possible teratogenic properties of lithium and anticonvulsants may want to consider use of a typical (older) antipsychotic agent, because they are not associated with fetal anomalies (157). However, there is also a risk of recurrence when a woman is switched from one medication to another.
Some patients may prefer to continue lithium throughout pregnancy. In this case, serum levels should be carefully monitored. Dosages may require adjustment upwards because of increases in extracellular fluid volume during pregnancy (and divided doses may be safer than once-daily dosing) (153). Dosage should be reduced after delivery to avoid lithium toxicity in the early postpartum period.
If the patient has psychotic symptoms, then a first-generation antipsychotic would be preferable to a mood stabilizer as the initial treatment and the latter can be added later. Patients with suicidal or homicidal ideation will need immediate assessment from a psychiatrist and may require inpatient treatment.
Many women with bipolar disorder present in the depressed phase of the illness. If an obstetrician elicits a history of bipolar disorder, then the initial treatment should optimally include either a mood stabilizer or an older antipsychotic agent because an antidepressant alone can trigger mania. If the initial treatment is insufficient, then an antidepressant can be added. Patients who strongly prefer monotherapy with an antidepressant agent because of concerns about teratogenicity should be monitored closely and switched to a mood stabilizer or antidepressant with mood stabilizer after they have completed their first trimester. As noted, electroconvulsive therapy is an excellent treatment for severe depression in patients with bipolar disorder.
Lactation
Women with bipolar disorder or a psychotic depression may require antipsychotic treatment. The atypical antipsychotics, such as olanzapine, for which we have less information, appear to be like older antipsychotics and can lead to extrapyramidal side effects in the infant (161). Lithium is detectable in breast milk and many counsel caution in its use while breastfeeding (162, 163), although the American Academy of Pediatrics (164) no longer designates lithium as absolutely contraindicated in breastfeeding women. If used, then the infant's lithium levels and complete blood count should be monitored. No adverse effects from the use of carbamazepine, valproate, or lamotrigine have been reported. However, serum concentrations of lamotrigine were 30% of maternal levels in one study, leading to recommendations that such infants be monitored for rash (124).
Conclusion
Unipolar and bipolar mood disorders, which are common in pregnant and postpartum women, deserve the attention of obstetric providers. Procedures to identify those at risk should begin in pregnancy if not in the preconceptional period. There is evidence to suggest familiality for perinatal risk among women with unipolar major depressive disorder and bipolar disorder. Psychotherapeutic counseling is beneficial for mild to moderately ill women with unipolar major depressive disorder, but medication is likely required for women with severe major depressive disorder or bipolar disorder. Obstetric management that coordinates care with psychiatric providers is likely to optimize outcomes for mothers and their children.
1 Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–105.Crossref, Google Scholar
2 Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 2005;62:1097–106.Crossref, Google Scholar
3 Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J Affect Disord 1993;29:77–84.Crossref, Google Scholar
4 American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed. Arlington (VA): American Psychiatric Association; 1994.Google Scholar
5 Gelenberg A, Freeman M, Markowitz J, Rosenbaum JF, Thase ME, Trivedi MH, et al. American Psychiatric Association practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Arlington (VA): American Psychiatric Association; 2010. p. 152.Google Scholar
6 Rush AJ, Golden WE, Hall GW, Herrera M, Houston A, Kathol RG, et al. Depression in primary care: volume 2. Treatment of major depression. Clinical Practice Guideline Number 5. Rockville (MD): Agency for Health Care Policy and Research; 1993.Google Scholar
7 Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: The Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry 2009; 66:287–96.Crossref, Google Scholar
8 Viguera AC, Cohen LS. The course and management of bipolar disorder during pregnancy. Psychopharmacol Bull 1998;34:339–46.Google Scholar
9 McNeil TF. A prospective study of postpartum psychoses in a high-risk group. 2. Relationships to demographic and psychiatric history characteristics. Acta Psychiatr Scand 1987;75:35–43.Crossref, Google Scholar
10 Robling SA, Paykel ES, Dunn VJ, Abbott R, Katona C. Long-term outcome of severe puerperal psychiatric illness: a 23 year follow-up study. Psychol Med 2000;30:1263–71.Crossref, Google Scholar
11 Schopf J, Bryois C, Jonquiere M, Le PK. On the nosology of severe psychiatric post-partum disorders. Results of a catamnestic investigation. Eur Arch Psychiatry Clin Neurosci 1984; 234:54–63.Crossref, Google Scholar
12 Videbech P, Gouliaev G. First admission with puerperal psychosis: 7–14 years of follow-up. Acta Psychiatr Scand 1995;91:167–73.Crossref, Google Scholar
13 Gaynes B, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal depression: prevalence, screening accuracy and screening outcomes. Evidence Report/Technology Assessment No. 119. AHRQ Publication No 5-E006–2. Rockville (MD): Agency for Healthcare Research and Quality; 2005.Google Scholar
14 Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 2005;106:1071–83.Crossref, Google Scholar
15 O'Hara MW. Social support, life events, and depression during pregnancy and the puerperium. Arch Gen Psychiatry 1986;43:569–73.Crossref, Google Scholar
16 Cooper PJ, Campbell EA, Day A, Kennerley H, Bond A. Non-psychotic psychiatric disorder after childbirth. A prospective study of prevalence, incidence, course and nature. Br J Psychiatry 1988;152:799–806.Crossref, Google Scholar
17 Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. Br J Psychiatry 1993;163:27–31.Crossref, Google Scholar
18 O'Hara MW, Zekoski EM, Philipps LH, Wright EJ. Controlled prospective study of postpartum mood disorders: comparison of childbearing and nonchildbearing women. J Abnorm Psychol 1990;99:3–15.Crossref, Google Scholar
19 Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord 2002;68:167–81.Crossref, Google Scholar
20 Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry 2007;164:1515–20.Crossref, Google Scholar
21 Mota N, Cox BJ, Enns MW, Calhoun L, Sareen J. The relationship between mental disorders, quality of life, and pregnancy: findings from a nationally representative sample. J Affect Disord 2008;109:300–4.Crossref, Google Scholar
22 Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA 2006;296:2582–9.Crossref, Google Scholar
23 Vesga-López O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry 2008;65:805–15.Crossref, Google Scholar
24 Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. Am J Obstet Gynecol 2005;192:522–6.Crossref, Google Scholar
25 Abiodun OA. Postnatal depression in primary care populations in Nigeria. Gen Hosp Psychiatry 28:133–6.Crossref, Google Scholar
26 Nakku JE, Nakasi G, Mirembe F. Postpartum major depression at six weeks in primary health care: prevalence and associated factors. Afr Health Sci 2006;6:207–14.Google Scholar
27 O'Hara MW, Swain AM. Rates and risk of postpartum depression-a meta-analysis. Int Rev Psychiatry 1996;8:37–54.Crossref, Google Scholar
28 Beck CT. A meta-analysis of predictors of postpartum depression. Nurs Res 1996;45:297–303.Crossref, Google Scholar
29 Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry 2004;26:289–95.Crossref, Google Scholar
30 Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol 2010;202:5–14.Crossref, Google Scholar
31 Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord 2009;117:1–17.Crossref, Google Scholar
32 Belmaker RH, Agam G. Major depressive disorder. N Engl J Med 2008;358:55–68.Crossref, Google Scholar
33 Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000;157:924–30.Crossref, Google Scholar
34 Ahokas A, Kaukoranta J, Aito M. Effect of oestradiol on postpartum depression. Psychopharmacology 1999;146:108–10.Crossref, Google Scholar
35 Gregoire AJP, Kumar R, Everitt B, Henderson AF, Studd JWW. Transdermal oestrogen for treatment of severe postnatal depression. Lancet 1996;347:930–3.Crossref, Google Scholar
36 Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, Bloomfield PM, et al. Elevated brain monoamine oxidase A binding in the early postpartum period. Arch Gen Psychiatry 2010;67:468–74.Crossref, Google Scholar
37 Maurer-Spurej E, Pittendreigh C, Misri S. Platelet serotonin levels support depression scores for women with postpartum depression. J Psychiatry Neurosci 2007;32:23–9.Google Scholar
38 Doornbos B, Fekkes D, Tanke MA, de Jongee P, Korf J. Sequential serotonin and noradrenalin associated processes involved in postpartum blues. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:1320–5.Crossref, Google Scholar
39 Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med 1998;129: 229–40.Crossref, Google Scholar
40 Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab 1996;81:1912–7.Google Scholar
41 Jolley SN, Elmore S, Barnard KE, Carr DB. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biological Research for Nursing 2007;8:210–22.Crossref, Google Scholar
42 Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry 2009;66:162–9.Crossref, Google Scholar
43 Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med 2006;68:931–7.Crossref, Google Scholar
44 Corwin EJ, Pajer K, Corwin EJ, Pajer K. The psychoneuro-immunology of postpartum depression. J Womens Health (Larchmt) 2008;17:1529–34.Crossref, Google Scholar
45 Maes M. The depressogenic effects of cytokines: Implications for the psychological and organic aetiology and treatment of depression. Int J Neuropsychopharmacol 2002;5:329–31.Crossref, Google Scholar
46 Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 1997;9:853–8.Crossref, Google Scholar
47 Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biological Research for Nursing 2008;10:128–33.Crossref, Google Scholar
48 Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology 2007;32:133–9.Crossref, Google Scholar
49 Bunevicius R, Kusminskas L, Mickuviene N, Bunevicius A, Pedersen CA, Pop VJ. Depressive disorder and thyroid axis functioning during pregnancy. World Journal of Biological Psychiatry 2009;10:324–9.Crossref, Google Scholar
50 McCoy SJ, Beal JM, Payton ME, Stewart AL, DeMers AM, Watson GH. Postpartum thyroid measures and depressive symptomology: a pilot study. J Am Osteopath Assoc 2008;108:503–7.Google Scholar
51 Harris B. Postpartum depression and thyroid antibody status. Thyroid 1999;9:699–703.Crossref, Google Scholar
52 Pedersen CA, Johnson JL, Silva S, Bunevicius R, Meltzer-Broday S, Hamer RM, et al. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology 2007;32:235–45.Crossref, Google Scholar
53 Pedersen CA, Stern RA, Pate J, Senger MA, Bowes WA, Mason GA. Thyroid and adrenal measures during late pregnancy and the puerperium in women who have been major depressed or who become dysphoric postpartum. J Affect Disord 1993;29:201–11.Crossref, Google Scholar
54 Kuijpens JL, Vader HL, Drexhage HA, Wiersinga WM, van Son MJ, Pop VJ. Thyroid peroxidase antibodies during gestation are a marker for subsequent depression postpartum. Eur J Endocrinol 2001;145:579–84.Crossref, Google Scholar
55 Oretti RG, Harris B, Lazarus JH, Parkes AB, Crownshaw T. Is there an association between life events, postnatal depression and thyroid dysfunction in thyroid antibody positive women? Int J Soc Psychiatry 2003;49:70–6.Crossref, Google Scholar
56 Pop VJ, de Rooy HA, Vader HL, van der Heide D, van Son MM, Komproe IH. Microsomal antibodies during gestation in relation to postpartum thyroid dysfunction and depression. Acta Endocrinol 1993;129:26–30.Crossref, Google Scholar
57 Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, et al. Plasma melatonin circadian rhythm disturbances during pregnancy and postpartum in depressed women and women with personal or family histories of depression. Am J Psychiatry 2008;165:1551–8.Crossref, Google Scholar
58 Parry BL, Curran ML, Stuenkel CA, Yokimozo M, Tam L, Powell KA, et al. Can critically timed sleep deprivation be useful in pregnancy and postpartum depressions? J Affect Disord 2000;60:201–12.Crossref, Google Scholar
59 Treloar SA, Martin NG, Bucholz KK, Madden PA, Heath AC. Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychol Med 1999;29:645–54.Crossref, Google Scholar
60 Davidson L, McComb JG, Bowen I, Krieger MD. Craniospinal Langerhans cell histiocytosis in children: 30 years' experience at a single institution. J Neurosurg Pediatr 2008;1:187–95.Crossref, Google Scholar
61 Murphy-Eberenz K, Zandi PP, March D, Crowe RR, Scheftner WA, Alexander M, et al. Is perinatal depression familial? J Affect Disord 2006;90:49–55.Crossref, Google Scholar
62 Sanjuan J, Martin-Santos R, Garcia-Esteve L, Carot JM, Guillamat R, Gutierrez-Zotes A, et al. Mood changes after delivery: role of the serotonin transporter gene. Br J Psychiatry 2008;193:383–8.Crossref, Google Scholar
63 Mahon PB, Payne JL, MacKinnon DF, Mondimore FM, Goes FS, Schweizer B, et al. Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am JPsychiatry 2009;166:1229–37.Crossref, Google Scholar
64 Doornbos B, Dijck-Brouwer DA, Kema IP, Tanke MA, van Goor SA, Muskiet FA, et al. The development of peripartum depressive symptoms is associated with gene polymorphisms of MAOA, 5-HTT and COMT. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:1250–4.Crossref, Google Scholar
65 Gilbody S, Sheldon T, House A. Screening and case-finding instruments for depression: a meta-analysis. CMAJ 2008;178:997–1003.Crossref, Google Scholar
66 Pignone MP, Gaynes BN, Rushton JL, Burchell CM, Orleans CT, Mulrow CD, et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventative Services Task Force. Ann Intern Med 2002;136:765–76.Crossref, Google Scholar
67 Georgiopoulos A, Bryan T, Wollan P, Yawn B. Routine screening for postpartum depression. J Fam Pract 2001;50:117–22.Google Scholar
68 Yonkers KA, Smith MV, Lin H, Howell HB, Shao L, Rosenheck RA. Depression screening of perinatal women: an evaluation of the healthy start depression initiative. Psychiatr Serv 2009;60:322–8.Crossref, Google Scholar
69 Screening for depression during and after pregnancy. ACOG Committee Opinion No. 453. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;115:394–5.Crossref, Google Scholar
70 Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13.Crossref, Google Scholar
71 Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782–6.Crossref, Google Scholar
72 Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth 2006;33:323–31.Crossref, Google Scholar
73 Morrell CJ, Slade P, Warner R, Paley G, Dixon S, Walters SJ, et al. Clinical effectiveness of health visitor training in psychologically informed approaches for depression in postnatal women: pragmatic cluster randomised trial in primary care. BMJ 2009;338:a3045.Crossref, Google Scholar
74 Dennis C. Psychosocial and psychological interventions for prevention of postnatal depression: A systematic review. BMJ 2005;331:15.Crossref, Google Scholar
75 Dennis CL, Hodnett E, Kenton L, Weston J, Zupancic J, Stewart DE, et al. Effect of peer support on prevention of postnatal depression among high risk women: multisite randomised controlled trial. BMJ 2009;338:a3064.Crossref, Google Scholar
76 Brugha TS, Wheatley S, Taub NA, Culverwell A, Friedman T, Kirwan P, et al. Pragmatic randomized trial of antenatal intervention to prevent post-natal depression by reducing psychosocial risk factors. Psychol Med 2000;30:1273–81.Crossref, Google Scholar
77 Reid M, Glazener C, Murray GD, Taylor GS. A two-centred pragmatic randomised controlled trial of two interventions of postnatal support. Bjog-an International J Obstet Gynaecol 2002;109:1164–70.Crossref, Google Scholar
78 Morgan M, Matthey S, Barnett B, Richardson C. A group programme for postnatally distressed women and their partners. J Adv Nurs 1997;26:913–20.Crossref, Google Scholar
79 Honey KL, Bennett P, Morgan M. A brief psycho-educational group intervention for postnatal depression. Br J Clin Psychol 2002;41:405–9.Crossref, Google Scholar
80 Misri S, Kostaras X, Fox D, Kostaras D. The impact of partner support in the treatment of postpartum depression. Canadian Journal of Psychiatry-Revue Canadienne De Psychiatrie 2000;45:554–8.Google Scholar
81 Ngai FW, Chan SW, Ip WY. The effects of a childbirth psychoeducation program on learned resourcefulness, maternal role competence and perinatal depression: a quasi-experiment. Int J Nurs Stud 2009;46:1298–306.Crossref, Google Scholar
82 Lavender T, Walkinshaw SA. Can midwives reduce postpartum psychological morbidity? A randomized trial. Birth 1998;25:215–9.Crossref, Google Scholar
83 Small R, Lumley J, Donohue L, Potter A, Waldenström U. Randomised controlled trial of midwife led debriefing to reduce maternal depression after operative childbirth. BMJ 2000;321:1043–7.Crossref, Google Scholar
84 Priest SR, Henderson J, Evans SF, Hagan R. Stress debriefing after childbirth: a randomised controlled trial. Med J Aust 2003;178:542–5.Crossref, Google Scholar
85 Holden JM, Sagovsky R, Cox JL. Counselling in a general practice setting: controlled study of health visitor intervention in treatment of postnatal depression. BMJ 1989;298:223–6.Crossref, Google Scholar
86 Wickberg B, Hwang CP. Counselling of postnatal depression: a controlled study on a population based Swedish sample. J Affect Disord 1996;39:209–16.Crossref, Google Scholar
87 Stuart S, O'Hara MW. Treatment of postpartum depression with interpersonal psychotherapy. Arch Gen Psychiatry 1995;52:75–6.Crossref, Google Scholar
88 Spinelli MG. Interpersonal psychotherapy for depressed antepartum women: a pilot study. Am J Psychiatry 1997;154:1028–30.Crossref, Google Scholar
89 Klier CM, Muzik M, Rosenblum K, Lenz G. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression: A pilot study. Infant Mental Health J 2000;21:381.Google Scholar
90 Zlotnick C, Miller IW, Pearlstein T, Howard M, Sweeney P. A preventive intervention for pregnant women on public assistance at risk for postpartum depression. Am J Psychiatry 2006;163:1443–5.Crossref, Google Scholar
91 Grote NK, Swartz HA, Geibel SL, Zuckoff A, Houck PR, Frank E. A randomized controlled trial of culturally relevant, brief interpersonal psychotherapy for perinatal depression. Psychiatr Serv 2009;60:313–21.Crossref, Google Scholar
92 Meager I, Milgrom J. Group treatment for postpartum depression: a pilot study. Aust N Z J Psychiatry 1996;30:852–60.Crossref, Google Scholar
93 Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ 1997;314:932–6.Crossref, Google Scholar
94 Chabrol H, Teissedre F, Saint-Jean M, Teisseyre N, Rogé B, Mullet E. Prevention and treatment of post-partum depression: a controlled randomized study on women at risk. Psychol Med 2002;32:1039–47.Crossref, Google Scholar
95 Cho HJ, Kwon JH, Lee JJ. Antenatal cognitive-behavioral therapy for prevention of postpartum depression: a pilot study. Yonsei Med J 2008;49:553–62.Crossref, Google Scholar
96 Cooper PJ, Murray L, Wilson A, Romaniuk H. Controlled trial of the short- and long-term effect of psychological treatment of post-partum depression. I. Impact on maternal mood. Br J Psychiatry 2003;182:412–9.Crossref, Google Scholar
97 Mulcahy R, Reay RE, Wilkinson RB, Owen C. A randomised control trial for the effectiveness of group interpersonal psychotherapy for postnatal depression. Arch Womens Mental Health 2010;13:125–39.Crossref, Google Scholar
98 O'Hara MW, Stuart S, Gorman LL, Wenzel A. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry 2000;57:1039–45.Crossref, Google Scholar
99 Einarson A. Antidepressants and pregnancy: complexities of producing evidence-based information. CMAJ 2010;182:1017–8.Crossref, Google Scholar
100 Steer RA, Scholl TO, Hediger ML, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol 1992;45:1093–9.Crossref, Google Scholar
101 Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry 2006;63:898–906.Crossref, Google Scholar
102 Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry 2007;164:1206–13.Crossref, Google Scholar
103 Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol 2009;114:703–13.Crossref, Google Scholar
104 Wisner KL, Perel JM, Wheeler SB. Tricyclic dose requirements across pregnancy. Am J Psychiatry 1993;150:1541–2.Crossref, Google Scholar
105 O'Brien L, Baumer C, Thieme D, Sachs H, Koren G. Changes in antidepressant metabolism in pregnancy evidenced by metabolic ratios in hair: a novel approach. Forensic Sci Int 2010;196:93–6.Crossref, Google Scholar
106 Greene MF. Teratogenicity of SSRIs-serious concern or much ado about little? N Engl J Med 2007;356:2732–3.Crossref, Google Scholar
107 Einarson A, Choi J, Einarson T, Koren G. Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiatry 2009;54:242–6.Crossref, Google Scholar
108 Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry 2009;31:403–13.Crossref, Google Scholar
109 Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354:579–87.Crossref, Google Scholar
110 Källén B, Olausson PO. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Safety 2008;17:801–6.Crossref, Google Scholar
111 Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med 2009;71:235–42.Crossref, Google Scholar
112 Loo CK, McFarquhar TF, Mitchell PB. A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. International Journal of Neuropsychopharmacology 2008;11:131–47.Crossref, Google Scholar
113 Harrison-Hohner J, Coste S, Dorato V, Curet LB, McCarron D, Hatton D. Prenatal calcium supplementation and postpartum depression: an ancillary study to a randomized trial of calcium for prevention of preeclampsia. Arch Women Ment Health 2001;3:141–6.Crossref, Google Scholar
114 Armstrong K, Edwards H. The effects of exercise and social support on mothers reporting depressive symptoms: a pilot randomized controlled trial. Int J Mental Health Nursing 2003;12:130–8.Crossref, Google Scholar
115 Onozawa K, Glover V, Adams D, Modi N, Kumar RC. Infant massage improves mother-infant interaction for mothers with postnatal depression. J Affect Disord 2001;63:201–7.Crossref, Google Scholar
116 Field T, Diego MA, Hernandez-Reif M, Schanberg S, Kuhn C. Massage therapy effects on depressed pregnant women. J Psychosom Obstet Gynecol 2004;25:115–22.Crossref, Google Scholar
117 Oren DA, Wisner KL, Spinelli M, Epperson CN, Peindl KS, Terman JS, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 2002;159:666–9.Crossref, Google Scholar
118 Epperson CN, Terman M, Terman JS, Hanusa BH, Oren DA, Peindl KS, et al. Randomized clinical trial of bright light therapy for antepartum depression: preliminary findings. J Clin Psychiatry 2004;65:421–5.Crossref, Google Scholar
119 Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 fatty acids: evidence basis for treatment and future research. J Clin Psychiatry 2006;67:1954–67.Crossref, Google Scholar
120 Rees AM, Austin MP, Parker G. Role of omega-3 fatty acids as a treatment for depression in the perinatal period. Aust N Z J Psychiatry 2005;39:274–80.Crossref, Google Scholar
121 Borja-Hart NL, Marino J. Role of omega-3 fatty acids for prevention or treatment of perinatal depression. Pharmacotherapy 2010;30:210–6.Crossref, Google Scholar
122 Stowe ZN, Owens MJ, Landry JC, Kilts CD, Ely T, Llewellyn A, et al. Sertraline and desmethylsertraline in human breast milk and nursing infants. Am J Psychiatry 1997;154:1255–60.Crossref, Google Scholar
123 Stowe ZN, Cohen LS, Hostetter A, Ritchie JC, Owens MJ, Nemeroff CB. Paroxetine in human breast milk and nursing infants. Am J Psychiatry 2000;157:185–9.Crossref, Google Scholar
124 Lanza di Scalea T, Wisner KL. Antidepressant medication use during breastfeeding. Clin Obstet Gynecol 2009;52:483–97.Crossref, Google Scholar
125 Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA 1996;276:293–9.Crossref, Google Scholar
126 Sharma V, Khan M, Corpse C, Sharma P. Missed bipolarity and psychiatric comorbidity in women with postpartum depression. Bipolar Disord 2008;10:742–7.Crossref, Google Scholar
127 Viguera AC, Nonacs R, Cohen LS, Tondo L, Murray A, Baldessarini RJ. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry 2000;157:179–84.Crossref, Google Scholar
128 Yonkers KA, Wisner KL, Stowe Z, Leibenluft E, Cohen L, Miller L, et al. Management of bipolar disorder during pregnancy and the postpartum period. Am J Psychiatry 2004;161:608–20.Crossref, Google Scholar
129 Viguera AC, Cohen LS, Bouffard S, Whitfield TH, Baldessarini RJ. Reproductive decisions by women with bipolar disorder after prepregnancy psychiatric consultation. Am J Psychiatry 2002;159:2102–4.Crossref, Google Scholar
130 Harlow BL, Vitonis AF, Sparen P, Cnattingius S, Joffe H, Hultman CM. Incidence of hospitalization for postpartum psychotic and bipolar episodes in women with and without prior prepregnancy or prenatal psychiatric hospitalizations. Arch Gen Psychiatry 2007;64:42–8.Crossref, Google Scholar
131 Akdeniz F, Vahip S, Pirildar S, Vahip I, Doganer I, Bulut I. Risk factors associated with childbearing-related episodes in women with bipolar disorder. Psychopathology 2003;36:234–8.Crossref, Google Scholar
132 Jones I, Craddock N. Familiality of the puerperal trigger in bipolar disorder: results of a family study. Am J Psychiatry 2001;158:913–7.Crossref, Google Scholar
133 Reich T, Winokur G. Postpartum psychoses in patients with manic depressive disease. J. Nerv Ment Dis 1970;151:60–8.Crossref, Google Scholar
134 Newport DJ, Stowe ZN, Viguera AC, Calamaras MR, Juric S, Knight B, et al. Lamotrigine in bipolar disorder: efficacy during pregnancy. Bipolar Disord 2008;10:432–6.Crossref, Google Scholar
135 Viguera AC, Whitfield T, Baldessarini RJ, Newport DJ, Stowe Z, Reminick A, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry 2007;164:1817–24.Crossref, Google Scholar
136 Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt) 2006;15:352–68.Crossref, Google Scholar
137 Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry 1987;150:662–73.Crossref, Google Scholar
138 Munk-Olsen T, Laursen TM, Mendelson T, Pedersen CB, Mors O, Mortensen PB. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry 2009;66:189–95.Crossref, Google Scholar
139 Marks MN, Wieck A, Checkley SA, Kumar R. Contribution of psychological and social factors to psychotic and non-psychotic relapse after childbirth in women with previous histories of affective disorder. J Affect Disord 1992;24:253–63.Crossref, Google Scholar
140 Valdimarsdottir U, Hultman CM, Harlow B, Cnattingius S, Sparen P. Psychotic illness in first-time mothers with no previous psychiatric hospitalizations: A population-based study. PLoS Med 2009;6:e1000013.Crossref, Google Scholar
141 Vinogradov S, Csernansky JG. Postpartum psychosis with abnormal movements: dopamine supersensitivity unmasked by withdrawal of endogenous estrogens? J Clin Psychiatry 1990;51:365–6.Google Scholar
142 Kumar R, Marks M, Wieck A, Hirst D, Campbell I, Checkley S. Neuroendocrine and psychosocial mechanisms in postpartum psychosis. Prog Neuropsychopharmacol Biol Psychiatry 1993;17:571–9.Crossref, Google Scholar
143 Meakin CJ, Brockington IF, Lynch S, Jones SR. Dopamine supersensitivity and hormonal status in puerperal psychosis. The British Journal of Psychiatry 1995;166:73–9.Crossref, Google Scholar
144 Sharma V, Mazmanian D. Sleep loss and postpartum psychosis. Bipolar Disord 2003;5:98–105.Crossref, Google Scholar
145 Hlastala SA, Frank E. Adapting interpersonal and social rhythm therapy to the developmental needs of adolescents with bipolar disorder. Dev Psychopathol 2006;18:1267–88.Crossref, Google Scholar
146 Sharma V, Smith A, Khan M. The relationship between duration of labour, time of delivery, and puerperal psychosis. J Affect Disord 2004;83:215–20.Crossref, Google Scholar
147 Payne JL, MacKinnon DF, Mondimore FM, McInnis MG, Schweizer B, Zamoiski RB, et al. Familial aggregation of postpartum mood symptoms in bipolar disorder pedigrees. Bipolar Disord 2008;10:38–44.Crossref, Google Scholar
148 Jones I, Hamshere M, Nangle JM, Bennett P, Green E, Heron J, et al. Bipolar affective puerperal psychosis: genome-wide significant evidence for linkage to chromosome 16. Am J Psychiatry 2007;164:1099–104.Crossref, Google Scholar
149 Cox JL, Connor Y, Kendell RE. Prospective study of the psychiatric disorders of childbirth. Br J Psychiatry 1982;140:111–7.Crossref, Google Scholar
150 Hirschfield RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE Jr, et al. develoment and validation of a screening instrument for bioplar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2000;157:1873–5.Crossref, Google Scholar
151 Hirschfield R, Bowden CL, Gitlin MJ, Keck PE, Suppes T, Thase ME, et al. American Psychiatric Association practice guideline for the treatment of patients with bipolar disorder. 2nd ed. Arlington (VA): American Psychiatric Publishing, Inc.; 2002.Google Scholar
152 Frank E, Kupfer D, Ehlers C, Monk T, Cornes C, Carter S. Interpersonal and social rhythm therapy for bipolar disorder: integrating interpersonal and behavioral approaches. Behavior Therapist 1994;17:143–9.Google Scholar
153 Stewart DE, Robinson GE. Psychotropic drugs and electroconvulsive therapy during pregnancy and lactation. In: Stotland NL, Stewart DE, editors. Psychological aspects of Women's health care. The interface between psychiatry and obstetrics and gynecology. 2nd ed. Washington (DC): American Psychiatric Press; 2001. pp. 67–94.Google Scholar
154 Meador, KJ, Baker GA, Finnell RH, Kalayjian LA, Liporace JD, Loring DW, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology 2006;67:407–12.Crossref, Google Scholar
155 Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract 2009;15:183–92.Crossref, Google Scholar
156 Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML. A reevaluation of risk of in utero exposure to lithium. J Am Med Assoc 1994;271:146–50.Crossref, Google Scholar
157 Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996;153:592–606.Crossref, Google Scholar
158 Stewart DE, Klompenhouwer JL, Kendell RE, van Hulst AM. Psophylactic lithium in puerperal psychosis. The experience of three centres. Br J Psychiatry 1991;158:393–7.Crossref, Google Scholar
159 Cohen LS, Sichel DA, Robertson LM, Heckscher E, Rosenbaum JF. Postpartum prophylaxis for women with bioplar disorder. Am J Psychiatry 1995;152:1641–5.Crossref, Google Scholar
160 Wisner KL, Hanusa BH, Peindl K, Perel J. Prevention of postpartum episodes in women with bipolar disorder Biol Psychiatry 2004;56:592–6.Google Scholar
161 Gentile S. Infant safety with antipsychotic therapy in breastfeeding: a systematic review. J Clin Psychiatry 2008;69:666–73.Crossref, Google Scholar
162 Eberhard-Gran M, Eskild A, Opjordsmoen S. Use of psychotropic medications in treating mood disorders during lactation: practical recommendations. CNS Drugs 2006;20:187–98.Crossref, Google Scholar
163 Gentile S. Prophylactic treatment of bipolar disorder in pregnancy and breastfeeding: focus on emerging mood stabilizers. Bipolar Disord 2006;8:207–20.Crossref, Google Scholar
164 American Academy of Pediatrics Committee on Drugs. Use of psychoactive medication during pregnancy and possible effects on the fetus and newborn. Pediatrics 2000;105:880–7.Crossref, Google Scholar



